An Overview of High-k Oxides on Hydrogenated-Diamond for Metal-Oxide-Semiconductor Capacitors and Field-Effect Transistors
Abstract
1. Introduction
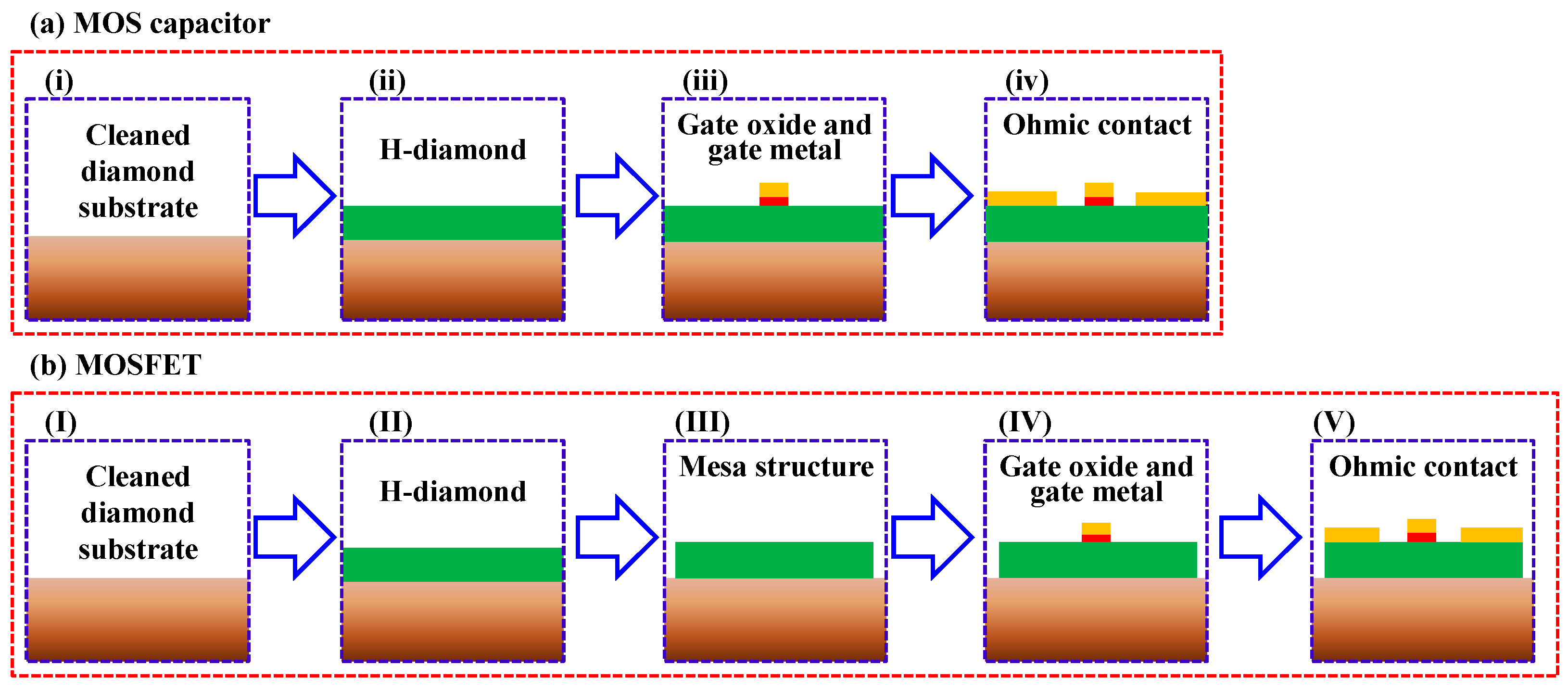
2. Materials and Methods
3. Results and Discussion
3.1. Band Configurations of High-k Oxide/H-Diamond Heterointerfaces
3.2. High-k Oxides on H-Diamond for MOS Capacitors
3.2.1. ALD-Al2O3 and ALD-HfO2 Single Layers
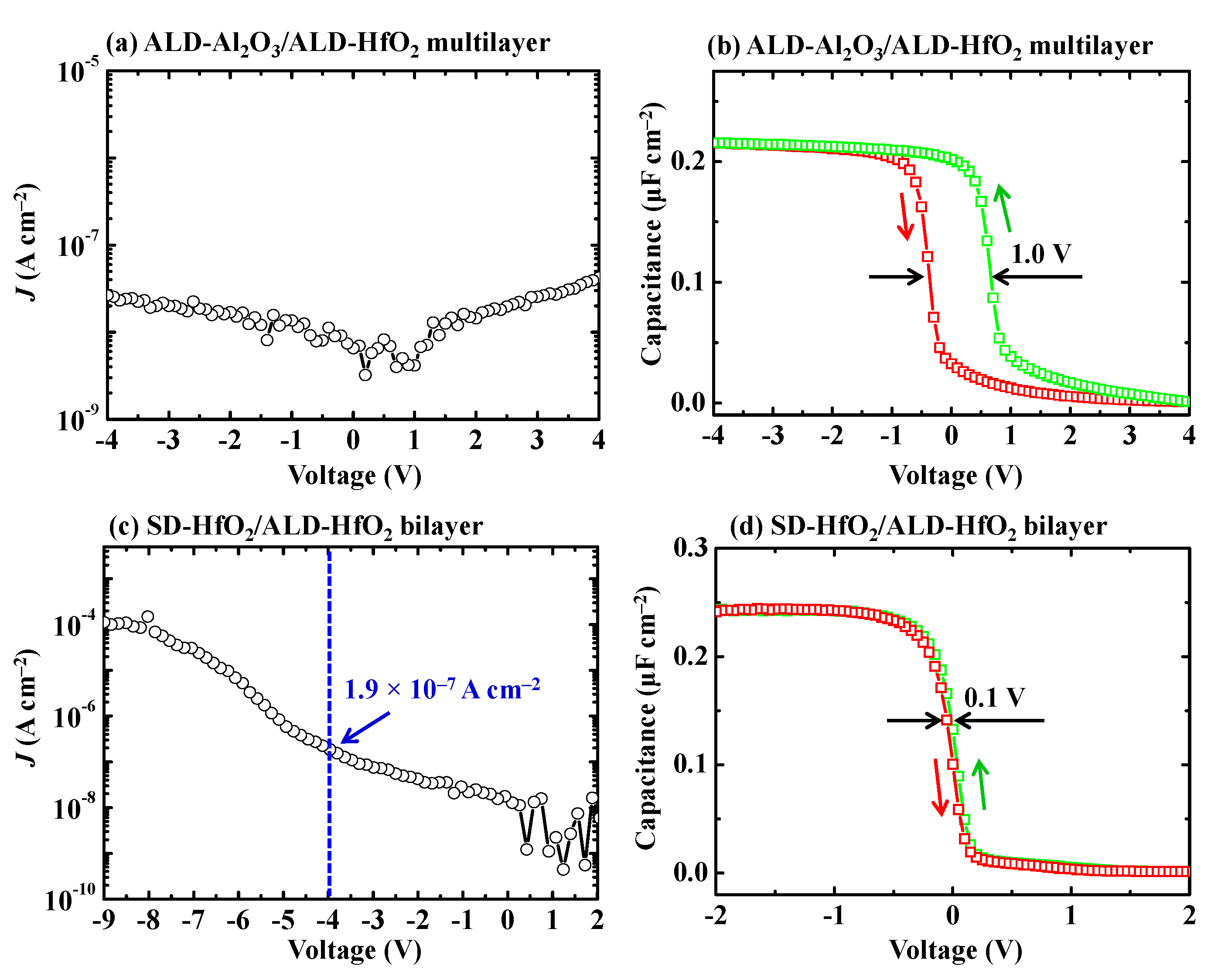
3.2.2. ALD-HfO2/ALD-Al2O3 Multilayer and SD-HfO2/ALD-HfO2 Bilayer
3.2.3. SD-TiO2/ALD-Al2O3 and ALD-TiO2/ALD-Al2O3 Bilayers
3.2.4. Discussion for High-k Oxide/H-Diamond MOS Capacitors
3.3. Electrical Properties of H-Diamond MOSFETs
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bhatnagar, M.; Baliga, B.J. Comparison of 6H-SiC, 3C-SiC, and Si for power devices. IEEE Tran. Electron Dev. 1993, 40, 645–655. [Google Scholar] [CrossRef]
- Saito, W.; Takada, Y.; Kuraguchi, M.; Tsuda, K.; Omura, I.; Ogura, T.; Ohashi, H. High breakdown voltage AlGaN-GaN power-HEMT design and high current density switching behavior. IEEE Tran. Electron Dev. 2013, 50, 2528–2531. [Google Scholar] [CrossRef]
- Umezawa, H.; Nagase, M.; Kato, Y.; Shikata, S.I. High temperature application of diamond power device. Diam. Relat. Mater. 2012, 24, 201–205. [Google Scholar] [CrossRef]
- Wort, C.J.H.; Balmer, R.S. Diamond as an electronic material. Mater. Today 2008, 11, 22–28. [Google Scholar] [CrossRef]
- Gaska, R.; Shur, M.S.; Bykhovski, A.D.; Orlov, A.O.; Snider, G.L. Electron mobility in modulation-doped AlGaN-GaN heterointerfaces. Appl. Phys. Lett. 1999, 74, 287–289. [Google Scholar] [CrossRef]
- Trew, R.J. SiC and GaN transistors-is there one winner for microwave power applications? Proc. IEEE 2002, 90, 1032–1047. [Google Scholar] [CrossRef]
- Song, K.; Zhang, G.; Nakamura, Y.; Furukawa, K.; Hiraki, T.; Yang, J.; Funatsu, T.; Ohdomari, I.; Kawarada, H. Label-free DNA sensors using ultrasensitive diamond field-effect transistors in solution. Phys. Rev. E 2006, 74, 041919. [Google Scholar] [CrossRef] [PubMed]
- Dankerl, M.; Eick, S.; Hofmann, B.; Hauf, M.; Ingebrandt, S.; Offenhausser, A.; Stutzmann, M.; Garrido, J.A. Diamond transistor array for extracellular recording from electrogenic cells. Adv. Funct. Mater. 2009, 19, 2915–2923. [Google Scholar] [CrossRef]
- Nebel, C.E.; Shin, D.; Rezek, B.; Tokuda, N.; Uetsuka, H.; Watanabe, H. Diamond and biology. J. R. Soc. Interface 2007, 4, 439–461. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, S.; Watanabe, K.; Hasegawa, M.; Kanda, H. Ultraviolet emission from a diamond pn junction. Science 2001, 292, 1899–1901. [Google Scholar] [CrossRef] [PubMed]
- Mainwood, A. Recent developments of diamond detectors for particles and UV radiation. Semicond. Sci. Technol. 2000, 15, R55–R63. [Google Scholar] [CrossRef]
- Gurbuz, Y.; Kang, W.P.; Davidson, J.L.; Kerns, D.V. High-temperature tolerant diamond diode for carbon monoxide gas detection. J. App. Phys. 1998, 84, 6935–6936. [Google Scholar] [CrossRef]
- Gurbuz, Y.; Kang, W.P.; Davidson, J.L.; Kinser, D.L.; Kerns, D.V. Diamond microelectronic gas sensors. Sens. Actuators B 1996, 33, 100–104. [Google Scholar] [CrossRef]
- Davidson, J.L.; Kang, W.P.; Gurbuz, Y.; Holmes, K.C.; Davis, L.G.; Wisitsora-at, A.; Kerns, D.V.; Eidson, R.L.; Henderson, T. Diamond as an active sensor material. Diam. Relat. Mater. 1999, 8, 1741–1747. [Google Scholar] [CrossRef]
- Pruvost, F.; Bustarret, E.; Deneuville, A. Characteristics of homoepitaxial heavily boron-doped diamond films from their Raman spectra. Diam. Relat. Mater. 2000, 9, 295–299. [Google Scholar] [CrossRef]
- Bustarret, E.; Gheeraert, E.; Watanabe, K. Optical and electronic properties of heavily boron-doped homo-epitaxial diamond. Phys. Status Solidi (a) 2003, 199, 9–18. [Google Scholar] [CrossRef]
- Strobel, P.; Riedel, M.; Ristein, J.; Ley, L. Surface transfer doping of diamond. Nature 2004, 430, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Yamanaka, S.; Okushi, H.; Kajimura, K. Study of the effect of hydrogen on transport properties in chemical vapor deposited diamond films by Hall measurements. Appl. Phys. Lett. 1996, 68, 376–378. [Google Scholar] [CrossRef]
- Pakes, C.I.; Garrido, J.A.; Kawarada, H. Diamond surface conductivity: Properties, devices, and sensors. MRS Bull. 2014, 39, 542–548. [Google Scholar] [CrossRef]
- Kubovic, M.; Kasu, M.; Kageshima, H.; Maeda, F. Electronic and surface properties of H-terminated diamond surface affected by NO2 gas. Diam. Relat. Mater. 2010, 19, 889–893. [Google Scholar] [CrossRef]
- Imura, M.; Hayakawa, R.; Watanabe, E.; Liao, M.; Koide, Y.; Amano, H. Demonstration of diamond field effect transistors by AlN/diamond heterostructure. Phys. Status Solidi (RRL) 2011, 5, 125–127. [Google Scholar] [CrossRef]
- Winquist, F.; Spetz, A.; Armgarth, M.; Nylander, C.; Lundström, I. Modified palladium metal-oxide-semiconductor structures with increased ammonia gas sensitivity. Appl. Phys. Lett. 1983, 43, 839–841. [Google Scholar] [CrossRef]
- Arbab, A.; Spetz, A.; Lundström, I. Gas sensors for high temperature operation based on metal oxide silicon carbide (MOSiC) devices. Sens. Actuators B Chem. 1993, 15, 19–23. [Google Scholar] [CrossRef]
- Mizsei, J. How can sensitive and selective semiconductor gas sensors be made? Sens. Actuators B Chem. 1995, 23, 173–176. [Google Scholar] [CrossRef]
- Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S. A review of gas sensors employed in electronic nose applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef]
- Wingbrant, H.; Svenningstorp, H.; Salomonsson, P.; Kubinski, D.; Visser, J.H.; Lofdahl, M.; Spetz, A.L. Using a MISiC-FET sensor for detecting NH/sub 3/in SCR systems. IEEE Sens. J. 2005, 5, 1099–1105. [Google Scholar] [CrossRef]
- Spetz, A.L.; Skoglundh, M.; Ojamäe, L. FET gas-sensing mechanism, experimental and theoretical studies. In Solid State Gas Sensing; Gomini, E., Faglia, G., Sberveglieri, G., Eds.; Springer: New York, NY, USA, 2009; pp. 153–197. [Google Scholar]
- Liu, J.W.; Liao, M.Y.; Imura, M.; Oosato, H.; Watanabe, E.; Koide, Y. Electrical properties of atomic layer deposited HfO2/Al2O3 multilayer on diamond. Diam. Relat. Mater. 2015, 54, 55–58. [Google Scholar] [CrossRef]
- Liu, J.W.; Liao, M.Y.; Imura, M.; Oosato, H.; Watanabe, E.; Koide, Y. Electrical characteristics of hydrogen-terminated diamond metal-oxide-semiconductor with atomic layer deposited HfO2 as gate dielectric. Appl. Phys. Lett. 2013, 102, 112910. [Google Scholar] [CrossRef]
- Liu, J.W.; Liao, M.Y.; Imura, M.; Koide, Y. Normally-off HfO2-gated diamond field effect transistors. Appl. Phys. Lett. 2013, 103, 092905. [Google Scholar] [CrossRef]
- Liu, J.W.; Liao, M.Y.; Imura, M.; Banal, R.G.; Koide, Y. Deposition of TiO2/Al2O3 bilayer on hydrogenated diamond for electronic devices: Capacitors, field-effect transistors, and logic inverters. J. Appl. Phys. 2017, 121, 224502. [Google Scholar] [CrossRef]
- Liu, J.W.; Liao, M.Y.; Imura, M.; Watanabe, E.; Oosato, H.; Koide, Y. Diamond field effect transistors with a high-dielectric constant Ta2O5 as gate material. J. Phys. D Appl. Phys. 2014, 47, 245102. [Google Scholar] [CrossRef]
- Liu, J.W.; Liao, M.Y.; Imura, M.; Tanaka, A.; Iwai, H.; Koide, Y. Low on-resistance diamond field effect transistor with high-k ZrO2 as dielectric. Sci. Rep. 2014, 4, 6395. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Ohsato, H.; Wang, X.; Liao, M.Y.; Koide, Y. Design and fabrication of high-performance diamond triple-gate field-effect transistors. Sci. Rep. 2016, 6, 34757. [Google Scholar] [CrossRef] [PubMed]
- Hirama, K.; Sato, H.; Harada, Y.; Yamamoto, H.; Kasu, M. Diamond field-effect transistors with 1.3 A/mm drain current density by Al2O3 passivation layer. Jpn. J. Appl. Phys. 2012, 51, 090112. [Google Scholar]
- Kawarada, H.; Tsuboi, H.; Naruo, T.; Yamada, T.; Xu, D.; Daicho, A.; Saito, T.; Hiraiwa, A. CH surface diamond field effect transistors for high temperature (400 °C) and high voltage (500 V) operation. Appl. Phys. Lett. 2014, 105, 013510. [Google Scholar] [CrossRef]
- Kitabayashi, Y.; Kudo, T.; Tsuboi, H.; Yamada, T.; Xu, D.; Shibata, M.; Matsumura, D.; Hayashi, Y.; Syamsul, M.; Inaba, M.; et al. Normally-Off C–H diamond MOSFETs with partial C–O channel achieving 2-kV breakdown voltage. IEEE Electron Dev. Lett. 2017, 38, 363–366. [Google Scholar] [CrossRef]
- Liu, J.W.; Oosato, H.; Liao, M.Y.; Koide, Y. Enhancement-mode hydrogenated diamond metal-oxide-semiconductor field-effect transistors with Y2O3 oxide insulator grown by electron beam evaporator. Appl. Phys. Lett. 2017, 110, 203502. [Google Scholar] [CrossRef]
- Liu, J.W.; Ohsato, H.; Liao, M.Y.; Imura, M.; Watanabe, E.; Koide, Y. Logic circuits with hydrogenated diamond field-effect transistors. IEEE Electron Dev. Lett. 2017, 38, 922–925. [Google Scholar] [CrossRef]
- Cheng, S.H.; Sang, L.; Liao, M.Y.; Liu, J.W.; Imura, M.; Li, H.; Koide, Y. Integration of high-dielectric constant Ta2O5 oxides on diamond for power devices. Appl. Phys. Lett. 2012, 101, 232907. [Google Scholar] [CrossRef]
- Yamasaki, S.; Gheeraert, E.; Koide, Y. Doping and interface of homoepitaxial diamond for electronic applications. MRS Bull. 2014, 39, 499–503. [Google Scholar] [CrossRef]
- Kordoš, P.; Gregušová, D.; Stoklas, R.; Gaži, Š.; Novák, J. Transport properties of AlGaN/GaN metal–oxide–semiconductor heterostructure field-effect transistors with Al2O3 of different thickness. Solid-State Electron. 2008, 52, 973–979. [Google Scholar] [CrossRef]
- Marinel, S.; Choi, D.H.; Heuguet, R.; Agrawal, D.; Lanagan, M. Broadband dielectric characterization of TiO2 ceramics sintered through microwave and conventional processes. Ceram. Int. 2013, 39, 299–306. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, J.W.; Sang, L.W.; Liao, M.Y.; Coathup, D.; Imura, M.; Shi, B.; Gu, C.Z.; Koide, Y.; Ye, H. Assembly of a high-dielectric constant thin TiOx layer directly on H-terminated semiconductor diamond. Appl. Phys. Lett. 2016, 108, 012105. [Google Scholar] [CrossRef]
- Banal, R.G.; Imura, M.; Liu, J.W.; Koide, Y. Structural properties and transfer characteristics of sputter deposition AlN and atomic layer deposition Al2O3 bilayer gate materials for H-terminated diamond field effect transistors. J. Appl. Phys. 2016, 120, 115307. [Google Scholar] [CrossRef]
- Liu, J.W.; Liao, M.Y.; Imura, M.; Koide, Y. Band offsets of Al2O3 and HfO2 oxides deposited by atomic layer deposition technique on hydrogenated diamond. Appl. Phys. Lett. 2012, 101, 252108. [Google Scholar] [CrossRef]
- Vanhove, E.; De Sanoit, J.; Arnault, J.C.; Saada, S.; Mer, C.; Mailley, P.; Bergonzo, P.; Nesladek, M. Stability of H-terminated BDD electrodes: An insight into the influence of the surface preparation. Phys. Status Solidi (a) 2007, 204, 2931–2939. [Google Scholar] [CrossRef]
- Shi, K.; Liu, X.L.; Li, D.B.; Wang, J.; Song, H.P.; Xu, X.Q.; Wei, H.Y.; Jiao, C.M.; Yang, S.Y.; Song, H.; et al. Valence band offset of GaN/diamond heterojunction measured by X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2011, 257, 8110–8112. [Google Scholar] [CrossRef]
- Liu, J.W.; Liao, M.Y.; Cheng, S.H.; Imura, M.; Koide, Y. Interfacial chemical bonding state and band alignment of CaF2/hydrogen-terminated diamond heterojunction. J. Appl. Phys. 2013, 113, 123706. [Google Scholar] [CrossRef]
- Liu, J.W.; Cheng, S.H.; Liao, M.Y.; Imura, M.; Tanaka, A.; Iwai, H.; Koide, Y. Interfacial electronic band alignment of Ta2O5/hydrogen-terminated diamond heterojunction determined by X-ray photoelectron spectroscopy. Diam. Relat. Mater. 2013, 38, 24–27. [Google Scholar] [CrossRef]
- Liu, G.X.; Shan, F.K.; Lee, W.J.; Shin, B.C. Growth temperature dependence of TiO2 thin films prepared by using plasma-enhanced atomic layer deposition method. J. Korean Phys. Soc. 2007, 50, 1827. [Google Scholar] [CrossRef]
- Deal, B.E. Standardized terminology for oxide charges associated with thermally oxidized silicon. IEEE Trans. Electron Dev. 1980, 27, 606–608. [Google Scholar] [CrossRef]
- Lai, B.C.M.; Kung, N.H.; Lee, J.Y.M. A study on the capacitance-voltage characteristics of metal-Ta2O5-silicon capacitors for very large scale integration metal-oxide-semiconductor gate oxide applications. J. Appl. Phys. 1999, 85, 4087. [Google Scholar]
- Chang, Y.C.; Chiu, H.C.; Lee, Y.J.; Huang, M.L.; Lee, K.Y.; Hong, M.; Chiu, Y.N.; Kwo, J.; Wang, Y.H. Structural and electrical characteristics of atomic layer deposited high κ HfO2 on GaN. Appl. Phys. Lett. 2007, 90, 232904. [Google Scholar] [CrossRef]
- Kukli, K.; Ritala, M.; Sajavaara, T.; Keinonen, J.; Leskelä, M. Comparison of hafnium oxide films grown by atomic layer deposition from iodide and chloride precursors. Thin Solid Films 2002, 416, 72–79. [Google Scholar] [CrossRef]
- Takeuchi, D.; Riedel, M.; Ristein, J.; Ley, L. Surface band bending and surface conductivity of hydrogenated diamond. Phys. Rev. B 2003, 68, 041304. [Google Scholar] [CrossRef]
- Maier, F.; Riedel, M.; Mantel, B.; Ristein, J.; Ley, L. Origin of surface conductivity in diamond. Phys. Rev. Lett. 2000, 85, 3472. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.W.; Liao, M.Y.; Imura, M.; Matsumoto, T.; Shibata, N.; Ikuhara, Y.; Koide, Y. Control of normally on/off characteristics in hydrogenated diamond metal-insulator-semiconductor field-effect transistors. J. Appl. Phys. 2015, 118, 115704. [Google Scholar] [CrossRef]







| Properties | Si | 4H-SiC | GaN | Diamond |
|---|---|---|---|---|
| Bandgap energy (eV) | 1.12 | 3.2 | 3.4 | 5.47 |
| Breakdown field (MV·cm−1) | 0.3 | 3 | 5 | 10 |
| Thermal conductivity (W·cm−1·K−1) | 1.5 | 5.0 | 1.3 | 24 |
| Electron mobility (cm2·V−1·s−1) | 1450 | 900 | 2000 | 4500 |
| Hole mobility (cm2·V−1·s−1) | 480 | 120 | 200 | 3800 |
| Saturation electron velocity (×107 cm−1) | 0.86 | 3 | 2.5 | 2 |
| Saturation hole velocity (×107 cm−1) | - | - | - | 0.8 |
| Sample | C 1s | Al 2p3/2 | Hf 4f7/2 | Ti 2p3/2 | VBM |
|---|---|---|---|---|---|
| H-diamond | 284.3 | 1.2 | |||
| Al2O3 (20 nm) | 76.3 | 5.4 | |||
| Al2O3 (4 nm) | 284.0 | 74.7 | |||
| HfO2 (20 nm) | 18.3 | 4.3 | |||
| HfO2 (4 nm) | 284.0 | 17.5 | |||
| TiO2 (25 nm)/Al2O3 | 459.2 | 3.4 | |||
| TiO2 (3 nm)/Al2O3 | 75.0 | 459.3 |
| Oxide Insulators | J at −4.0 V (A·cm−2) | k | Hysteresis Loop Voltage (V) | Voltage Shift Related to 0 V (V) |
|---|---|---|---|---|
| ALD-Al2O3 | 1.0 × 10−7 | 5.4 | 0 | small |
| ALD-HfO2 (300 °C annealing) | 8.5 × 10−9 | 11.2 | 0.5 | large |
| ALD-HfO2/ALD-Al2O3 multilayer | 2.7 × 10−8 | 7.6 | 1.0 | - |
| SD-HfO2/ALD-HfO2 bilayer | 1.9 × 10−7 | 9.1 | 0.1 | small |
| SD-TiO2 (O2: 0%)/ALD-Al2O3 bilayer | 1.0 × 10−2 | 22.5 | 0.3 | large |
| ALD-TiO2/ALD-Al2O3 (4 nm) bilayer | 6.0 × 10−6 | 27.2 | 0.06 | large |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Koide, Y. An Overview of High-k Oxides on Hydrogenated-Diamond for Metal-Oxide-Semiconductor Capacitors and Field-Effect Transistors. Sensors 2018, 18, 1813. https://doi.org/10.3390/s18061813
Liu J, Koide Y. An Overview of High-k Oxides on Hydrogenated-Diamond for Metal-Oxide-Semiconductor Capacitors and Field-Effect Transistors. Sensors. 2018; 18(6):1813. https://doi.org/10.3390/s18061813
Chicago/Turabian StyleLiu, Jiangwei, and Yasuo Koide. 2018. "An Overview of High-k Oxides on Hydrogenated-Diamond for Metal-Oxide-Semiconductor Capacitors and Field-Effect Transistors" Sensors 18, no. 6: 1813. https://doi.org/10.3390/s18061813
APA StyleLiu, J., & Koide, Y. (2018). An Overview of High-k Oxides on Hydrogenated-Diamond for Metal-Oxide-Semiconductor Capacitors and Field-Effect Transistors. Sensors, 18(6), 1813. https://doi.org/10.3390/s18061813






