Electrochemical Determination of Norepinephrine by Means of Modified Glassy Carbon Electrodes with Carbon Nanotubes and Magnetic Nanoparticles of Cobalt Ferrite
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Procedures
2.2. Instrumentation and Measurement
2.3. Preparation of Electrodes
2.4. Preparation and Analysis of Norepinephrine
3. Results and Discussion
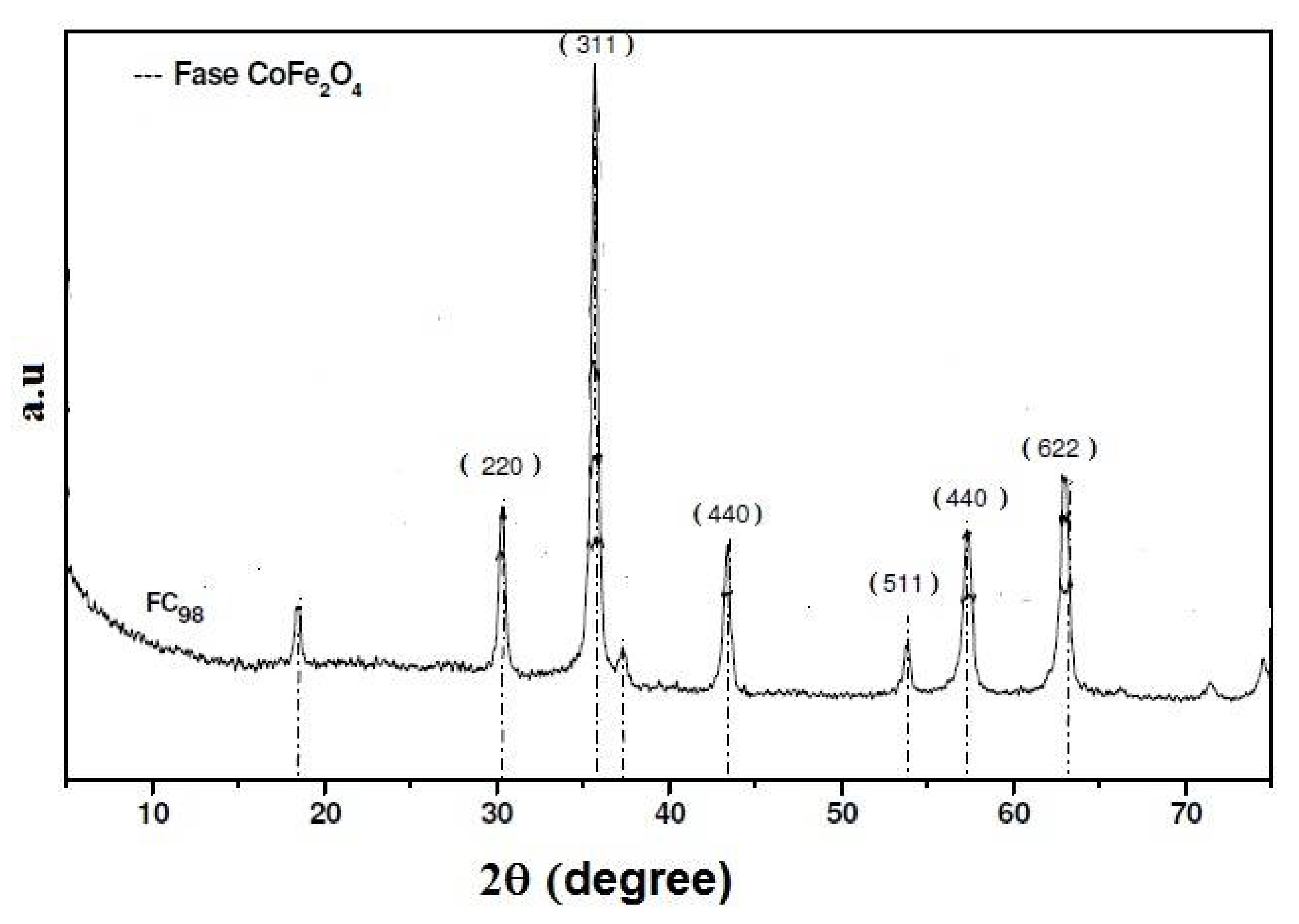
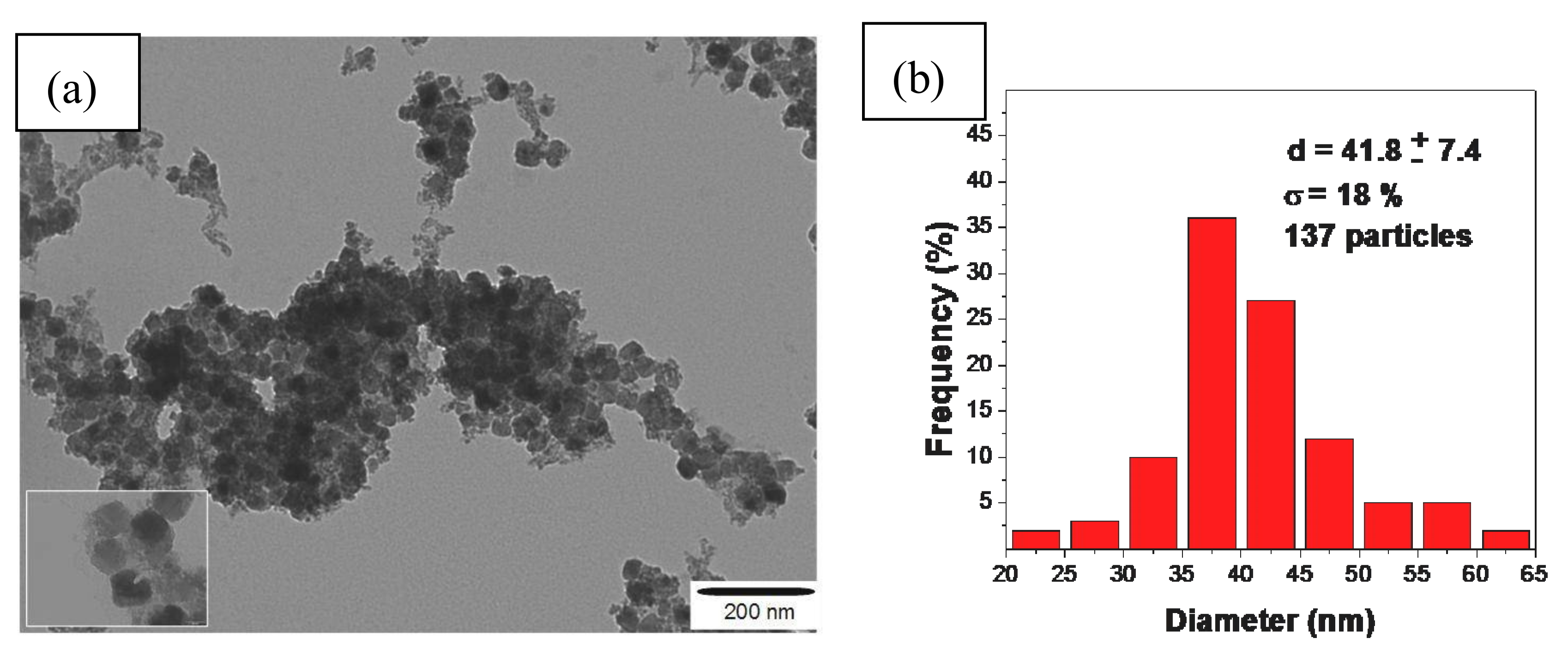
3.1. Synthesis and Characterization of Magnetic Particles by a Co-Precipitation CoFe2O4 Process
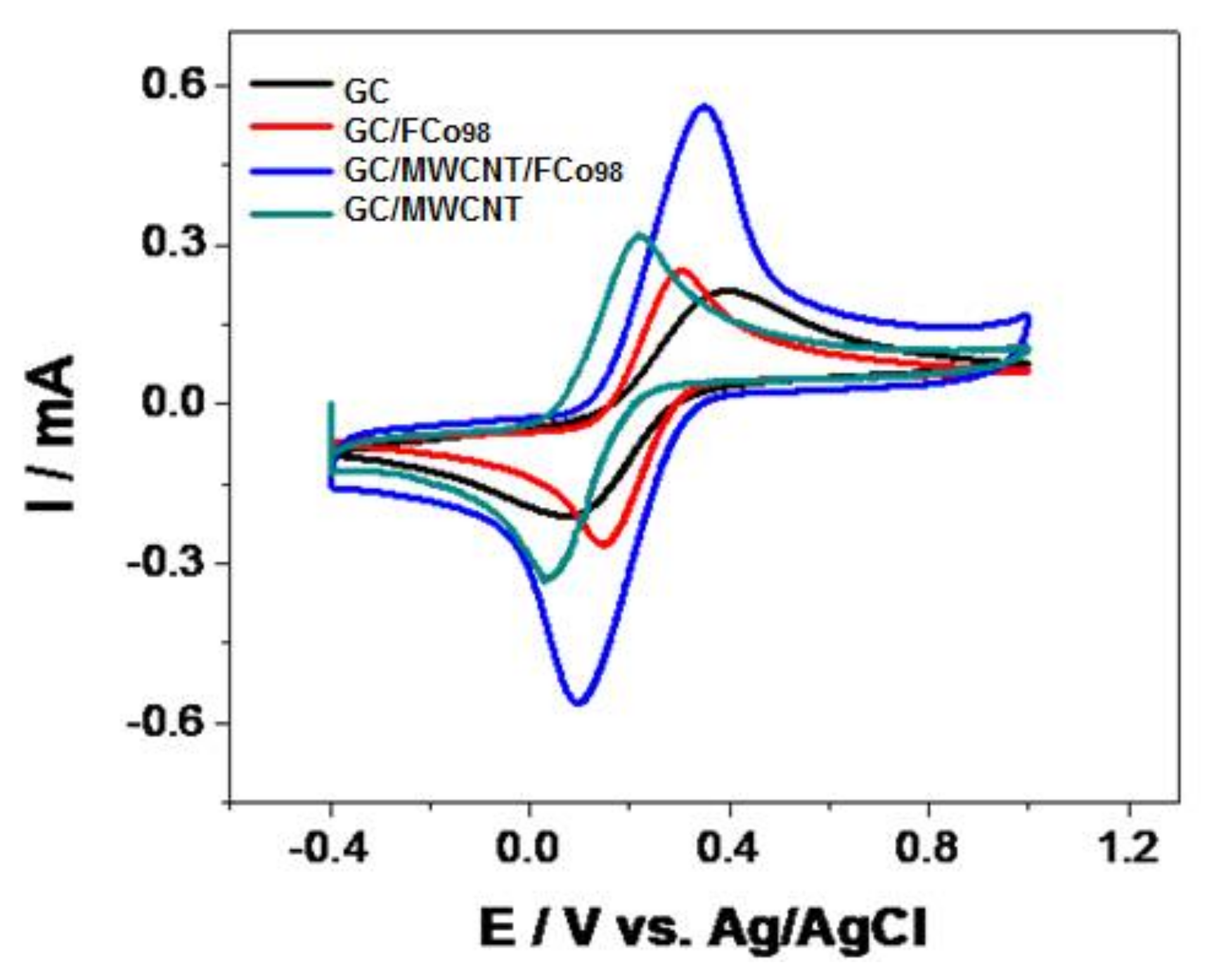
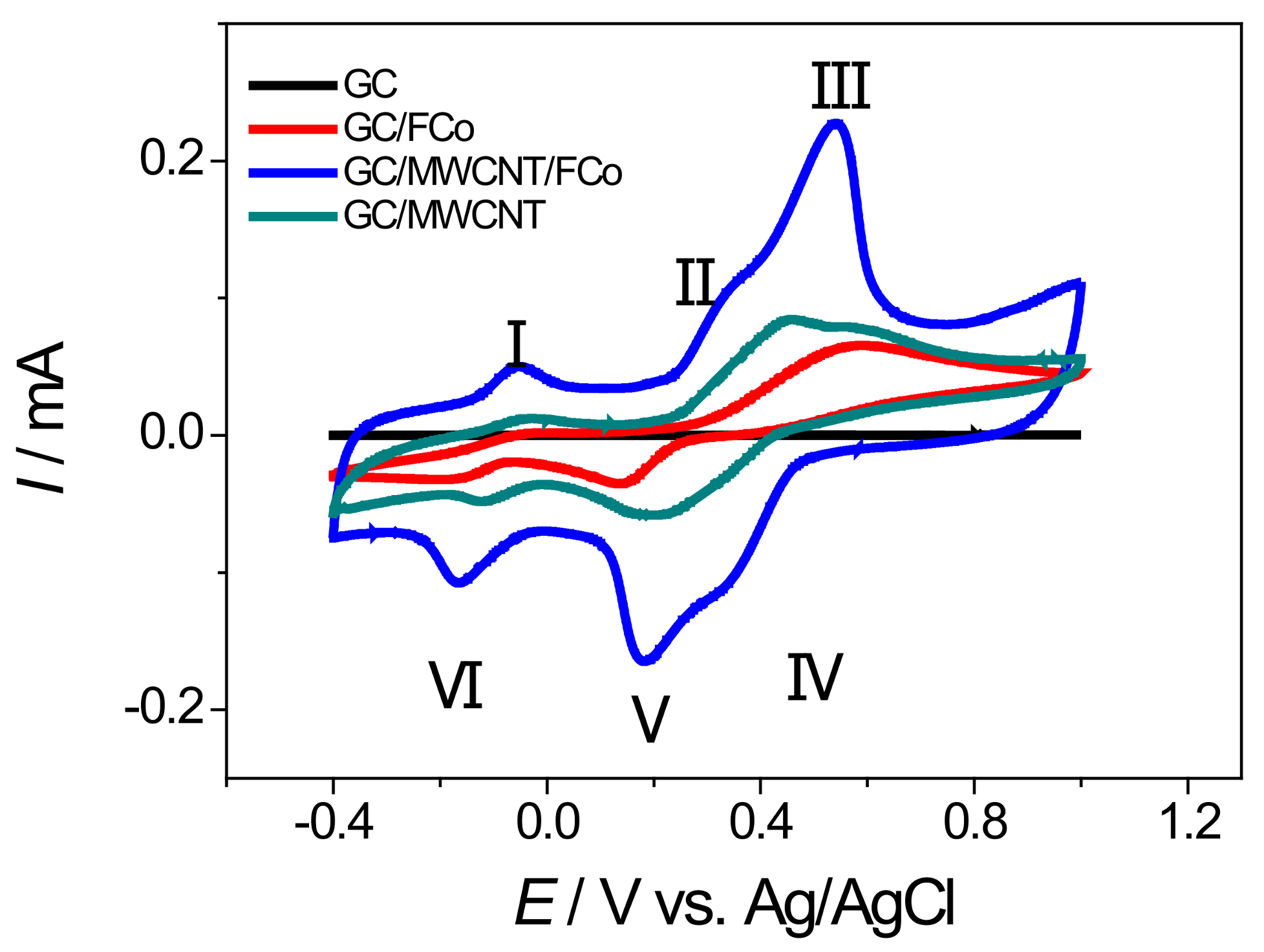
3.2. Electrochemical Behavior GC/MWCNT/FCo98
3.3. Detection of Norepinephrine with a Modified GC/MWCNT/FCo98 Electrode


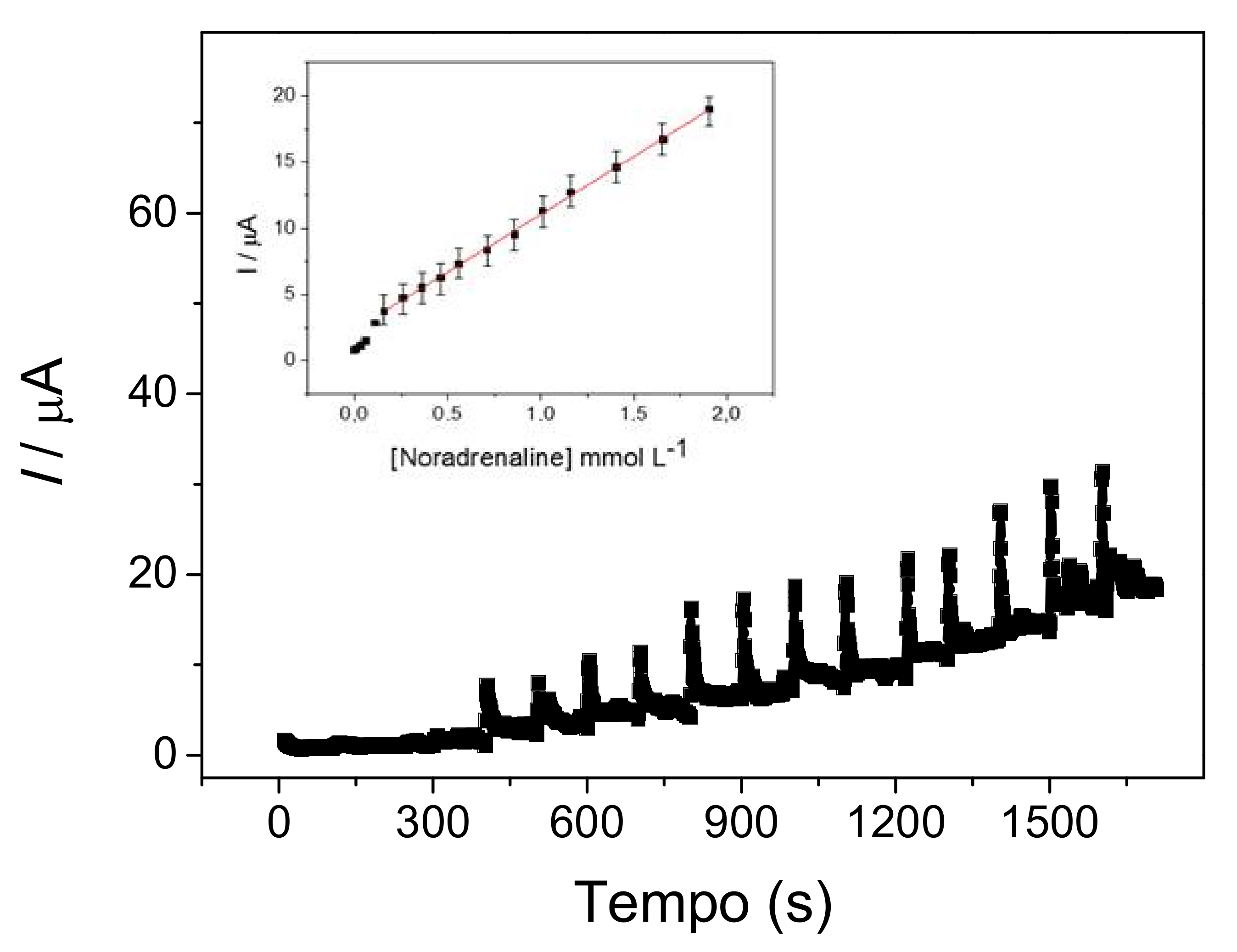
3.4. Amperometric Measurement of Noradrenaline
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vafafard, A.; Goharshenasan, S.; Nozari, N.; Mortezapour, A.; Mahmoudi, M. Phase-Dependent Optical Bistability in the Quantum Dot Nanostructure Molecules via Inter-Dot Tunneling. J. Lumin. 2013, 134, 900–905. [Google Scholar] [CrossRef]
- Rangasamy, M. Nano Technology: A Review. J. Appl. Pharm. Sci. 2011, 1, 8–16. [Google Scholar]
- El-Sayed, M.A. Some Interesting Properties of Metals Confined in Time and Nanometer Space of Different Shapes. Acc. Chem. Res. 2001, 34, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.; Li, Q.; Kennedy, R.T. Review of Recent Advances in Analytical Techniques for the Determination of Neurotransmitters. Anal. Chim. Acta 2009, 653, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kozikowski, A.P. On the Path from Chemistry to Neuroscience: Early Explorations in Chemical Medicine under the Mentorship of Dr. Erminio Costa, a Neuroscientist with a Big Brain and a Bigger Heart. Pharmacol. Res. 2011, 64, 327–329. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Y.; Xu, Y.; Wang, M.; Zhu, Q.; Li, W.; Wang, X.; Liu, J.; Ma, B.; Wu, K. Neurotransmitter Noradrenaline Downregulate Cytoskeletal Protein Expression of Vsmcs. Exp. Mol. Pathol. 2013, 94, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Taei, M.; Ramazani, G. Simultaneous determination of norepinephrine, acetaminophen and tyrosine by differential pulse voltammetry using Au-nanoparticles/poly(2-amino-2-hydroxymethyl-propane-1,3-diol) film modified glassy carbon electrode. Colloids Surf. B Biointerfaces 2014, 123, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, K.; Zhao, L.; Chai, S.; Zhang, J.; Zhang, X.; Zou, Q. A novel sensor made of Antimony Doped Tin Oxide-silica composite sol on a glassy carbon electrode modified by single-walled carbon nanotubes for detection of norepinephrine. Mater. Sci. Eng. C 2017, 80, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Mahdavi, M.R.V.; Roghani, M.; Baluchnejadmojarad, T. The Role of Adrenergic and Angiotensinergic Systems in Vascular Effect of Alcoholic of Extract Trigonella Foenum-Graecum Seed in Diabetic Rats. Iran. J. Pharm. Res. 2011, 10, 83–88. [Google Scholar]
- Xie, Y.C.; Huang, H.W.; Zhang, Q.M.; Jin, S.H. Quantitative Determination of Adrenaline Hydrochloride Injection and Noradrenaline Bitartrate Injection by a New Hplc Method Via Substitute for Reference Substance. Chem. Res. Chin. Univ. 2009, 25, 433–438. [Google Scholar]
- Gutiérrez, A.; Primo, E.N.; Eguílaz, M.; Parrado, C.; Rubianes, M.D.; Rivas, G.A. Quantification of neurotransmitters and metabolically related compounds at glassy carbon electrodes modified with bamboo-like carbon nanotubes dispersed in double stranded DNA. Microchem. J. 2017, 130, 40–46. [Google Scholar] [CrossRef]
- Lavanya, N.; Sekar, C. Electrochemical sensor for simultaneous determination of epinephrine and norepinephrine based on cetyltrimethylammonium bromide assisted SnO2 nanoparticles. J. Electroanal. Chem. 2017, 801, 503–510. [Google Scholar] [CrossRef]
- Chen, F.N.; Zhang, Y.X.; Zhang, Z.J. Simultaneous Determination of Epinephrine, Noradrenaline and Dopamine in Human Serum Samples by High Performance Liquid Chromatography with Chemiluminescence Detection. Chin. J. Chem. 2007, 25, 942–946. [Google Scholar] [CrossRef]
- Thomas, A.; Geyer, H.; Mester, H.J.; Schänzer, W.; Zimmermann, E.; Thevis, M. Quantitative Determination of Adrenaline and Noradrenaline in Urine Using Liquid Chromatography-Tandem Mass Spectrometry. Chromatographia 2006, 64, 587–591. [Google Scholar] [CrossRef]
- Amiri-Aref, M.; Raoof, J.B.; Ojani, R. A highly sensitive electrochemical sensor for simultaneous voltammetric determination of noradrenaline, acetaminophen, xanthine and caffeine based on a flavonoid nanostructured modified glassy carbon electrode. Sens. Actuators B Chem. 2014, 192, 634–641. [Google Scholar] [CrossRef]
- Pahlavan, A.; Gupta, V.K.; Sanati, A.L.; Karimi, F.; Yoosefian, M.; Ghadami, M. ZnO/CNTs nanocomposite/ionic liquid carbon paste electrode for determination of noradrenaline in human samples. Electrochim. Acta 2014, 123, 456–462. [Google Scholar] [CrossRef]
- Silva, J.B.; Brito, W.; Mohallem, N.D.S. Influence of heat tretment on cobalt ferrit powders. Mater. Sci. Eng. B 2004, 112, 182–187. [Google Scholar] [CrossRef]
- Da Silva, H.; Pacheco, J.G.; Magalhães, J.M.; Viswanathan, S.; Delerue-Matos, C. Mip-Graphene-Modified Glassy Carbon Electrode for the Determination of Trimethoprim. Biosens. Bioelectron. 2014, 52, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Rejandra, M.; Pullar, R.C.; Bhattacharya, A.K.; Das, D.; Chintalapudi, S.N.; Majumdar, K.C. Magnetic properties of nanocrystalline CoFe2O4 powders prepared at room temperature: Variation with crystallite size. J. Magn. Magn. Mater. 2001, 232, 71–83. [Google Scholar]
- Li, S.; John, V.T.; O’Connor, C.; Harris, V.; Carpenter, E. Cobalt ferrite nanoparticles: Struture, cation distributions and magnetic properties. J. Appl. Phys. 2000, 87, 6223–6225. [Google Scholar] [CrossRef]
- Yi, Y.; Weinberg, G.; Prenzel, M.; Greiner, M.; Heumann, S.; Becker, S.; Schlögl, R. Electrochemical corrosion of a glassy carbon electrode. Catal. Today 2017, 295, 32–40. [Google Scholar] [CrossRef]
- Fang, W.; Linder, M.B.; Laaksonen, P. Modification of carbon nanotubes by amphiphilic glycosylated proteins. J. Colloid Interface Sci. 2018, 512, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Sabatani, E.; Rubinstein, I.; Maoz, R.; Sagiv, J. Organized Self-Assembling Monolayers on Electrodes. Part I. Octadecyl Derivatives on Gold. J. Electroanal. Chem. 1987, 219, 365–371. [Google Scholar] [CrossRef]
- Kim, S.; Na, J.; Lee, S.K.; Song, M.J.; Kang, P.; Huh, J.; Lim, D.S.; Kim, G.T. Geometrical Effects of Nanowire Electrodes for Amperometric Enzyme Biosensors. Sens. Actuators B Chem. 2013, 183, 222–229. [Google Scholar] [CrossRef]
- Kikandi, S.N.; Okello, V.A.; Wang, Q.; Sadik, O.A.; Varner, K.E.; Burns, S.A. Size-Exclusive Nanosensor for Quantitative Analysis of Fullerene C 60. Environ. Sci. Technol. 2011, 45, 5294–5300. [Google Scholar] [CrossRef] [PubMed]
- Moraes, F.C.; Cesarino, I.; Coelho, D.; Pedrosa, V.A.; Machado, S.A.S. Highly Sensitive Neurotransmitters Analysis at Platinum-Ultramicroelectrodes Arrays. Electroanalysis 2012, 24, 1115–1120. [Google Scholar] [CrossRef]
- Łuczak, T. Structure—Reactivity Relationships: The Oxidation of Aliphatic Amines on the Gold Electrode. J. Appl. Electrochem. 2007, 37, 269–274. [Google Scholar] [CrossRef]
- Chen, L.; Lu, G. Direct Electrochemistry and Electrocatalysis of Hybrid Film Assembled by Polyelectrolyte-Surfactant Polymer, Carbon Nanotubes and Hemoglobin. J. Electroanal. Chem. 2006, 597, 51–59. [Google Scholar] [CrossRef]
- Laviron, E. Adsorption, Autoinhibition and Autocatalysis in Polarography and in Linear Potential Sweep Voltammetry. J. Electroanal. Chem. 1974, 52, 355–393. [Google Scholar] [CrossRef]
- Liu, S.Q.; Ju, H.X. Renewable Reagentless Hydrogen Peroxide Sensor Based on Direct Electron Transfer of Horseradish Peroxidase Immobilized on Colloidal Gold-Modified Electrode. Anal. Biochem. 2002, 307, 110–116. [Google Scholar] [CrossRef]
- Cosio, M.S.; Pellicanò, A.; Brunetti, B.; Fuenmayor, C.A. A simple hydroxylated multi-walled carbon nanotubes modified glassy carbon electrode for rapid amperometric detection of bisphenol A. Sens. Actuators B Chem. 2017, 246, 673–679. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry; Pearson Education: Dorset, UK, 2005; p. 263. [Google Scholar]
- Goldstein, D.S.; Zimlichman, R.; Stull, R.; Keiser, H.R.; Kopin, I.J. Estimation of intrasynaptic norepinephrine concentrations in humans. Hypertension 1986, 8, 471–475. [Google Scholar] [CrossRef] [PubMed]
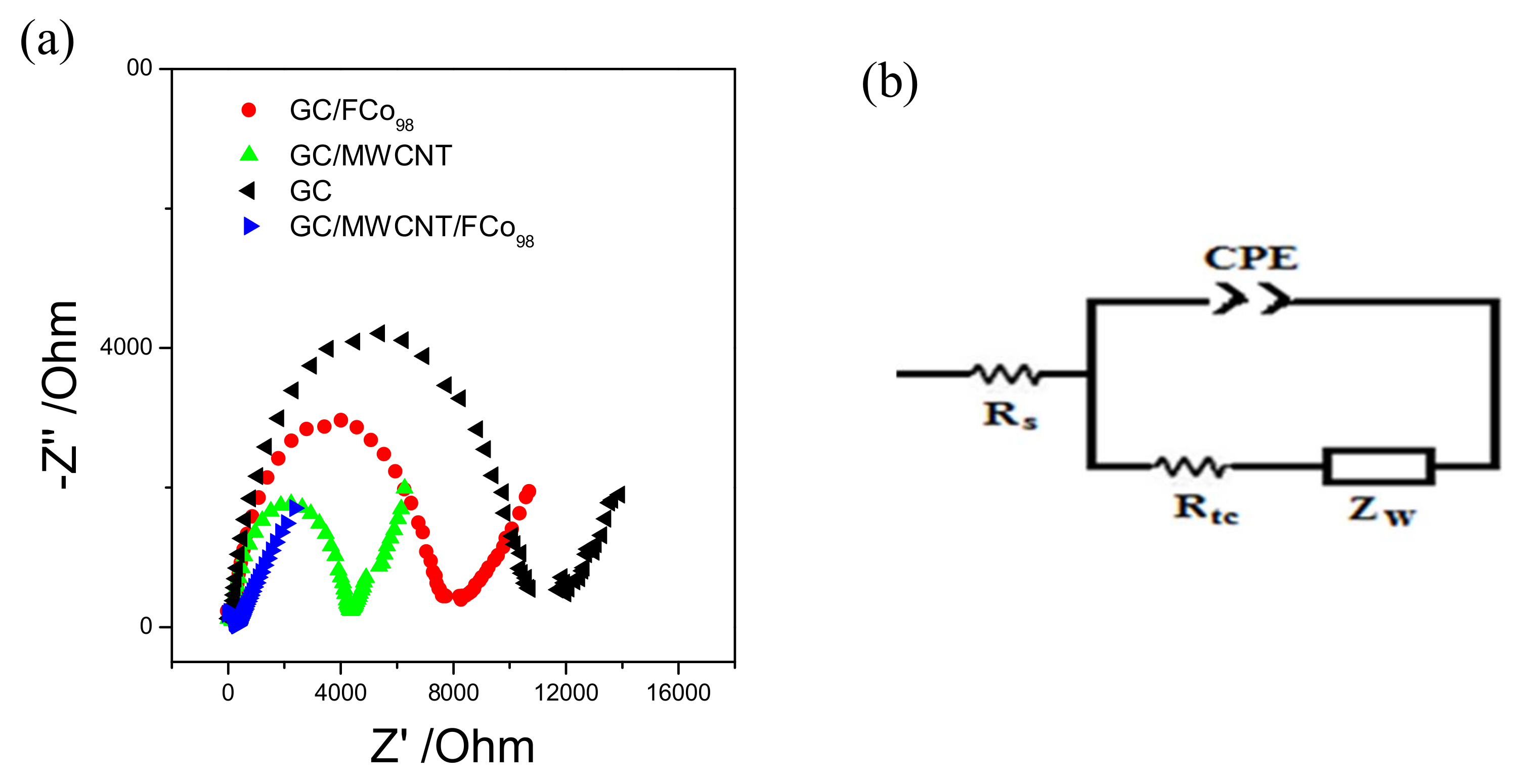
| Electrode | Rs (Ω) | CPE (µF) | Rtc (Ω) | α | Kapp (µcm s−1) |
|---|---|---|---|---|---|
| GC/FCo98 | 28 | 3.31 | 4892 | 0.91 | 0.08 |
| GC | 29 | 1.17 | 10,678 | 0.98 | 0.04 |
| GC/MWCNT | 31 | 1.9 | 2322 | 0.95 | 0.16 |
| GC/MWCNT/FCo98 | 31 | 230 | 107 | 0.92 | 3.5 |
| Parameter | Optimum Value |
|---|---|
| Composition | 4 uL FCo98 and 10 uL MWCNT |
| Supporting electrolyte and pH | 0.1 mol L−1 PBS (pH 7.0) |
| Potential range and Scan rate | Scan rate of 100 mV s−1 between −0.4 and 1.0 V vs. Ag/AgCl. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Queiroz, D.F.d.; Dadamos, T.R.d.L.; Machado, S.A.S.; Martines, M.A.U. Electrochemical Determination of Norepinephrine by Means of Modified Glassy Carbon Electrodes with Carbon Nanotubes and Magnetic Nanoparticles of Cobalt Ferrite. Sensors 2018, 18, 1223. https://doi.org/10.3390/s18041223
Queiroz DFd, Dadamos TRdL, Machado SAS, Martines MAU. Electrochemical Determination of Norepinephrine by Means of Modified Glassy Carbon Electrodes with Carbon Nanotubes and Magnetic Nanoparticles of Cobalt Ferrite. Sensors. 2018; 18(4):1223. https://doi.org/10.3390/s18041223
Chicago/Turabian StyleQueiroz, Daniely Ferreira de, Tony Rogério de Lima Dadamos, Sergio Antonio Spinola Machado, and Marco Antonio Utrera Martines. 2018. "Electrochemical Determination of Norepinephrine by Means of Modified Glassy Carbon Electrodes with Carbon Nanotubes and Magnetic Nanoparticles of Cobalt Ferrite" Sensors 18, no. 4: 1223. https://doi.org/10.3390/s18041223





