A Multi-Region Magnetoimpedance-Based Bio-Analytical System for Ultrasensitive Simultaneous Determination of Cardiac Biomarkers Myoglobin and C-Reactive Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
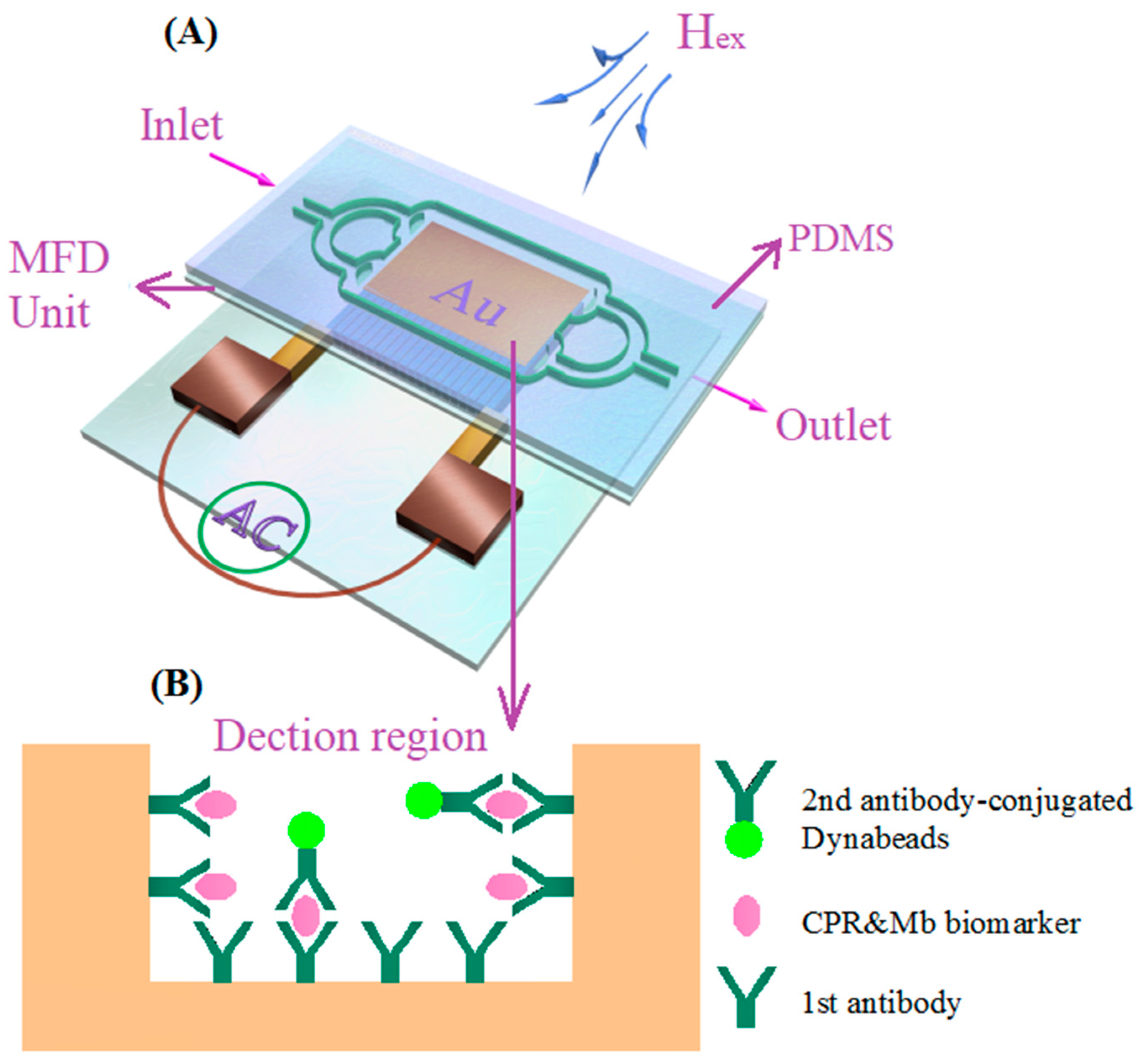
2.2. Microfabrication of MI Element and MR-MFD
- Fabrication of gold layer: First a chromium adhesion layer (~60 nm) was deposited on the glass wafer with a thickness of 1 mm at a rate of 1 Å s−1, followed by ~240 nm of gold at a rate of 2–3 Å s−1 by a radio frequency sputtering system (LH-Z550, Shanghai, China).
- Patterning of the gold film: A photoresist layer with a thickness of 10 μm was spun onto the Au layer and afterwards patterned to several small MFD units through the mask.
- Deleting the uncovered part of the gold layer: the uncovered part of the Au layer was removed by wet etching in the KI, I2 and H2O mixed solution for 45 s.
- Deleting the photoresist: The whole glass substrate with Au film was immersed in acetone solution for 25 s.
- SU-8 layers preparation: The SU-8 photoresist with a thickness of 500 μm was spin coated on Au film, soft baked, patterned with a mask, and developed with an SU-8 developer. Then, the same thickness of the SU-8 photoresist was spun again, finally a 5 × 3 mm2 rectangular microcavity with a depth of 1 mm was achieved. Figure 1B showed the SEM of gold nanofilm.
- PDMS casting: The pre-polymer and the curing agent of PDMS were mixed in a 10:1 ratio by weight. After thermal coagulation, the PDMS can be obtained.
- Bonding of the SU-8 with the PDMS: The surface-treated PDMS was tightly bound to the Su-8 surface and then opened the inlets and waste chamber. Figure 1A (inset) shows the fabricated microfluidic device without PDMS.
2.3. Sandwich Immunoassay for Capturing CBs
2.4. Determination of CBs Using MI-Based Bio-Analytic System
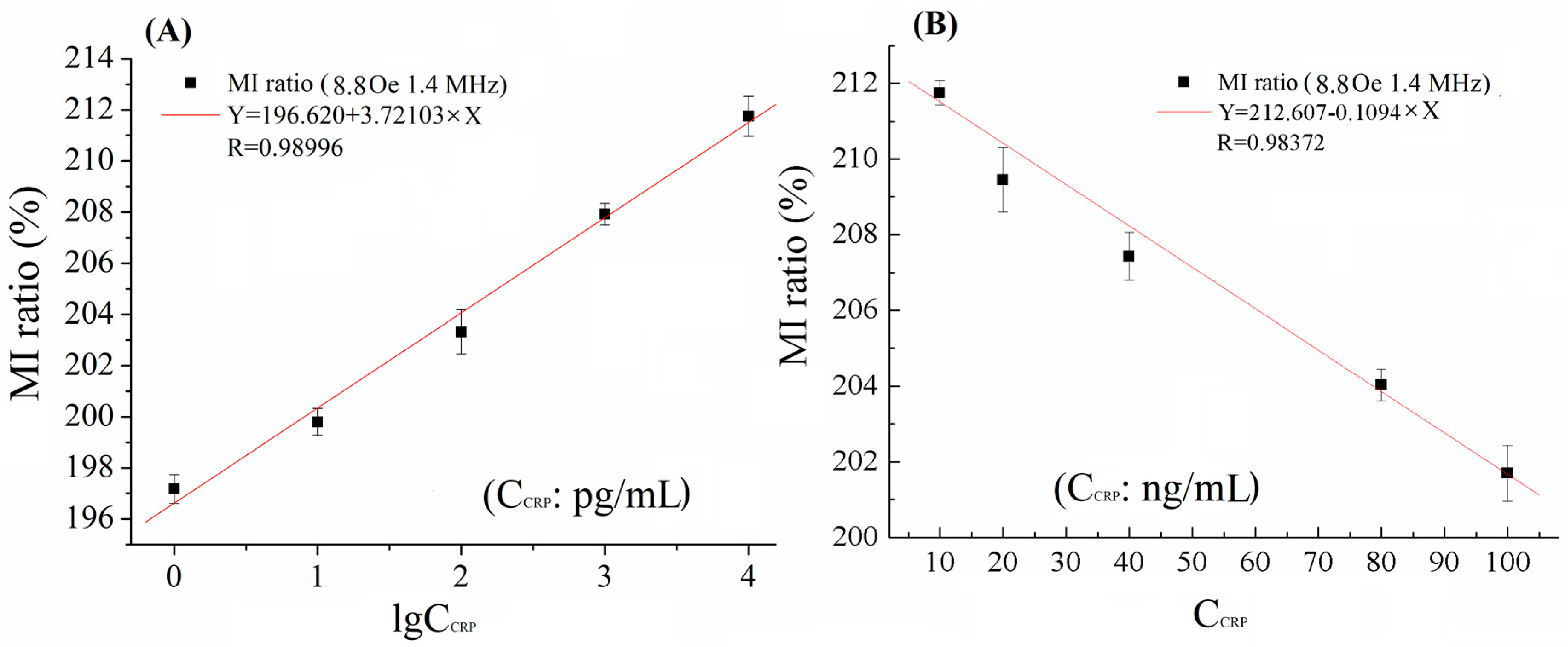
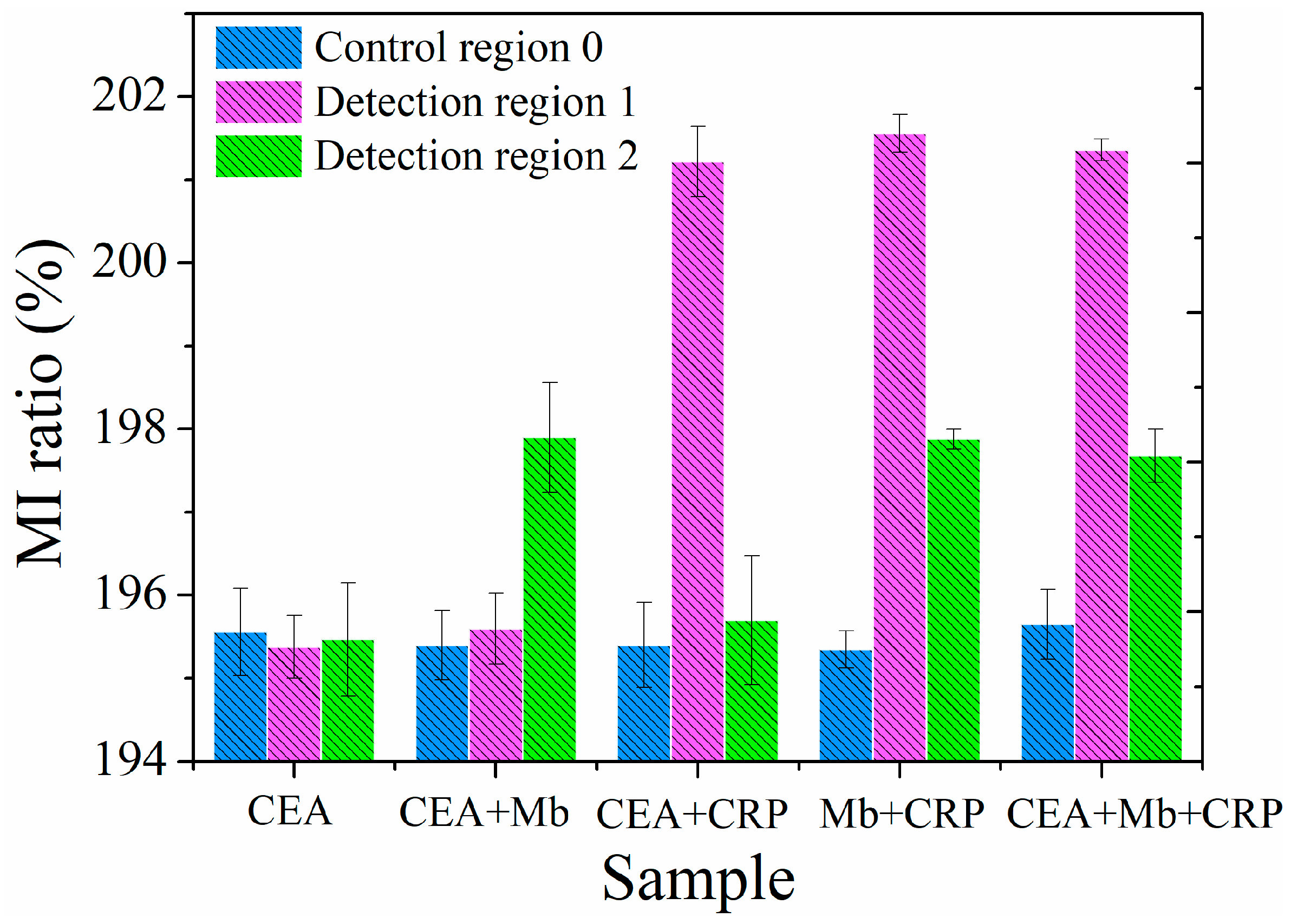
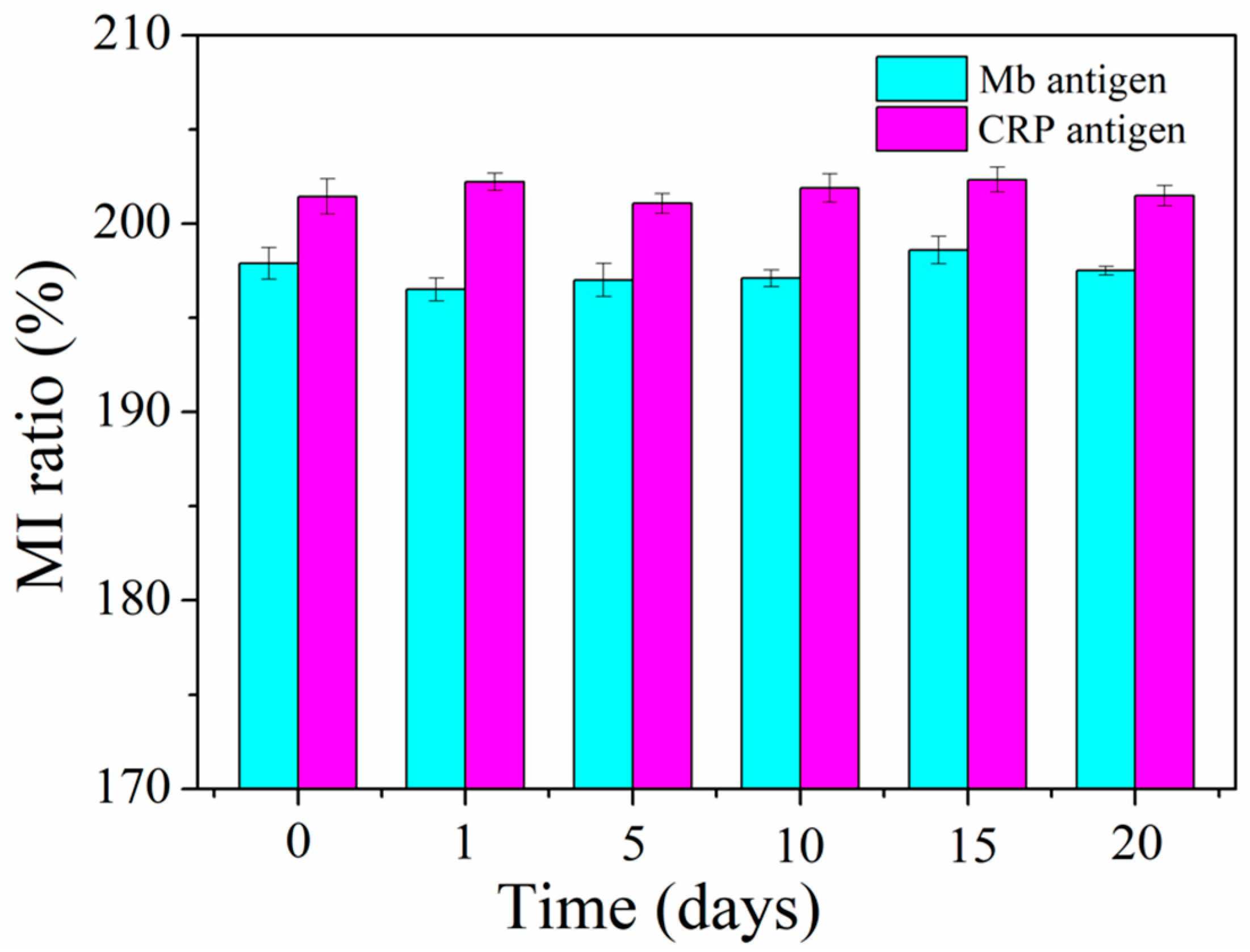
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, W.B.; Chen, Y.H.; Lin, H.I.; Shiesh, S.C.; Lee, G.B. An integrated microfluidic system for fast, automatic detection of C-reactive protein. Biosens. Bioelectron. 2009, 24, 3091–3096. [Google Scholar] [CrossRef]
- Kushner, I.; Sehgal, A.R. Is high-sensitivity C-reactive protein an effective screening test for cardiovascular risk? Arch. Intern. Med. 2002, 162, 867–869. [Google Scholar] [CrossRef] [PubMed]
- Black, S.; Kushner, I.; Samols, D. C-reactive protein. J. Biol. Chem. 2004, 47, 48487–48490. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Gurbuz, Y.; Niazi, J.H. Biosensors for cardiac biomarkers detection: A review. Sens. Actuators B Chem. 2012, 171, 62–76. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Liu, F.; Yang, X.H.; Wang, K.M.; Wang, H.; Deng, X. Sensitive point-of-care monitoring of cardiac biomarker myoglobin using aptamer and ubiquitous personµal glucose meter. Biosens. Bioelectron. 2015, 64, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Sallach, S.M.; Nowak, R.; Hudson, M.P.; Tokarski, G.; Khoury, N.; Tomlanovich, M.C.; Jacobsen, G.; de Lemos, J.A.; McCord, J. A Change in Serum Myoglobin to Detect Acute Myocardial Infarction in Patients with Normal Troponin I Levels. Am. J. Cardiol. 2004, 94, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.H.F.; Hartmann, M.; Krgoldse, H.J.; Blankenstein, G.; Mueller-Chorus, B.; Oster, J.; Miethe, P.; Keusgen, M. CRP determination based on a novel magnetic biosensor. Biosens. Bioelectron. 2007, 22, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Baselt, D.R.; Lee, G.U.; Natesan, M.; Metzger, S.W.; Sheehan, P.E.; Colton, R.J. A biosensor based on magnetoresistance technology. Biosens. Bioelectron. 1998, 13, 731–739. [Google Scholar] [CrossRef]
- Gnedenko, O.V.; Mezentsev, Y.V.; Molnar, A.A.; Lisitsa, A.V.; Ivanov, A.S.; Archakov, A.I. Highly sensitive detection of human cardiac myoglobin using a reverse sandwich immunoassay with a gold nanoparticle-enhanced surface plasmon resonance biosensor. Anal. Chim. Acta 2013, 759, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Shorie, M.; Kumar, V.; Sabherwal, P.; Ganguli, A.K. Carbon quantum dots-mediated direct fluorescence assay for the detection of cardiac marker myoglobin. Curr. Sci. 2015, 108, 1595–1596. [Google Scholar]
- Yang, Y.N.; Lin, H.I.; Wang, J.H.; Shiesh, S.C.; Lee, G.B. An integrated microfluidic system for C-reactive protein measurement. Biosens. Bioelectron. 2009, 24, 3091–3096. [Google Scholar] [CrossRef] [PubMed]
- Rifai, N.; Tracy, R.P.; Ridker, P.M. Clinical efficacy of an automated high-sensitivity C-reactive protein assay. Clin. Chem. 1999, 45, 2136–2141. [Google Scholar] [PubMed]
- Xie, M.J.; Huang, H.; Hang, J.F.; Dong, Z.N.; Xiao, D.Y.; Xu, P.; Zhu, C.Y.; Xu, W.W. Evaluation of the Analytical and Clinical Performances of Time-resolved Fluoroimmunoassay for Detecting Carcinoma Antigen 50. J. Immunoass. Immunochem. 2015, 3, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Bedatty Fernandes, F.C.; Patil, A.V.; Bueno, P.R.; Davis, J.J. Optimized Diagnostic Assays Based on Redox Tagged Bioreceptive Interfaces. Anal. Chem. 2015, 87, 12137–12144. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.H.; Zhang, Y.; Sun, Y.Q.; Gao, L.L. Development of Electrochemical Impedance Immunosensor for Sensitive Determination of Myoglobin. Int. J. Electrochem. Sci. 2017, 12, 7765–7776. [Google Scholar] [CrossRef]
- Chiriac, H.; Herea, D.D.; Corodeanu, S. Microwire array for giant magnetoimpedance detection of magnetic particles for biosensor prototype. J. Magn. Magn. Mater. 2007, 311, 425–428. [Google Scholar] [CrossRef]
- Kurlyandskaya, G.V.; Levit, V.I. Advanced materials for drug delivery and biosensors based on magnetic label detection. Mater. Sci. Eng. C 2007, 27, 495–503. [Google Scholar] [CrossRef]
- Beach, R.S.; Berkowitz, A.E. Sensitive field-and frequency-dependent impedance spectra of amorphous FeCoSiB wire and ribbon. J. Appl. Phys. 1994, 76, 6209–6213. [Google Scholar] [CrossRef]
- Kraus, L. GMI modelling and material optimization. Sens. Actuators A 2003, 106, 187–194. [Google Scholar] [CrossRef]
- Nishibe, Y.; Ohta, N.; Tsukada, K.; Yamadera, H.; Nomomura, Y.; Mohri, K.; Uchiyama, T. Sensing of passing vehicles using a lane marker on road with built-in thin film MI sensor and power source. IEEE Trans. Veh. Technol. 2004, 53, 1827–1834. [Google Scholar] [CrossRef]
- Wang, T.; Yang, Z.; Lei, C.; Lei, J.; Zhou, Y. An integrated giant magnetoimpedance biosensor for detection of biomarker. Biosens. Bioelectron. 2014, 58, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Blyakhman, F.A.; Safronov, A.P.; Zubarev, A.Y.; Shklyar, T.F.; Makeyev, O.G.; Makarova, E.B.; Melekhin, V.V.; Larrañaga, A.; Kurlyandskaya, G.V. Polyacrylamide ferrogels with embedded maghemite nanoparticles for biomedical engineering. Results Phys. 2017, 7, 3624–3633. [Google Scholar] [CrossRef]
- Blyakhman, F.A.; Buznikov, N.A.; Sklyar, T.F.; Safronov, A.P.; Golubeva, E.V.; Svalov, A.V.; Sokolov, S.Y.; Melnikov, G.Y.; Orue, I.; Kurlyandskaya, G.V. Mechanical, Electrical and Magnetic Properties of Ferrogels with Embedded Iron Oxide Nanoparticles Obtained by Laser Target Evaporation: Focus on Multifunctional Biosensor Applications. Sensors 2018, 18, 872. [Google Scholar] [CrossRef] [PubMed]
- Kurlyandskaya, G.V.; Fernandez, E.; Safronov, A.P.; Blyakhman, F.A.; Svalov, A.V.; Burgoa Beitia, A.; Beketov, I.V. Magnetoimpedance biosensor prototype for ferrogel detection. J. Magn. Magn. Mater. 2017, 441, 650–655. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Y.; Lei, C.; Lei, J.; Yang, Z. Development of an ingenious method for determination of Dynabeads protein A based on a giant magnetoimpedance sensor. Sens. Actuators B Chem. 2013, 186, 727–733. [Google Scholar] [CrossRef]
- Safronov, A.P.; Mikhnevich, E.A.; Lotfollahi, Z.; Blyakhman, F.A.; Sklyar, T.F.; Larrañaga Varga, A.; Medvedev, A.I.; Fernández Armas, S.; Kurlyandskaya, G.V. Polyacrylamide ferrogels with magnetite or strontium hexaferrite: Next step in the development of soft biomimetic matter for biosensor applications. Sensors 2018, 18, 257. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, Y.; Lei, C.; Sun, X.C.; Zhou, Y. Ultrasensitive detection and quantification of E. coli O157:H7 using a giant magnetoimpedance sensor in an open-surface microfluidic cavity covered with an antibody-modified gold surface. Microchim. Acta 2016, 183, 1831–1837. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Y.; Lei, C.; Luo, J.; Xie, S.R.; Pu, H.Y. Magnetic impedance biosensor: A review. Biosens. Bioelectron. 2017, 90, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O.; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of Inflammation and Cardiovascular Disease. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apple, F.S.; Murakami, M.M.; Pearce, L.A.; Herzog, C.A. Multi-Biomarker Risk Stratification of N-Terminal Pro-B-Type Natriuretic Peptide, High-Sensitivity C-Reactive Protein, and Cardiac Troponin T and I in End-Stage Renal Disease for All-Cause Death. Clin. Chem. 2004, 50, 2279–2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Xia, H.S.; Sun, Z.D.; Lin, Y.; Wang, K.; Yu, J.; Tang, H.; Pang, D.W.; Zhang, Z.L. On-chip dual detection of cancer biomarkers directly in serum based on self-assembled magnetic bead patterns and quantum dots. Biosens. Bioelectron. 2013, 41, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Han, K.N.; Li, C.A.; Seong, G.H. Microfluidic Chips for Immunoassays. Annu. Rev. Anal. Chem. 2013, 6, 119–141. [Google Scholar] [CrossRef] [PubMed]
- Maeng, J.H.; Lee, B.C.; Ko, Y.J.; Cho, W.; Ahn, Y.; Cho, N.G.; Lee, S.H.; Hwang, S.Y. A novel microfluidic biosensor based on an electrical detection system for alpha-fetoprotein. Biosens. Bioelectron. 2008, 23, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.D.; Dong, M.L.; Santos, S.; Rigatto, C.; Liu, Y.; Lin, F. Lab-on-a-Chip platforms for detection of cardiovascular disease and cancer biomarkers. Sensors 2017, 17, 2934. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Zhou, W.W.; Shi, X.Z.; Gao, Y.H. Development of Integrated Microfluidic Magnetic Biosensor for Multi-biomarker Detection. Chin. J. Anal. Chem. 2013, 9, 1302–1307. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Y.; Zhou, Z.M.; Ding, W. Giant magnetoimpedance effects in patterned Co-based ribbons with a meander structure. Phys. Status Solidi (a) 2009, 206, 1594–1598. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Y.; Zhou, Z.M.; Ding, W. Enhancement of magnetoimpedance effect in Co-based amorphous ribbon with a meander structure. Phys. Status Solidi (a) 2010, 207, 448–451. [Google Scholar] [CrossRef]
- Rivero, M.A.; Maicas, M.; Lopez, E.; Aroca, C.; Sanchez, M.C.; Sanchez, P.J. Influence of the sensor shape on permalloy/Cu/permalloy magnetoimpedance. Magn. Magn. Mater. 2003, 254, 636–640. [Google Scholar] [CrossRef]
- Morikawa, T.; Nishibe, Y.; Yamadera, H.; Nonomura, Y.; Takeuchi, M.; Sakata, J.; Taga, Y. Enhancement of giant magneto-impedance in layered film by insulator separation. IEEE Trans. Magn. 1996, 32, 4965–4967. [Google Scholar] [CrossRef]
- Lodewijk, K.J.; Fernandez, E.; Garcia-Arribas, A.; Kurlyandskaya, G.V.; Lepalovskij, V.N.; Safronov, A.P.; Kooi, B.J. Magnetoimpedance of thin film meander with composite coating layer containing Ni nanoparticles. J. Appl. Phys. 2014, 115, 17A323. [Google Scholar] [CrossRef]
- Wang, T.; Lei, C.; Lei, J.; Yang, Z.; Zhou, Y. Preparation of meander thin-film microsensor and investigation the influence of structural parameters on the giant magnetoimpedance effect. Appl. Phys. A 2012, 109, 205–211. [Google Scholar] [CrossRef]
- Park, J.Y.; Allen, M.G. Integrated electroplated micromachined magnetic devices using low temperature fabrication processes. IEEE Trans. Electron. Packag. Manuf. 2000, 23, 48–55. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, Y.; Lei, C.; Lei, J.; Yang, Z. Ultrasensitive detection of Dynabeads protein A using the giant magnetoimpedance effect. Microchim. Acta 2013, 180, 1211–1216. [Google Scholar] [CrossRef]
- Miller, M.M.; Prinz, G.A.; Cheng, S.F.; Bounnak, S. Detection of a micron-sized magnetic sphere using a ring-shaped anisotropic magnetoresistance-based sensor: A model for a magnetoresistance-based biosensor. Appl. Phys. Lett. 2002, 81, 2211–2213. [Google Scholar] [CrossRef]
- Ferreira, H.A.; Graham, D.L.; Freitas, P.P.; Cabral, J.M.S. Biodetection using magnetically labeled biomolecules and arrays of spin valve sensors. J. Appl. Phys. 2002, 93, 7281–7286. [Google Scholar] [CrossRef]
- Besse, P.A.; Boero, G.; Demierre, M.; Pott, V.; Popovic, R. Detection of single magnetic microbead using a miniaturized silicon Hall sensor. Appl. Phys. Lett. 2002, 80, 4199–4201. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Y.; Lei, C.; Sun, X.C.; Zhou, Y. A flexible giant magnetoimpedance-based biosensor for the determination of the biomarker C-reactive protein. Microchim. Acta 2015, 182, 2411–2417. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, H.H.; Dong, X.W.; Yan, H.L.; Lei, C.; Luo, Y.S. Giant magnetoimpedance based immunoassay for cardiac biomarker myoglobin. Anal. Methods 2017, 9, 3636–3642. [Google Scholar] [CrossRef]
- Shorie, M.; Kumar, V.; Kaur, H.; Singh, K.; Tomer, V.K.; Sabherwal, P. Plasmonic DNA hotspots made from tungsten disulfide nanosheets and gold nanoparticles for ultrasensitive aptamer-based SERS detection of myoglobin. Microchim. Acta 2018, 185, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, P.; Sun, Z.X.; Sun, C.; Bu, H.Y.; Han, J.; Chen, S.P.; Xie, G. NH2-Ni-MOF electrocatalysts with tunable size/morphology for ultrasensitive C-reactive protein detection via an aptamer binding induced DNA walker–antibody sandwich assay. J. Mater. Chem. B. 2018, 6, 2426–2431. [Google Scholar] [CrossRef]


| Solution | Detail | Company |
|---|---|---|
| Mercaptopropionic acid | Concentration 20 mmol/L | Aladdin Chemistry Co. Ltd (Beijing, China) |
| EDC | Concentration 0.2 mol/L | Aladdin Chemistry Co. Ltd (Beijingi, China). |
| NHS | Concentration 0.05 mol/L | Shanghai Medpep Co. Ltd (Shanghai, China) |
| Mouse Mb &CRP 1st antibody | Concentration 1 mg/mL | Linc-Bio Science Co. Ltd. (Shanghai, China) |
| BSA | 1% BSA, 0.2% tween 20 | Via-gene pro bio Technologies Co. Ltd. (Shanghai, China) |
| Human Mb &CRP antigen | 0.1–100 pg/mL, 1–100 ng/mL | Linc-Bio Science Co. Ltd. (Shanghai, China) |
| Mb &CRP 2 nd antibody | Concentration 1 mg/mL | Linc-Bio Science Co. Ltd. (Shanghai, China) |
| Dynabeads® C1 | Concentration 10 μg/mL | Invitrogen Co. Ltd. (Shanghai, China) |
| NaOH | Concentration 1 mol/L | Pinghu Chemical Reagent (Pinghu, China) |
| HCL | Concentration 1 mol/L | Sinpharm Chemical Reagent Co. Ltd. (Shanghai, China) |
| PBS | PH = 7.4 | Medicago AB (Uppsala, Sweden) |
| C3H6O C2H5OH | AR | LingFeng Chemical Reagent Co. Ltd. (Shanghai, China) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Wang, H.; Guo, P.; Ding, Y.; Lei, C.; Luo, Y. A Multi-Region Magnetoimpedance-Based Bio-Analytical System for Ultrasensitive Simultaneous Determination of Cardiac Biomarkers Myoglobin and C-Reactive Protein. Sensors 2018, 18, 1765. https://doi.org/10.3390/s18061765
Yang Z, Wang H, Guo P, Ding Y, Lei C, Luo Y. A Multi-Region Magnetoimpedance-Based Bio-Analytical System for Ultrasensitive Simultaneous Determination of Cardiac Biomarkers Myoglobin and C-Reactive Protein. Sensors. 2018; 18(6):1765. https://doi.org/10.3390/s18061765
Chicago/Turabian StyleYang, Zhen, Huanhuan Wang, Pengfei Guo, Yuanyuan Ding, Chong Lei, and Yongsong Luo. 2018. "A Multi-Region Magnetoimpedance-Based Bio-Analytical System for Ultrasensitive Simultaneous Determination of Cardiac Biomarkers Myoglobin and C-Reactive Protein" Sensors 18, no. 6: 1765. https://doi.org/10.3390/s18061765





