A Fluorescent Cy7-Mercaptopyridine for the Selective Detection of Glutathione over Homocysteine and Cysteine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. UV-Vis Absorption and Fluorescence Spectroscopic Methods
2.3. Quantum Yield (ФF) Measurement
2.4. Cell Culture
2.5. Cell Viability
2.6. Confocal Microscope Imaging Analysis
2.7. Computational Study
2.8. Synthesis
2.8.1. Synthesis of 2
2.8.2. Synthesis of 1
3. Results and Discussion
3.1. Photophysical Study of 1 for the Detection of GSH over Cys/Hcy
3.2. Theoretical Study of Probe 1 and the Interaction with GSH and Cys
3.3. Frontier Molecular Orbitals of N- and S-Substituted Cy7 Compounds
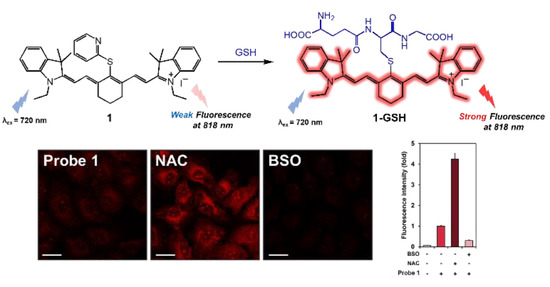
3.4. Fluorescence Imaging of the Cellular GSH Using Probe 1 in Live Human Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Dalton, T.P.; Shertzer, H.G.; Puga, A. Regulation of gene expression by reactive oxygen. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 67–101. [Google Scholar] [CrossRef] [PubMed]
- Meister, A.; Anderson, M.E. Glutathione. Annu. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.M.; Ortega, A.; Obrador, E. Glutathione in cancer biology and therapy. Crit. Rev. Clin. Lab. Sci. 2006, 43, 143–181. [Google Scholar] [CrossRef] [PubMed]
- Dooley, C.T.; Dore, T.M.; Hanson, G.T.; Jackson, W.C.; Remington, S.J.; Tsien, R.Y. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 2004, 279, 22284–22293. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.S.; Chen, X.; Kim, J.S.; Yoon, J. Recent progress in luminescent and colorimetric chemosensors for detection of thiols. Chem. Soc. Rev. 2013, 42, 6019–6031. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Qiang, M.; Gao, W.; Su, R.; Li, N.; Gao, Y.; Xie, Y.; Kong, F.; Tang, B. A near-infrared reversible fluorescent probe for real-time imaging of redox status changes in vivo. Chem. Sci. 2013, 4, 1079–1086. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, X.; Miao, Y.; Hu, Y.; Kwon, N.; Wu, X.; Yoon, J. A reversible fluorescent probe for real-time quantitative monitoring of cellular glutathione. Angew. Chem. Int. Ed. 2017, 56, 5812–5816. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Sharma, A.; Jang, J.H.; Shin, W.S.; Lee, J.H.; Kim, J.S. Real time OFF–ON monitoring of glutathione (GSH) in living cell. J. Incl. Phenom. Macrocycl. 2015, 82, 117–122. [Google Scholar] [CrossRef]
- Niu, L.Y.; Guan, Y.S.; Chen, Y.Z.; Wu, L.Z.; Tung, C.H.; Yang, Q.Z. BODIPY-based ratiometric fluorescent sensor for highly selective detection of glutathione over cysteine and homocysteine. J. Am. Chem. Soc. 2012, 134, 18928–18931. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Xu, Q.; Liu, Y.; Wei, H.; Tang, Y.; Lin, W. Coumarin-based turn-on fluorescence probe for specific detection of glutathione over cysteine and homocysteine. ACS Appl. Mater. Interfaces 2015, 7, 12809–12813. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.Y.; Guan, Y.S.; Chen, Y.Z.; Wu, L.Z.; Tung, C.H.; Yang, Q.Z. A turn-on fluorescent sensor for the discrimination of cystein from homocystein and glutathione. Chem. Commun. 2013, 49, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shao, X.; Wang, Y.; Pan, F.; Kang, R.; Peng, F.; Huang, Z.; Zhang, W.; Zhao, W. Dual emission channels for sensitive discrimination of Cys/Hcy and GSH in plasma and cells. Chem. Commun. 2015, 51, 4245–4248. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wu, X.; Shi, W.; Ma, H. Comparison of N-acetylcysteine and cysteine in their ability to replenish intracellular cysteine by a specific fluorescent probe. Chem. Commun. 2016, 52, 9410–9413. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Huang, Q.; Zhong, Y.; Li, Z.; Li, H.; Lowry, M.; Escobedo, J.O.; Strongin, R.M. A dual emission fluorescent probe enables simultaneous detection of glutathione and cysteine/homocysteine. Chem. Sci. 2014, 5, 2177–2183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Sun, Y.Q.; Huo, Y.; Zhang, H.; Wang, L.; Zhang, P.; Song, D.; Shi, Y.; Guo, W. Simultaneous fluorescence sensing of Cys and GSH from different emission channels. J. Am. Chem. Soc. 2017, 136, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Kwon, Y.; Kim, D.; Lee, D.; Kim, G.; Hu, Y.; Ryu, J.H.; Yoon, J. Cyanine-based fluorescent probe for highly selective detection of glutathione in cell cultures and live mouse tissues. J. Am. Chem. Soc. 2014, 136, 5351–5358. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Hong, K.H.; Kim, D.I.; Kwon, H.; Kim, H.J. Tunable heptamethine-azo dye conjugate as an NIR fluorescent probe for the selective detection of mitochondrial glutathione over cysteine and homocysteine. J. Am. Chem. Soc. 2014, 136, 7018–7025. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Kwon, Y.; Kim, D.; Lee, D.; Kim, G.; Hu, Y.; Ryu, J.-H.; Yoon, J. Preparation of a cyanine-based fluorescent probe for highly selective detection of glutathione and its use in living cells and tissues of mice. Nat. Protoc. 2015, 10, 1742–1754. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Chen, D.; Cao, M.; Zhang, Y.; Han, X.; Chen, X.; Liu, S.; Chen, H.; Yin, J. A near infrared cyanine-based fluorescent probe for highly selectively detecting glutathione in living cells. Chin. J. Chem. 2016, 34, 594–598. [Google Scholar] [CrossRef]
- Lee, D.; Jeong, K.; Luo, X.; Kim, G.; Yang, Y.; Chen, X.; Kim, S.; Yoon, J. Near-infrared fluorescent probes for the detection of glutathione and their application in the fluorescence imaging of living cells and tumor-bearing mice. J. Mater. Chem. B 2018, 6, 2541–2546. [Google Scholar] [CrossRef]
- Liu, C.-H.; Qi, F.-P.; Wen, F.-B.; Long, L.-P.; Liu, A.-J.; Yang, R.-H. Fluorescence detection of glutathione and oxidized glutathione in blood with a NIR-excitable cyanine probe. Methods Appl. Fluoresc. 2018, 6, 024001. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, Y.Q.; Zhang, H.; Huo, Y.; Shi, Y.; Guo, W. Simultaneous fluorescent imaging of Cys/Hcy and GSH from different emission channels. Chem. Sci. 2014, 5, 3183–3188. [Google Scholar] [CrossRef]
- Wang, X.; Lv, J.; Yao, X.; Li, Y.; Huang, F.; Li, M.; Yang, J.; Ruan, X.; Tang, B. Screening and investigation of a cyanine fluorescent probe for simultaneous sensing of glutathione and cysteine under single excitation. Chem. Commun. 2014, 50, 15439–15442. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent development of chemosensors based on cyanine platforms. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H. Bis(naphthalimide-piperazine)-based off-on fluorescent probe for acids. J. Fluoresc. 2016, 26, 807–811. [Google Scholar] [CrossRef] [PubMed]
- Oushiki, D.; Kojima, H.; Terai, T.; Arita, M.; Hanaoka, K.; Urano, Y.; Nagano, T. Development and application of a near-infrared fluorescence probe for oxidative stress based on differential reactivity of linked cyanine dyes. J. Am. Chem. Soc. 2010, 132, 2795–2801. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, E.; Kojima, H.; Nishimatsu, H.; Urano, Y.; Kikuchi, K.; Hirata, Y.; Nagano, T. Highly sensitive near-infrared fluorescent probes for nitric oxide and their application to isolated organs. J. Am. Chem. Soc. 2005, 127, 3684–3685. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Raghavachari, K.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XX. Basis set for correlated wave-functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian-basis sets for molecular calculations. 1. 2nd row atoms, Z = 11 − 18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; von Ragué Schleyer, P. Efficient diffuse function-augmented basis-sets for anion calculations. 3. The 3-21 + G basis set for 1st-row elements, Li-F. J. Comp. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-Consistent Molecular Orbital Methods. 25. Supplementary Functions for Gaussian Basis Sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Lipparini, F.; Scalmani, G.; Mennucci, B.; Cances, E.; Caricato, M.; Frisch, M.J. A variational formulation of the polarizable continuum model. J. Chem. Phys. 2010, 133, 014106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Allouche, A.R. Gabedit—A graphical user interface for computational chemistry softwares. J. Comp. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Y.; Li, J.; Su, Q.; Yuan, W.; Dai, Y.; Han, C.; Wang, Q.; Feng, W.; Li, F. Ultrasensitive near-infrared fluorescence-enhanced probe for in vivo nitroreductase imaging. J. Am. Chem. Soc. 2015, 137, 6407–6416. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, W.; Park, S.H.; Han, J.; Lee, J.; Kang, C.; Lee, M.H. An endoplasmic reticulum-selective ratiometric fluorescent probe for imaging a copper pool. Chem. Commun. 2017, 53, 4457–4460. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Jami Alahmadi, Y.; Rowley, C.N. Range-separated DFT functionals are necessary to model thio-Michael additions. J. Chem. Theory Comput. 2013, 9, 4860–4865. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Gausson, V.; Nguyen, A.T.; Touam, M.; Drüeke, T.; Santangelo, F.; Descamps-Latscha, B. AOPP-induced activation of human neutrophil and monocyte oxidative metabolism: A potential target for N-acetylcysteine treatment in dialysis patients. Kidney Int. 2003, 64, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.X.; Zhang, H.Y.; Meng, X.; Guan, Y.; Wang, H.Q. Role of oxidative stress and intracellular glutathione in the sensitivity to apoptosis induced by proteasome inhibitor in thyroid cancer cells. BMC Cancer 2009, 9, 56–66. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, S.A.; Kim, W.; Sharma, A.; Verwilst, P.; Won, M.; Lee, M.H. A Fluorescent Cy7-Mercaptopyridine for the Selective Detection of Glutathione over Homocysteine and Cysteine. Sensors 2018, 18, 2897. https://doi.org/10.3390/s18092897
Yoon SA, Kim W, Sharma A, Verwilst P, Won M, Lee MH. A Fluorescent Cy7-Mercaptopyridine for the Selective Detection of Glutathione over Homocysteine and Cysteine. Sensors. 2018; 18(9):2897. https://doi.org/10.3390/s18092897
Chicago/Turabian StyleYoon, Shin A, Wantae Kim, Amit Sharma, Peter Verwilst, Miae Won, and Min Hee Lee. 2018. "A Fluorescent Cy7-Mercaptopyridine for the Selective Detection of Glutathione over Homocysteine and Cysteine" Sensors 18, no. 9: 2897. https://doi.org/10.3390/s18092897