Electrochemical Sensor for Bilirubin Detection Using Screen Printed Electrodes Functionalized with Carbon Nanotubes and Graphene
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Measurement of BR in Blood Serum Samples
2.3. Instrumentation and Measurements
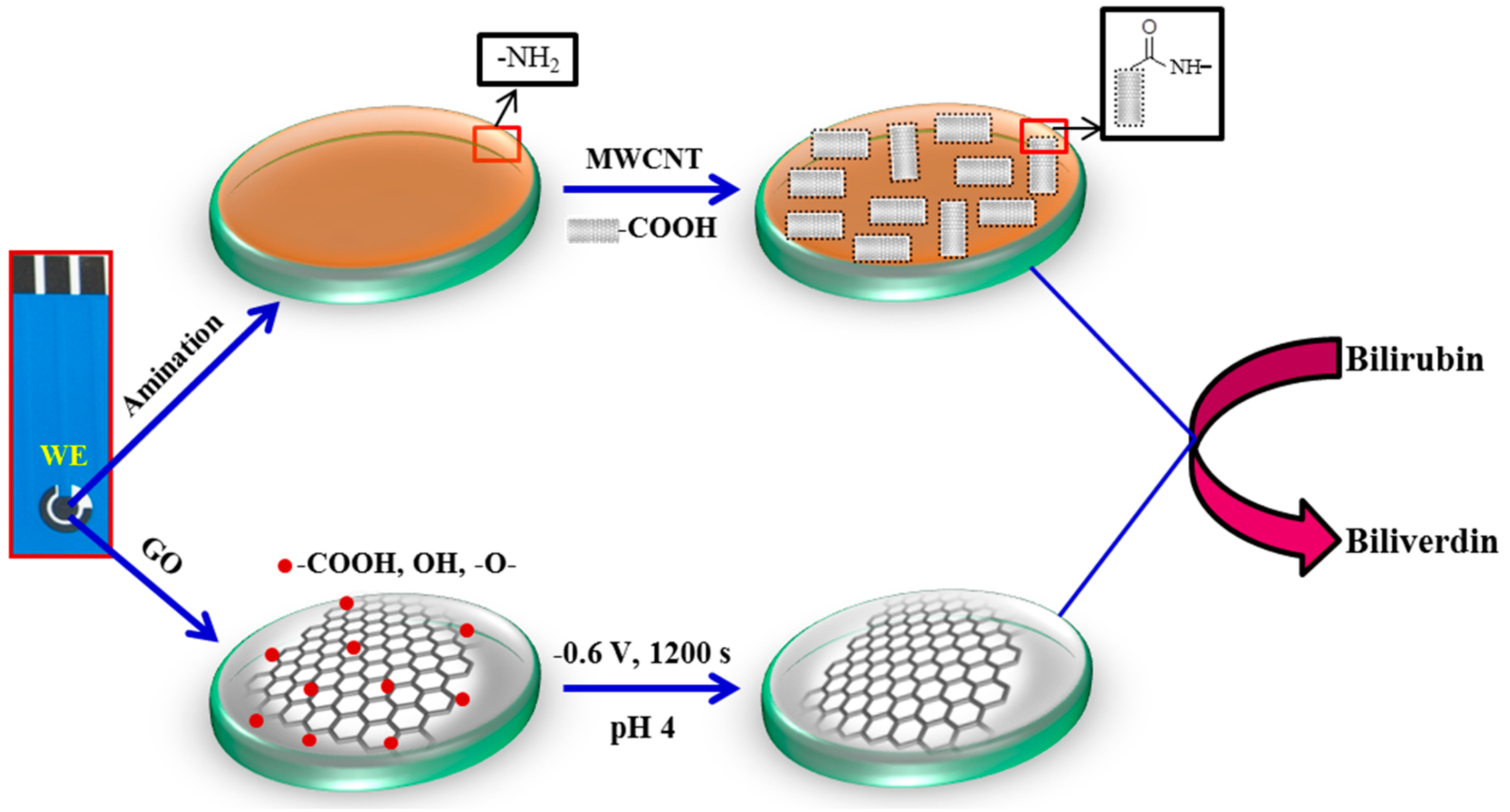
2.4. Sensor Fabrication
3. Results
3.1. Morphological Characterization
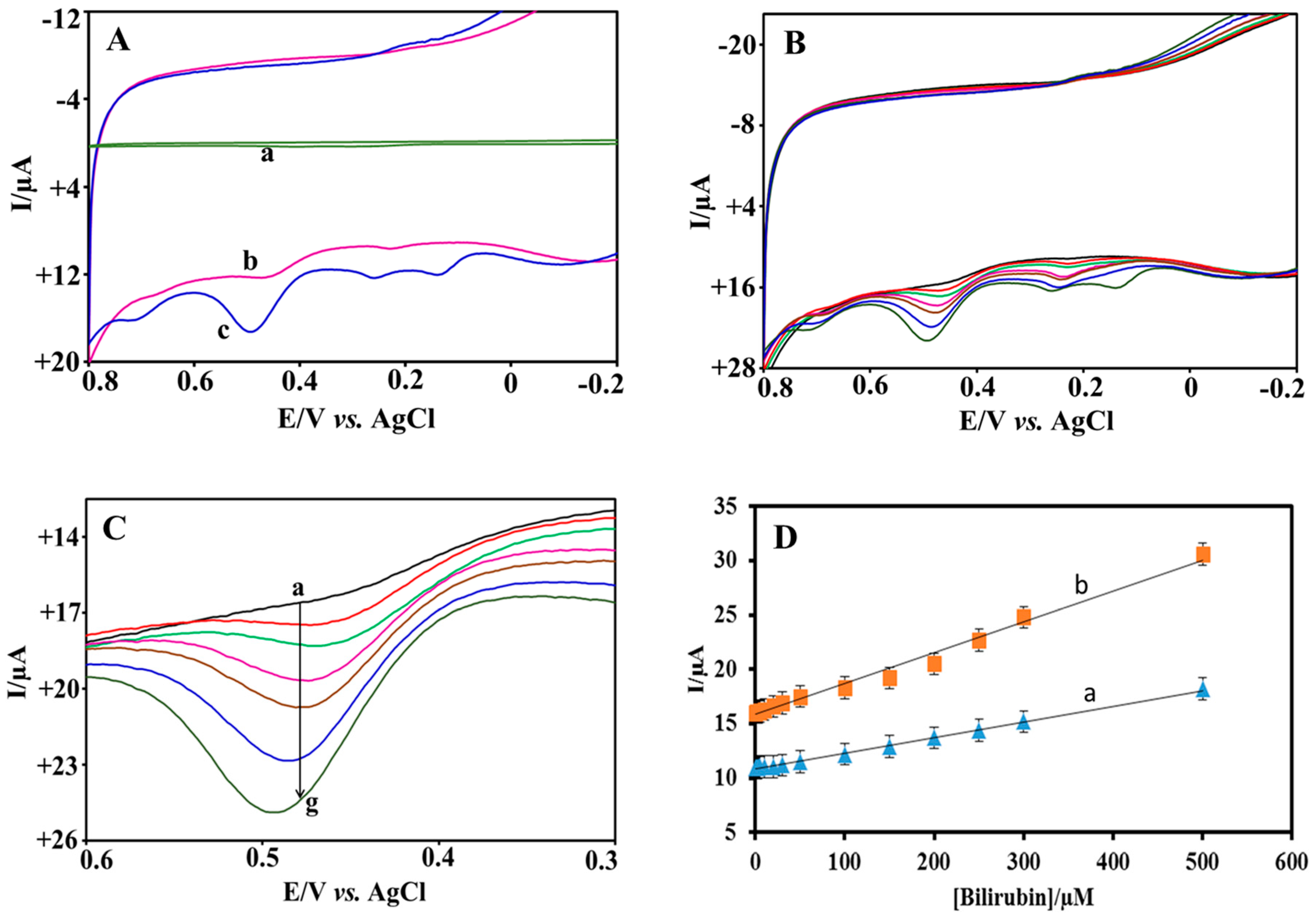
3.2. Electrochemical Characterization
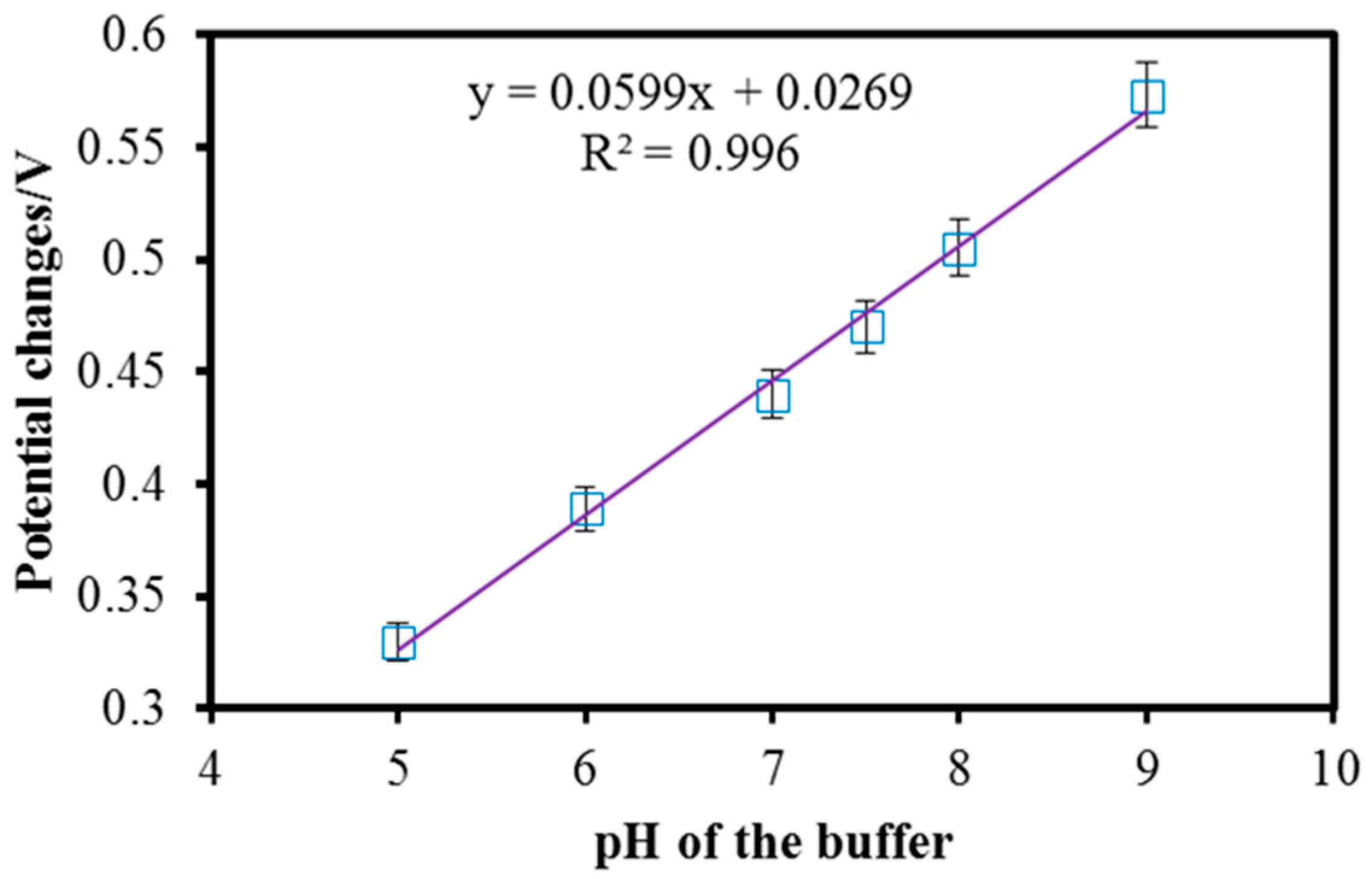
3.3. Effect of pH
3.4. Electrocatalytic Oxidation of BR
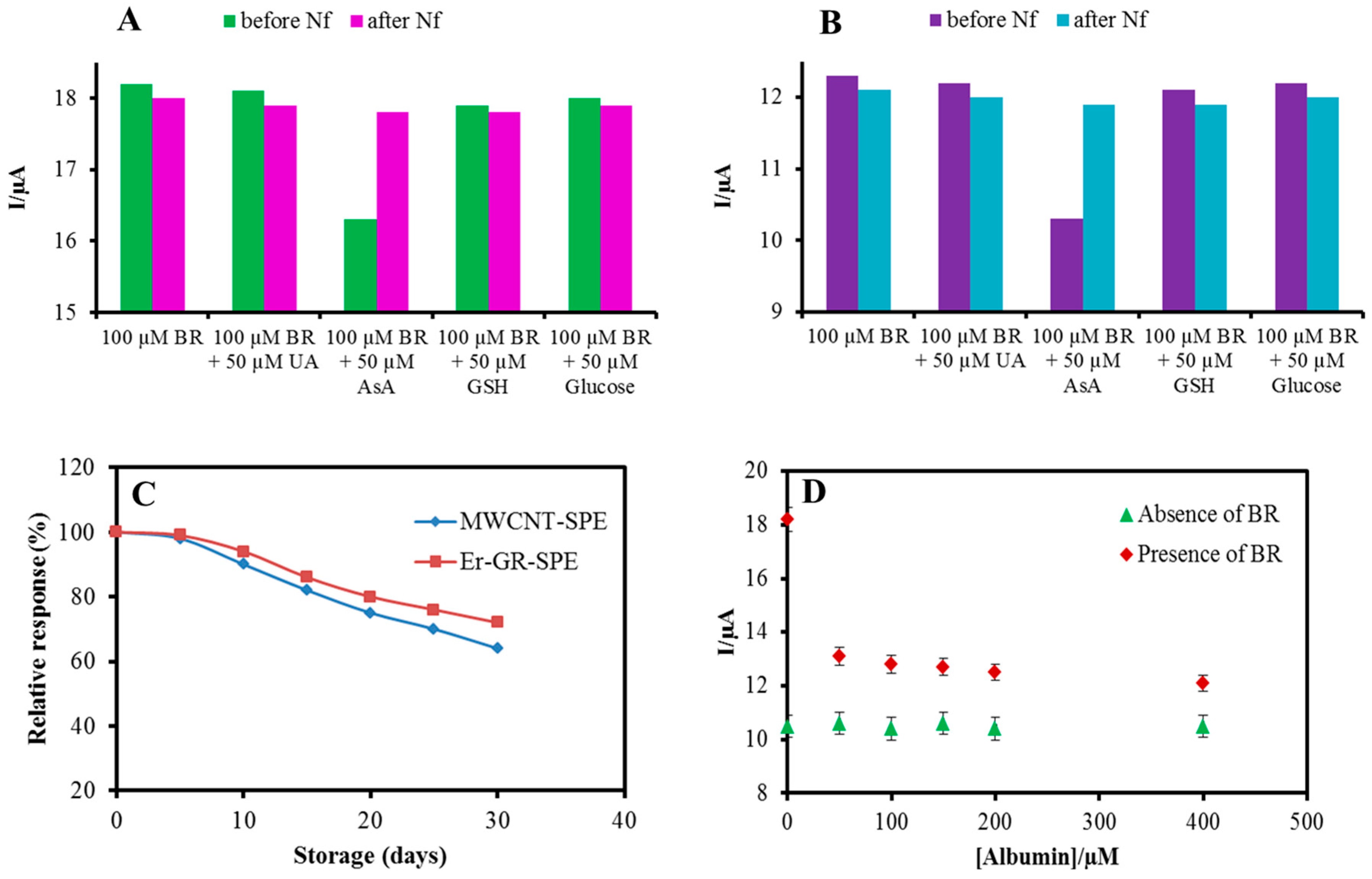
3.5. Specificity
3.6. Stability, Repeatability, Reproducibility
3.7. Real Sample Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Wang, X.; Chowdhury, J.R.; Chowdhury, N.R. Bilirubin metabolism: Applied physiology. Curr. Paediatr. 2006, 16, 70–74. [Google Scholar] [CrossRef]
- Stocker, R.; Yamamoto, Y.; McDonagh, A.F.; Glazer, A.N.; Ames, B.N. Bilirubin is an antioxidant of possible physiological importance. Science 1987, 235, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Brito, M.A.; Silva, R.F.M.; Brites, D. Bilirubin toxicity to human erythrocytes: A review. Clin. Chim. Acta 2006, 374, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Ellairaja, S.; Shenbagavalli, K.; Ponmariappan, S.; Vasantha, V.S. A green and facile approach for synthesizing imine to develop optical biosensor for wide range detection of bilirubin in human biofluids. Biosens. Bioelectron. 2017, 91, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Cashore, W.J. Bilirubin and jaundice in the micropremie. Clin. Perinatol. 2000, 27, 171–179. [Google Scholar] [CrossRef]
- Maisels, M.J. Bilirubin: On understanding and influencing its metabolism in the newborn infant. Pediatr. Clin. N. Am. 1972, 19, 447–501. [Google Scholar] [CrossRef]
- Harrison, S.P.; Barlow, I.M. Three direct spectrophotometric methods for determination of total bilirubin in neonatal and adult serum, adapted to the Technicon RA-1000 analyzer. Clin. Chem. 1989, 35, 1980–1986. [Google Scholar] [PubMed]
- Westwood, A. The analysis of bilirubin in serum. Ann. Clin. Biochem. 1991, 28, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Liu, Z.; Zhou, X. Polarographic investigation and determination of bilirubin. Anal. Sci. 1994, 10, 95–99. [Google Scholar] [CrossRef]
- Senthilkumar, T.; Asha, S.K. Selective and Sensitive Sensing of Free Bilirubin in Human Serum Using Water-Soluble Polyfluorene as Fluorescent Probe. Macromolecules 2015, 48, 3449–3461. [Google Scholar] [CrossRef]
- Li, X.; Rosenzweig, Z. A fiber optic sensor for rapid analysis of bilirubin in serum. Anal. Chim. Acta 1997, 353, 263–273. [Google Scholar] [CrossRef]
- Chauhan, N.; Rawal, R.; Hooda, V.; Jain, U. Electrochemical biosensor with graphene oxide nanoparticles and polypyrrole interface for the detection of bilirubin. RSC Adv. 2016, 6, 63624–63633. [Google Scholar] [CrossRef]
- Batra, B.; Lata, S.; Rana, J.S.; Pundir, C.S. Construction of an amperometric bilirubin biosensor based on covalent immobilization of bilirubin oxidase onto zirconia coated silica nanoparticles/chitosan hybrid film. Biosens. Bioelectron. 2013, 44, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Jianhong, P.; Mary, M.L.; Chung Chang, Y. Bilirubin sensor. U.S. Patent US7749766, 6 July 2010. [Google Scholar]
- Kannan, P.; Chen, H.; Lee, V.T.-W.; Kim, D.-H. Highly sensitive amperometric detection of bilirubin using enzyme and gold nanoparticles on sol–gel film modified electrode. Talanta 2011, 86, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ju, H.; Wang, J. Electrochemical Sensors, Biosensors and Their Biomedical Applications; Academic Press: Cambridge, MA, USA, 2011; ISBN 008055489X. [Google Scholar]
- Klemm, J.; Prodromidis, M.I.; Karayannis, M.I. An enzymic method for the determination of bilirubin using an oxygen electrode. Electroanalysis 2000, 12, 292–295. [Google Scholar] [CrossRef]
- Fortuney, A.; Guilbault, G.G. Enzyme electrode for the determination of bilirubin. Electroanalysis 1996, 8, 229–232. [Google Scholar] [CrossRef]
- Santhosh, M.; Chinnadayyala, S.R.; Singh, N.K.; Goswami, P. Human serum albumin-stabilized gold nanoclusters act as an electron transfer bridge supporting specific electrocatalysis of bilirubin useful for biosensing applications. Bioelectrochemistry 2016, 111, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yan, J.; Zhang, C. Piezoelectric detection of bilirubin based on bilirubin-imprinted titania film electrode. Anal. Biochem. 2012, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shang, X.; Zhang, C.; Zhu, J. Photoelectrochemical bilirubin biosensor based on Fe3O4/hydroxyapatite/molecularly imprinted polypyrrole nanoparticles. Sens. Actuators B Chem. 2014, 201, 167–172. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Syu, M.-J.; Chang, Y.-S.; Chang, C.-H.; Chou, T.-C.; Liu, B.-D. A portable potentiostat for the bilirubin-specific sensor prepared from molecular imprinting. Biosens. Bioelectron. 2007, 22, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bai, W.; Yang, Z. A novel photoelectrochemical sensor for bilirubin based on porous transparent TiO2 and molecularly imprinted polypyrrole. Electrochim. Acta 2016, 187, 451–456. [Google Scholar] [CrossRef]
- Wang, C.; Wang, G.; Fang, B. Electrocatalytic oxidation of bilirubin at ferrocenecarboxamide modified MWCNT–gold nanocomposite electrodes. Microchim. Acta 2009, 164, 113. [Google Scholar] [CrossRef]
- Taurino, I.; Van Hoof, V.; De Micheli, G.; Carrara, S. Superior sensing performance of multi-walled carbon nanotube-based electrodes to detect unconjugated bilirubin. Thin Solid Films 2013, 548, 546–550. [Google Scholar] [CrossRef]
- Feng, Q.; Du, Y.; Zhang, C.; Zheng, Z.; Hu, F.; Wang, Z.; Wang, C. Synthesis of the multi-walled carbon nanotubes-COOH/graphene/gold nanoparticles nanocomposite for simple determination of Bilirubin in human blood serum. Sens. Actuators B Chem. 2013, 185, 337–344. [Google Scholar] [CrossRef]
- Balamurugan, T.; Berchmans, S. Non-enzymatic detection of bilirubin based on a graphene–polystyrene sulfonate composite. RSC Adv. 2015, 5, 50470–50477. [Google Scholar] [CrossRef]
- Hayat, A.; Marty, J.L. Disposable screen printed electrochemical sensors: Tools for environmental monitoring. Sensors 2014, 14, 10432–10453. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, M.; Madasamy, T.; Pandiaraj, M.; Bhargava, K.; Sethy, N.K.; Karunakaran, C. Electrochemical assay for the determination of nitric oxide metabolites using copper(II) chlorophyllin modified screen printed electrodes. Anal. Biochem. 2015, 478, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, K.; Vestergaard, M.C.; Tamiya, E. Printable Electrochemical Biosensors: A Focus on Screen-Printed Electrodes and Their Application. Sensors 2016, 16, 1761. [Google Scholar] [CrossRef] [PubMed]
- Zaporotskova, I.V.; Boroznina, N.P.; Parkhomenko, Y.N.; Kozhitov, L.V. Carbon nanotubes: Sensor properties. A review. Mod. Electron. Mater. 2016, 2, 95–105. [Google Scholar] [CrossRef]
- Wang, F.; Liu, L.; Li, W.J. Graphene-Based Glucose Sensors: A Brief Review. IEEE Trans. Nanobiosci. 2015, 14, 818–834. [Google Scholar] [CrossRef] [PubMed]
- Madasamy, T.; Santschi, C.; Martin, O.J.F. A miniaturized electrochemical assay for homocysteine using screen-printed electrodes with cytochrome c anchored gold nanoparticles. Analyst 2015, 140, 6071–6078. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, W.; Zhang, Y.; Zhang, Z.-H.; Tiam Tan, S.; Yi, X.; Wang, G.; Sun, X.; Zhu, H.; Volkan Demir, H. Graphene-based transparent conductive electrodes for GaN-based light emitting diodes: Challenges and countermeasures. Nano Energy 2015, 12, 419–436. [Google Scholar] [CrossRef]
- Eckermann, A.L.; Feld, D.J.; Shaw, J.A.; Meade, T.J. Electrochemistry of redox-active self-assembled monolayers. Coord. Chem. Rev. 2010, 254, 1769–1802. [Google Scholar] [CrossRef] [PubMed]
- Zahn, R.; Coullerez, G.; Voros, J.; Zambelli, T. Effect of polyelectrolyte interdiffusion on electron transport in redox-active polyelectrolyte multilayers. J. Mater. Chem. 2012, 22, 11073–11078. [Google Scholar] [CrossRef]
- Ye, J.; Xiong, H.; Wang, Q.; Zhang, X.; Wang, S. Voltammetric Behavior of Bilirubin Based on [bmim][PF6] As the Supporting Electrolyte in Organic Solvent and Its Analytical Application. Am. J. Biomed. Sci. 2011, 191–198. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lee, K.-S.; Park, D.-S.; Won, M.-S.; Shim, Y.-B. An amperometric bilirubin biosensor based on a conductive poly-terthiophene-Mn(II) complex. Biosens. Bioelectron. 2008, 23, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Noh, H.-B.; Won, M.-S.; Shim, Y.-B. Selective nonenzymatic bilirubin detection in blood samples using a Nafion/Mn-Cu sensor. Biosens. Bioelectron. 2014, 61, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, C. Molecularly imprinted hydroxyapatite thin film for bilirubin recognition. Biosens. Bioelectron. 2011, 29, 167–171. [Google Scholar] [CrossRef] [PubMed]


| Human Blood Serum | BR Added (µM) | BR Found (µM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Sample 1 | 5 | 5.08 | 101.6 | 2.4 |
| Sample 2 | 10 | 9.72 | 97.2 | 1.9 |
| Sample 3 | 15 | 14.10 | 94.0 | 3.1 |
| Sample 4 | 20 | 21.3 | 106.5 | 2.0 |
| Sample 5 | 25 | 24.3 | 97.2 | 1.4 |
| Sample 6 | 30 | 28.7 | 95.6 | 2.7 |
| Sensing Element | Linearity Range (µM) | Detection Limit | Sensitivity (µA µM−1 cm−2) | Reference |
|---|---|---|---|---|
| BOx/SiO2@ZrONPs/Au | 0.02–250 | 0.1 nM | --- | Batra_2013 |
| PolyTTCA–Mn(II) | 0.1–50 | 40 ± 3.8 nM | --- | Rahman_2008 |
| MWCNT/SPE | 4.2 ± 0.1 µM | 52.2 ± 2.4 | Taurino_2013 | |
| BOx/Pt | 100–200 | 8 µM | --- | Klemm_2000 |
| FcAI/NG/MWCNT/GCE | 1–100 | 0.12 µM | --- | Wang_2009 |
| PSS-RGO/GCE | 0–450 | 2 | 0.16 | Balamurugan_2015 |
| BOx/GONP@Ppy/FTO | 0.01–500 | 2.6 nM | --- | Chauhan_2016 |
| BOx/MWCNT/GNs/AuNPs/GCE | 1.33–71.56 | 0.34 µM | 0.327 | Feng_2013 |
| BOx/AuNPs/MPTS/Au | 1–5000 | 1.4 nM | --- | Kannan_2011 |
| Nafion/Mn-Cu/GCE | 1.2–420 | 25 ± 1.8 nM | --- | Noh _2014 |
| HSA/AuNCs/ITO | 0.2–7 | 86.32 nM | 0.34 | Santhosh_2016 |
| HAP/QC | 0.05–80 | 0.01 µM | Yang_2011 | |
| Er-GR/SPE | 0.1–600 | 0.1 ± 0.018 µM | 0.030 | Present work |
| MWCNT/SPE | 0.5–500 | 0.3 ± 0.022 µM | 0.015 | Present work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thangamuthu, M.; Gabriel, W.E.; Santschi, C.; Martin, O.J.F. Electrochemical Sensor for Bilirubin Detection Using Screen Printed Electrodes Functionalized with Carbon Nanotubes and Graphene. Sensors 2018, 18, 800. https://doi.org/10.3390/s18030800
Thangamuthu M, Gabriel WE, Santschi C, Martin OJF. Electrochemical Sensor for Bilirubin Detection Using Screen Printed Electrodes Functionalized with Carbon Nanotubes and Graphene. Sensors. 2018; 18(3):800. https://doi.org/10.3390/s18030800
Chicago/Turabian StyleThangamuthu, Madasamy, Willimann Eric Gabriel, Christian Santschi, and Olivier J. F. Martin. 2018. "Electrochemical Sensor for Bilirubin Detection Using Screen Printed Electrodes Functionalized with Carbon Nanotubes and Graphene" Sensors 18, no. 3: 800. https://doi.org/10.3390/s18030800





