Radio Frequency Detection and Characterization of Water-Ethanol Solution through Spiral-Coupled Passive Micro-Resonator Sensor
Abstract
:1. Introduction
2. Materials and Methods
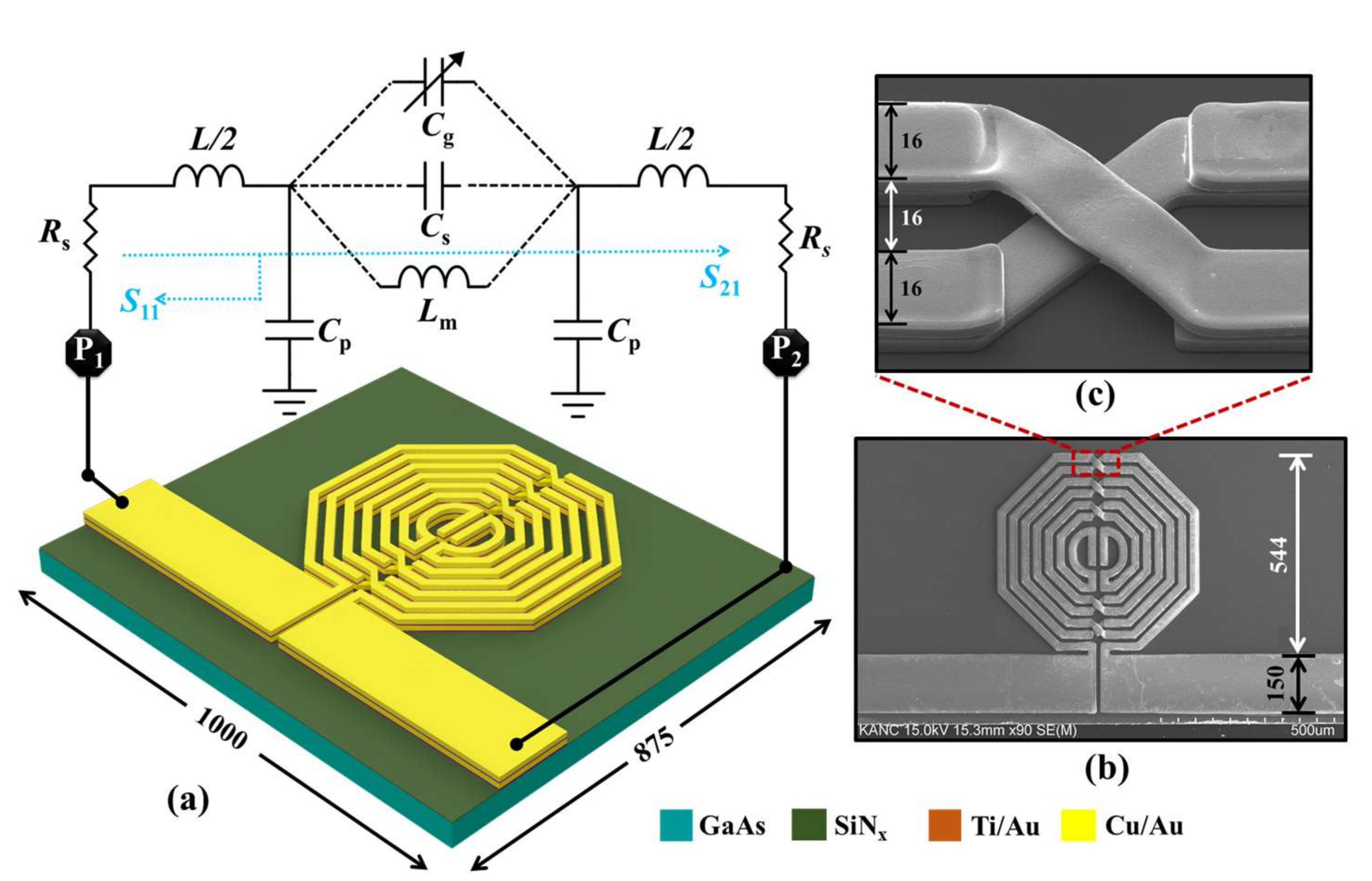
2.1. Design and Fabrication
2.2. Radio Frequency Modeling
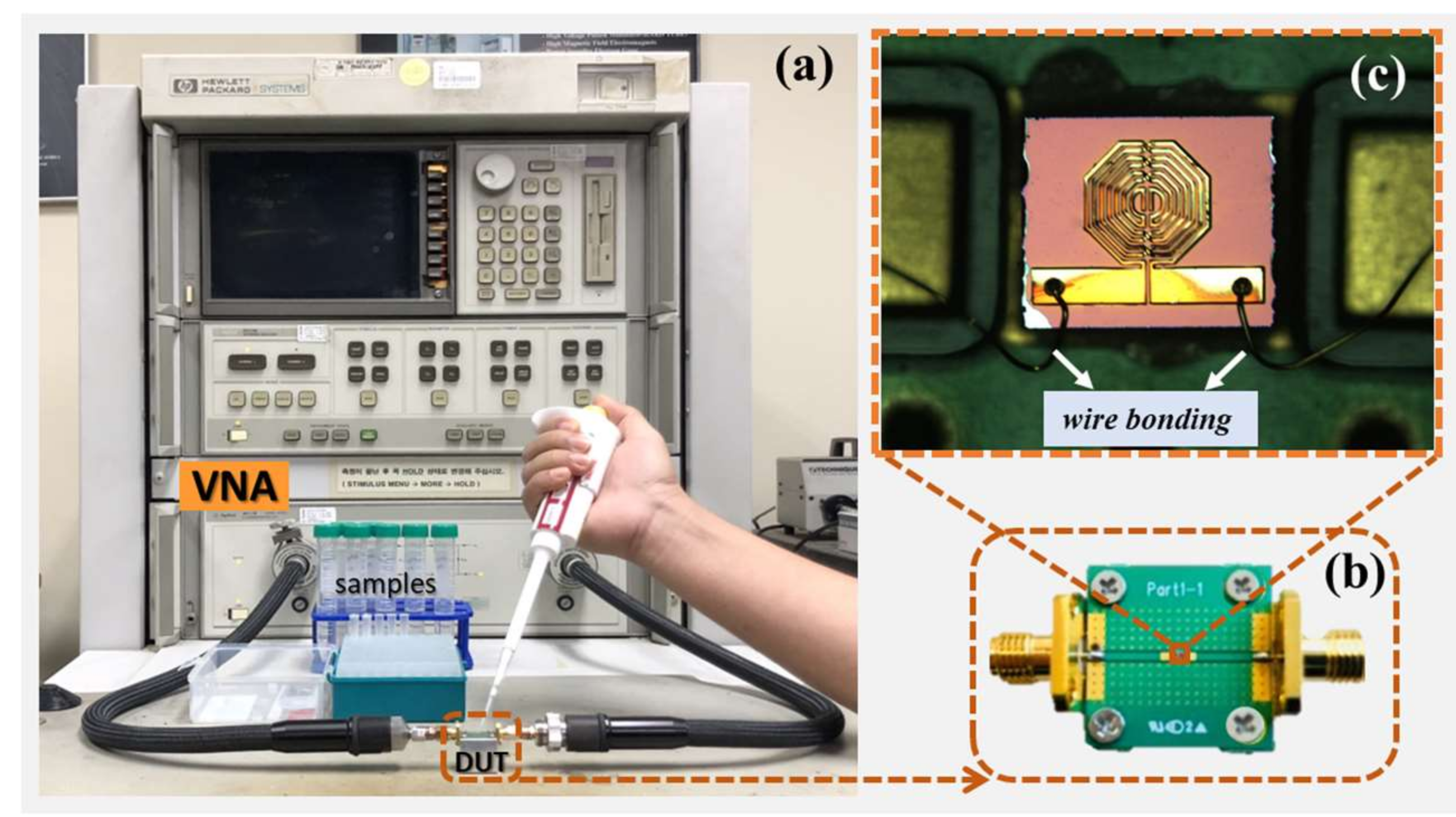
2.3. Sensor Characterization
3. Results
3.1. Capacitive Effect
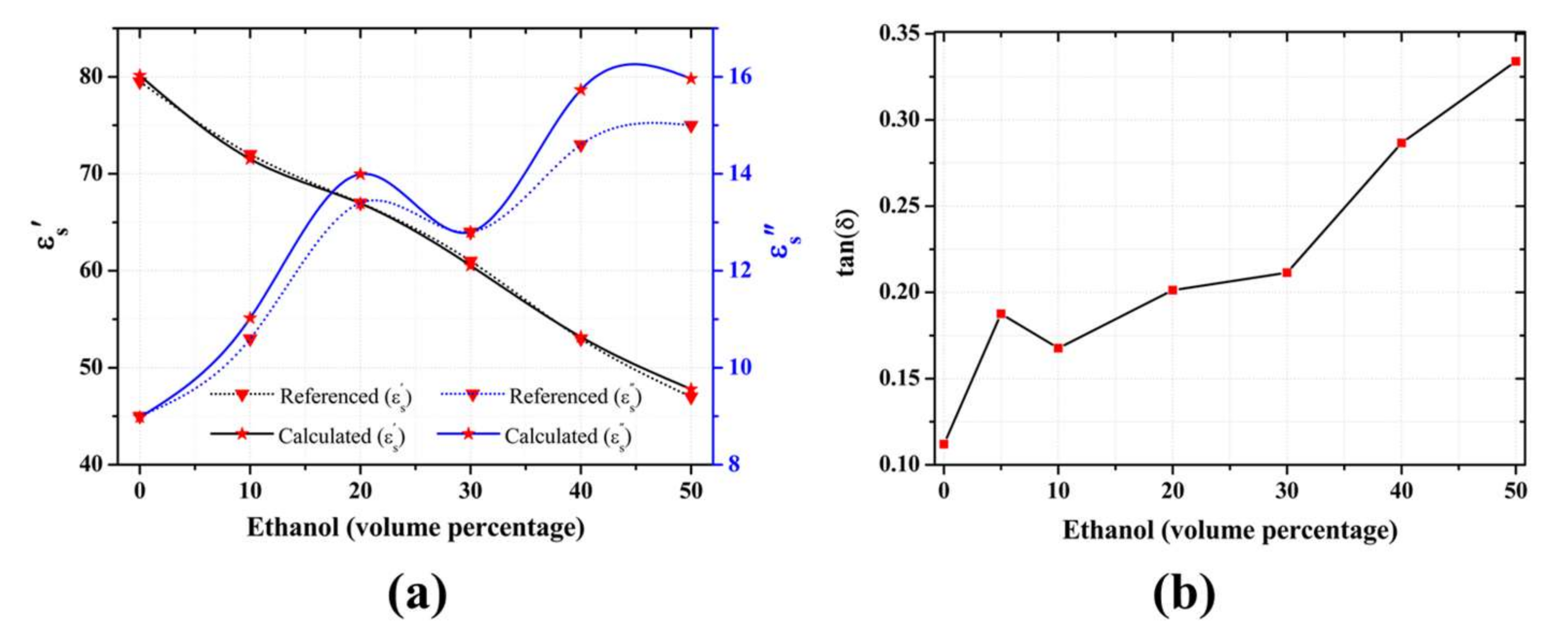
3.2. Complex Permittivity Analysis
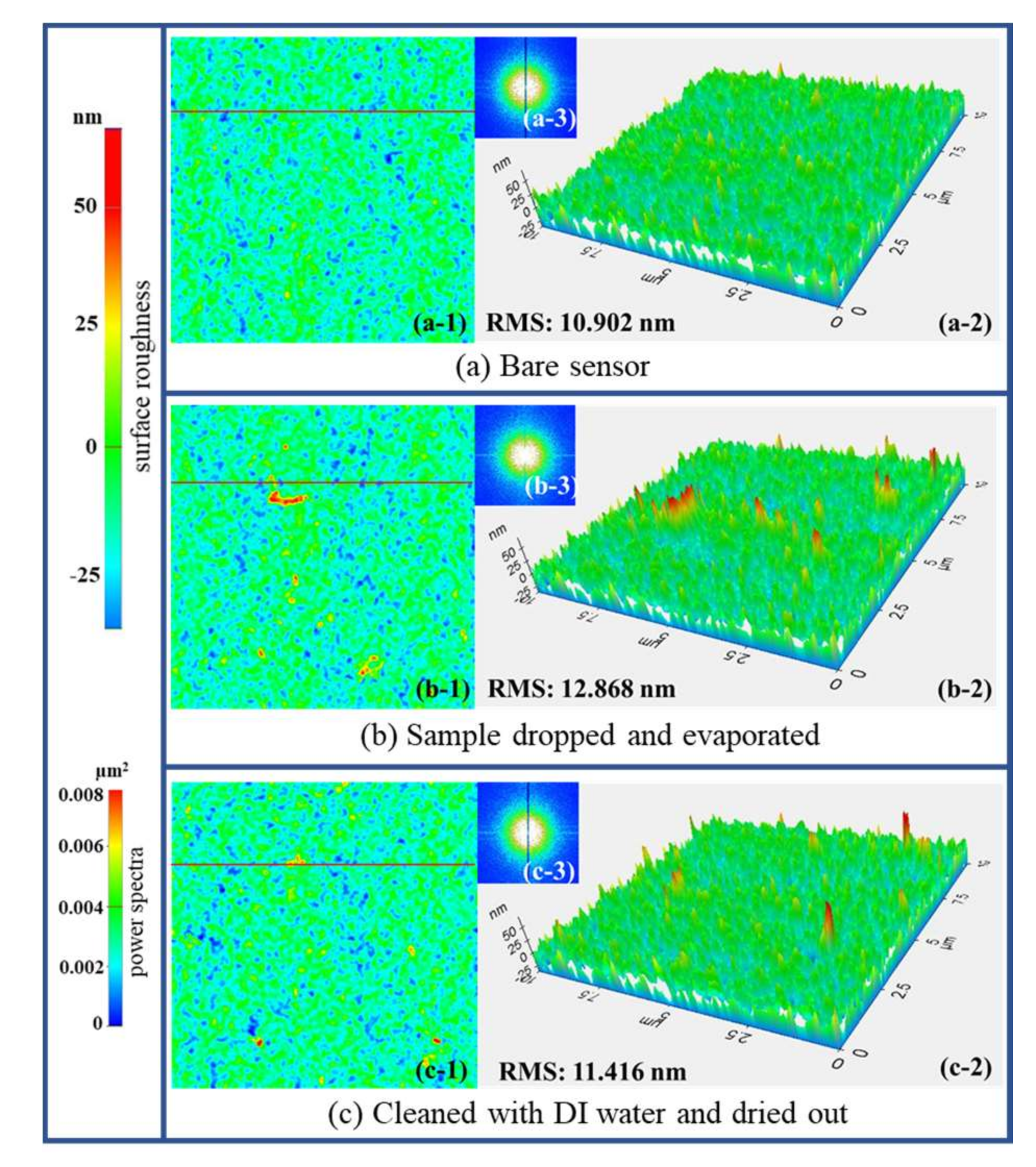
3.3. Reproducibility
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Park, H.; Seo Yoon, H.; Patil, U.; Anoop, R.; Lee, J.; Lim, J.; Lee, W.; Chan Jun, S. Radio frequency based label-free detection of glucose. Biosens. Bioelectron. 2014, 54, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Uttamchandani, D. A microwave dielectric biosensor based on suspended distributed MEMS transmission lines. IEEE Sens. J. 2009, 9, 1825–1830. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.Y.; Dhakal, R.; Adhikari, K.K.; Kim, E.S.; Wang, C. A reusable robust radio frequency biosensor using microwave resonator by integrated passive device technology for quantitative detection of glucose level. Biosens. Bioelectron. 2015, 67, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Kuo, L.S.; Chen, P.H.; Yang, C.R.; Tsai, Z.M. Development of a multilayered polymeric DNA biosensor using radio frequency technology with gold and magnetic nanoparticles. Biosens. Bioelectron. 2012, 31, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, J.H.; Moon, H.S.; Jang, I.S.; Choi, J.S.; Yook, J.G.; Jung, H. Il A planar split-ring resonator-based microwave biosensor for label-free detection of biomolecules. Sens. Actuators B Chem. 2012, 169, 26–31. [Google Scholar] [CrossRef]
- Chretiennot, T.; Dubuc, D.; Grenier, K. A Microwave and Microfluidic Planar Resonator for Efficient and Accurate Complex Permittivity Characterization of Aqueous Solutions. IEEE Trans. Microw. Theory Tech. 2013, 61, 972–978. [Google Scholar] [CrossRef]
- Cui, Y.; Sun, J.; He, Y.; Wang, Z.; Wang, P. A simple, tunable, and highly sensitive radio-frequency sensor. Appl. Phys. Lett. 2013, 103, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Cagnin, S.; Caraballo, M.; Guiducci, C.; Martini, P.; Ross, M.; SantaAna, M.; Danley, D.; West, T.; Lanfranchi, G. Overview of Electrochemical DNA Biosensors: New Approaches to Detect the Expression of Life. Sensors 2009, 9, 3122–3148. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.; Yetisen, A.K.; Butt, H. High Numerical Aperture Hexagonal Stacked Ring-Based Bidirectional Flexible Polymer Microlens Array. ACS Nano 2017, 11, 3155–3165. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Butt, H.; Volpatti, L.R.; Pavlichenko, I.; Humar, M.; Kwok, S.J.J.; Koo, H.; Kim, K.S.; Naydenova, I.; Khademhosseini, A.; et al. Photonic hydrogel sensors. Biotechnol. Adv. 2016, 34, 250–271. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dong, M.; Santos, S.; Rigatto, C.; Liu, Y.; Lin, F. Lab-on-a-Chip Platforms for Detection of Cardiovascular Disease and Cancer Biomarkers. Sensors 2017, 17, 2934. [Google Scholar] [CrossRef] [PubMed]
- Torun, H.; Cagri Top, F.; Dundar, G.; Yalcinkaya, A.D. An antenna-coupled split-ring resonator for biosensing. J. Appl. Phys. 2014, 116. [Google Scholar] [CrossRef]
- Salim, A.; Lim, S. Complementary split-ring resonator-loaded microfluidic ethanol chemical sensor. Sensors 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Adhikari, K.K.; Dhakal, R.; Chuluunbaatar, Z.; Wang, C.; Kim, E.S. Rapid, sensitive, and reusable detection of glucose by a robust radiofrequency integrated passive device biosensor chip. Sci. Rep. 2015, 5, 7807. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.R.; Kang, S.W. Highly sensitive multi-channel IDC sensor array for low concentration taste detection. Sensors 2015, 15, 13201–13221. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, R.; Wang, C.; Kim, E.S.; Kim, N.Y. Complex permittivity characterization of serum with an air-bridge enhanced capacitor for quantifiable detection of glucose. Appl. Phys. Lett. 2015, 106, 1–6. [Google Scholar] [CrossRef]
- McKee, J.M.; Johnson, B.P. Real-time chemical sensing of aqueous ethanol glucose mixtures. IEEE Trans. Instrum. Meas. 2000, 49, 114–119. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Withayachumnankul, W.; Al-Sarawi, S.; Abbott, D. High-Sensitivity Metamaterial-Inspired Sensor for Microfluidic Dielectric Characterization. IEEE Sens. J. 2014, 1, 1345–1351. [Google Scholar] [CrossRef]
- Proekt, L.; Cangellaris, A.C. Investigation of the Impact of Conductor Surface Roughness on Interconnect Frequency-Dependent Ohmic. In Proceedings of the Electron. Components Technology Conference, New Orleans, LA, USA, 27–30 May 2003; pp. 1004–1010. [Google Scholar]
- Qiang, T.; Wang, C.; Kim, N.Y. Quantitative detection of glucose level based on radiofrequency patch biosensor combined with volume-fixed structures. Biosens. Bioelectron. 2017, 98, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, C.; Kim, N. Design of Very Compact Bandpass Filters Based on Differential Transformers. IEEE Microw. Wirel. Compon. Lett. 2015, 25, 439–441. [Google Scholar] [CrossRef]
- Carchon, G.; Vaesen, K.; Sun, X.; Brebels, S.; Imaoka, T.; Sawai, T.; Inoue, Y.; Description, T. Design Library Components and Models. Components 2006, 1732–1739. [Google Scholar]
- Endres, H.E.; Drost, S. Optimization of the geometry of gas-sensitive interdigital capacitors. Sens. Actuators B. Chem. 1991, 4, 95–98. [Google Scholar] [CrossRef]
- Megriche, A.; Belhadj, A.; Mgaidi, A. Microwave dielectric properties of binary solvent water- alcohol, alcohol-alcohol mixtures at temperatures between −35 °C and +35 °C and dielectric relaxation studies. Mediterr. J. Chem. 2012, 1, 200–209. [Google Scholar] [CrossRef]
- Campos, D.C.; Dall’Oglio, E.L.; De Sousa, P.T.; Vasconcelos, L.G.; Kuhnen, C.A. Investigation of dielectric properties of the reaction mixture during the acid-catalyzed transesterification of Brazil nut oil for biodiesel production. Fuel 2014, 117, 957–965. [Google Scholar] [CrossRef]
- Hofmann, M.; Fischer, G.; Weigel, R.; Kissinger, D. Microwave-based noninvasive concentration measurements for biomedical applications. IEEE Trans. Microw. Theory Tech. 2013, 61, 2195–2204. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Bounaix Morand Du Puch, C.; Dalmay, C.; Lacroix, A.; Landoulsi, A.; Leroy, J.; Mélin, C.; Lalloué, F.; Battu, S.; Lautrette, C.; et al. Discrimination of colorectal cancer cell lines using microwave biosensors. Sens. Actuators A Phys. 2014, 216, 405–416. [Google Scholar] [CrossRef]
- Bao, J.; Swicord, M.L.; Davis, C.C. Microwave dielectric characterization of binary mixtures of water, methanol, and ethanol. J. Chem. Phys. 1996, 104, 4441–4450. [Google Scholar] [CrossRef]
- Ryyniinen, S. The Electromagnetic Properties of Food Materials: A Review of the Basic Principles. J. Food Eng. 1995, 26, 409–429. [Google Scholar] [CrossRef]
- Routray, W.; Orsat, V. Dielectric properties of concentration-dependent ethanol + acids solutions at different temperatures. J. Chem. Eng. Data 2013, 58, 1650–1661. [Google Scholar] [CrossRef]



| Reference | Design Concept | Frequency Band of Operation | Sensitivity (MHz/Ethanol Percentage) | Selectivity (in dB) | Reproducibility |
|---|---|---|---|---|---|
| [6] | Planar resonator | Ku-/K-band | 70.72 * | <−8.5 * | NA |
| [17] | Microstrip BPF | <L-band | 2.88 * | NA | NA |
| [18] | Split ring resonator | L-/S-band | 3.86 * | <−6 * | NA |
| This work | Spiral-coupled resonator | L-/S-band | 10.4 | <−14 | Yes |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koirala, G.R.; Dhakal, R.; Kim, E.-S.; Yao, Z.; Kim, N.-Y. Radio Frequency Detection and Characterization of Water-Ethanol Solution through Spiral-Coupled Passive Micro-Resonator Sensor. Sensors 2018, 18, 1075. https://doi.org/10.3390/s18041075
Koirala GR, Dhakal R, Kim E-S, Yao Z, Kim N-Y. Radio Frequency Detection and Characterization of Water-Ethanol Solution through Spiral-Coupled Passive Micro-Resonator Sensor. Sensors. 2018; 18(4):1075. https://doi.org/10.3390/s18041075
Chicago/Turabian StyleKoirala, Gyan Raj, Rajendra Dhakal, Eun-Seong Kim, Zhao Yao, and Nam-Young Kim. 2018. "Radio Frequency Detection and Characterization of Water-Ethanol Solution through Spiral-Coupled Passive Micro-Resonator Sensor" Sensors 18, no. 4: 1075. https://doi.org/10.3390/s18041075
APA StyleKoirala, G. R., Dhakal, R., Kim, E.-S., Yao, Z., & Kim, N.-Y. (2018). Radio Frequency Detection and Characterization of Water-Ethanol Solution through Spiral-Coupled Passive Micro-Resonator Sensor. Sensors, 18(4), 1075. https://doi.org/10.3390/s18041075







