Thermal Treatment of Aerosol Deposited NiMn2O4 NTC Thermistors for Improved Aging Stability
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Film Characterization in Deposited State
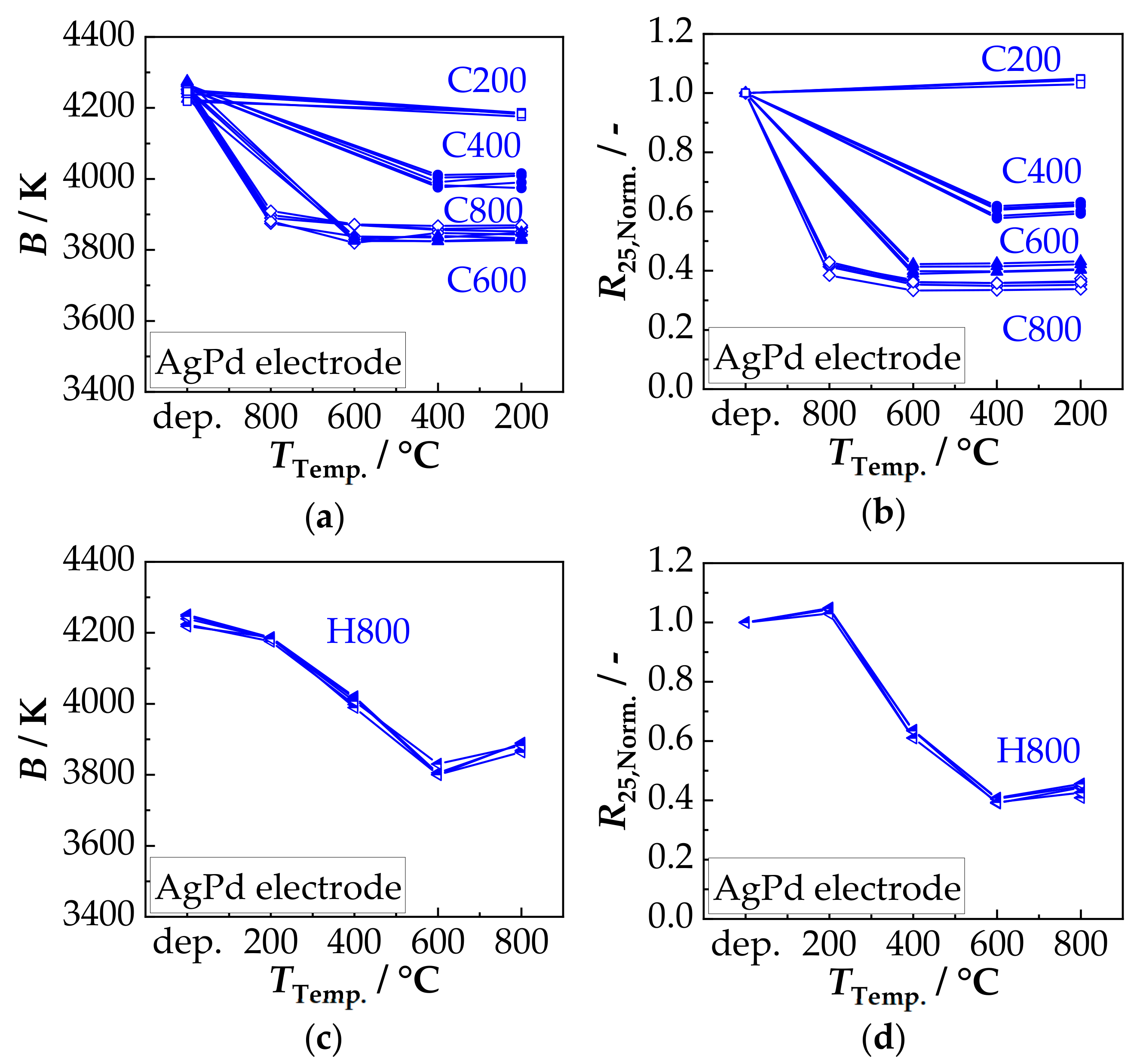
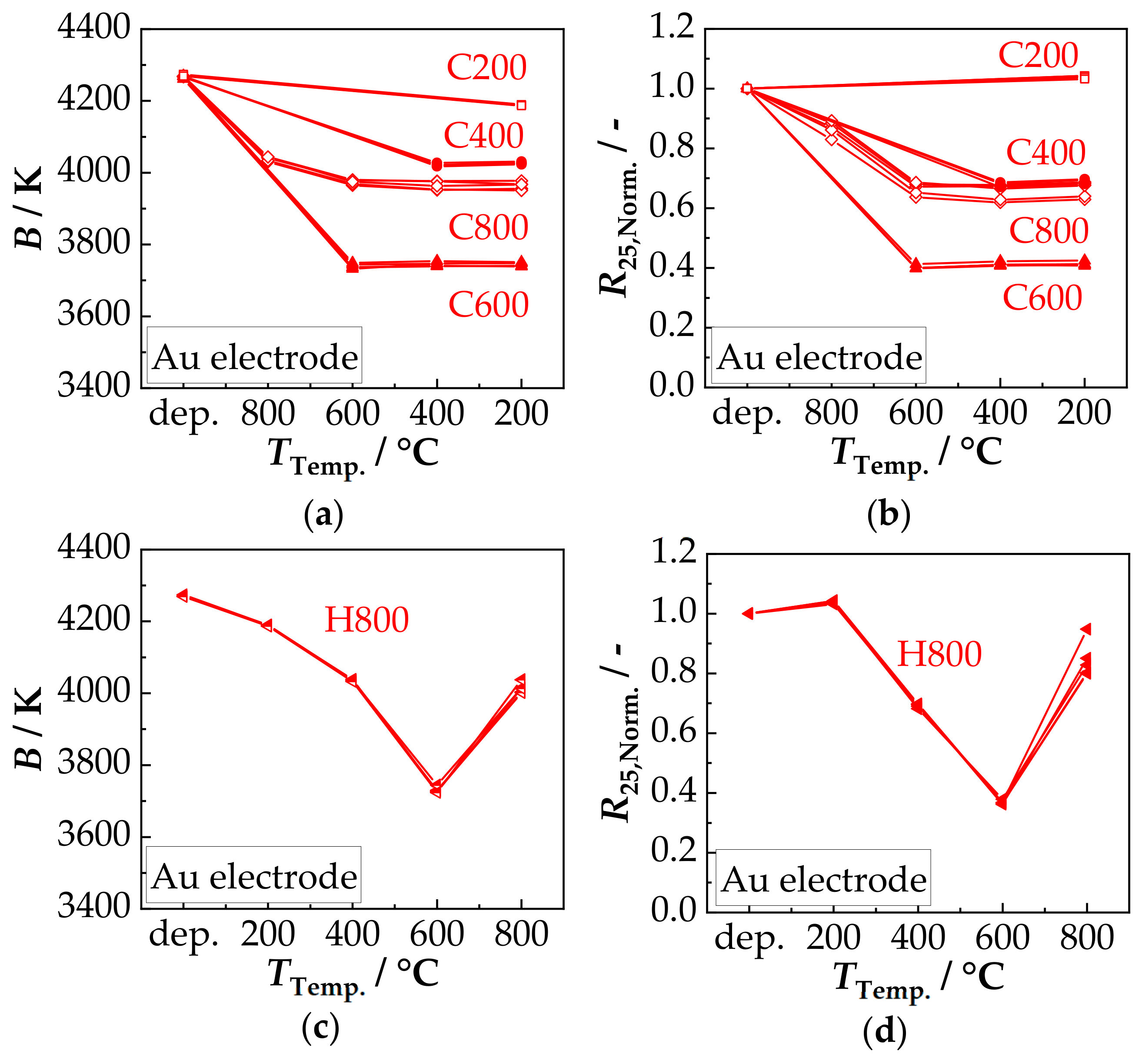
3.2. Film Characterization in the Tempered State—Thermal Stability
3.3. Film Characterization in Aged State—Aging Stability
4. Discussion
5. Conclusions
6. Further Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parlak, M.; Hashemi, T.; Hogan, M.J.; Brinkman, A.W. Effect of heat treatment on nickel manganite thin film thermistors deposited by electron beam evaporation. Thin Solid Films 1999, 345, 307–311. [Google Scholar] [CrossRef]
- Díez, A.; Schmidt, R.; Sagua, A.E.; Frechero, M.A.; Matesanz, E.; Leon, C.; Morán, E. Structure and physical properties of nickel manganite NiMn2O4 obtained from nickel permanganate precursor. J. Eur. Ceram. Soc. 2010, 30, 2617–2624. [Google Scholar] [CrossRef]
- Martin De Vidales, J.L.; Garcia-Chain, P.; Rojas, R.M.; Vila, E.; Garcia-Martinez, O. Preparation and characterization of spinel-type Mn–Ni–Co–O negative temperature coefficient ceramic thermistors. J. Mater. Sci. 1998, 33, 1491–1496. [Google Scholar] [CrossRef]
- Gao, H.; Ma, C.; Sun, B. Preparation and characterization of NiMn2O4 negative temperature coefficient ceramics by solid-state coordination reaction. J. Mater. Sci. Mater. Electron. 2014, 25, 3990–3995. [Google Scholar] [CrossRef]
- Csete de Györgyfalva, G.D.C.; Reaney, I.M. Decomposition of NiMn2O4 spinel: An NTC thermistor material. J. Eur. Ceram. Soc. 2001, 21, 2145–2148. [Google Scholar] [CrossRef]
- Fang, D.-I.; Zheng, C.-H.; Chen, C.-S.; Winnubst, A.J.A. Aging of nickel manganite NTC ceramics. J. Electroceram. 2009, 22, 421–427. [Google Scholar] [CrossRef]
- Schmidt, R.; Basu, A.; Brinkman, A.W. Small polaron hopping in spinel manganates. Phys. Rev. B 2005, 72, 115101. [Google Scholar] [CrossRef]
- Fau, P.; Bonino, J.P.; Demai, J.J.; Rousset, A. Thin films of nickel manganese oxide for NTC thermistor applications. Appl. Surf. Sci. 1993, 65–66, 319–324. [Google Scholar] [CrossRef]
- Park, K.; Bang, D.Y. Electrical properties of Ni-Mn-Co-(Fe) oxide thick-film NTC thermistors prepared by screen printing. J. Mater. Sci. Mater. Electron. 2003, 14, 81–87. [Google Scholar] [CrossRef]
- Schmidt, R.; Basu, A.; Brinkman, A.W. Production of NTCR thermistor devices based on NiMn2O4+δ. J. Eur. Ceram. Soc. 2004, 24, 1233–1236. [Google Scholar] [CrossRef]
- Schaumburg, H. Keramik: Werkstoffe und Bauelemente der Elektrotechnik; Vieweg + Teubner Verlag: Wiesbaden, Germany, 1994. [Google Scholar]
- Kamat, R.K.; Naik, G.M. Thermistors—In search of new applications, manufacturers cultivate advanced NTC techniques. Sens. Rev. 2002, 22, 334–340. [Google Scholar] [CrossRef]
- Schmidt, R.; Brinkman, A.W. Electrical properties of screen-printed NiMn2O4+δ. J. Eur. Ceram. Soc. 2005, 25, 3027–3031. [Google Scholar] [CrossRef]
- Schmidt, R.; Stiegelschmitt, A.; Roosen, A.; Brinkman, A.W. Screen printing of co-precipitated NiMn2O4+δ for production of NTCR thermistors. J. Eur. Ceram. Soc. 2003, 23, 1549–1558. [Google Scholar] [CrossRef]
- Ryu, J.; Park, D.-S.; Schmidt, R. In-plane impedance spectroscopy in aerosol deposited NiMn2O4 negative temperature coefficient thermistor films. J. Appl. Phys. 2011, 109, 113722. [Google Scholar] [CrossRef] [Green Version]
- Ryu, J.; Han, G.; Lee, Y.-P.; Lim, Y.-S.; Park, D.-S.; Jeong, D.-Y. Co and Fe Doping Effect on Negative Temperature Coefficient Characteristics of Nano-Grained NiMn2O4 Thick Films Fabricated by Aerosol-Deposition. J. Nanosci. Nanotechnol. 2013, 13, 3422–3426. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Kim, K.-Y.; Choi, J.-J.; Hahn, B.-D.; Yoon, W.-H.; Lee, B.-K.; Park, D.-S.; Park, C. Highly dense and nanograined NiMn2O4 negative temperature coefficient thermistor thick films Fabricated by Aerosol-Deposition. J. Am. Ceram. Soc. 2009, 92, 3084–3087. [Google Scholar] [CrossRef]
- Kang, J.-E.; Ryu, J.; Han, G.; Choi, J.-J.; Yoon, W.-H.; Hahn, B.-D.; Kim, J.-W.; Ahn, C.-W.; Choi, J.H.; Park, D.-S. LaNiO3 conducting particle dispersed NiMn2O4 nanocomposite NTC thermistor thick films by aerosol deposition. J. Alloys Compd. 2012, 534, 70–73. [Google Scholar] [CrossRef]
- Schubert, M.; Münch, C.; Schuurman, S.; Poulain, V.; Kita, J.; Moos, R. Characterization of nickel manganite NTC thermistor films prepared by aerosol deposition at room temperature. J. Eur. Ceram. Soc. 2018, 38, 613–619. [Google Scholar] [CrossRef]
- Schubert, M.; Kita, J.; Münch, C.; Moos, R. Analysis of the characteristics of thick-film NTC thermistor devices manufactured by screen-printing and firing technique and by room temperature aerosol deposition method (ADM). Funct. Mater. Lett. 2017, 10, 1750073. [Google Scholar] [CrossRef]
- Hanft, D.; Exner, J.; Schubert, M.; Stöcker, T.; Fuierer, P.; Moos, R. An Overview of the Aerosol Deposition Method: Process Fundamentals and New Trends in Materials Applications. J. Ceram. Sci. Technol. 2015, 6, 147–182. [Google Scholar]
- Akedo, J. Room Temperature Impact Consolidation (RTIC) of Fine Ceramic Powder by Aerosol Deposition Method and Applications to Microdevices. J. Therm. Spray Technol. 2008, 17, 181–198. [Google Scholar] [CrossRef]
- Rousset, A.; Tenailleau, C.; Dufour, P.; Bordeneuve, H.; Pasquet, I.; Guillemet-Fritsch, S.; Poulain, V.; Schuurman, S. Electrical Properties of Mn3−x CoxO4 (0 ≤ x ≤ 3) Ceramics: An Interesting System for Negative Temperature Coefficient Thermistors. Int. J. Appl. Ceram. Technol. 2013, 10, 175–185. [Google Scholar] [CrossRef]
- Fritsch, S.; Sarrias, J.; Brieu, M.; Couderc, J.J.; Baudour, J.L.; Snoeck, E.; Rousset, A. Correlation between the structure, the microstructure and the electrical properties of nickel manganite negative temperature coefficient (NTC) thermistors. Solid State Ionics 1998, 109, 229–237. [Google Scholar] [CrossRef]
- Verwey, E.J.W.; Braun, P.B.; Gorter, E.W.; Romeijn, F.C.; van Santen, J.H. Die Verteilung der Metallionen im Spinellgitter und deren Einfluß auf die physikalischen Eigenschaften. Z. Phys. Chem. 1951, 198, 6–22. [Google Scholar] [CrossRef]
- Groen, W.A.; Metzmacher, C.; Zaspalis, V.; Huppertz, P.; Schuurman, S. Aging of NTC ceramics in the system Mn–Ni–Fe–O. J. Eur. Ceram. Soc. 2001, 21, 1793–1796. [Google Scholar] [CrossRef] [Green Version]
- Metz, R. Electrical properties of N.T.C. thermistors made of manganite ceramics of general spinel structure: Mn3–x–x′MxNx′O4 (0 ≤ x + x′ ≤ 1; M and N beeing Ni, Co or Cu). Aging phenomenon study. J. Mater. Sci. 2000, 35, 4705–4711. [Google Scholar] [CrossRef]
- He, L.; Ling, Z.Y.; Huang, Y.T.; Liu, Y.S. Effects of annealing temperature on microstructure and electrical properties of Mn–Co–Ni–O thin films. Mater. Lett. 2011, 65, 1632–1635. [Google Scholar] [CrossRef]
- Exner, J.; Fuierer, P.; Moos, R. Aerosol deposition of (Cu, Ti) substituted bismuth vanadate films. Thin Solid Films 2014, 573, 185–190. [Google Scholar] [CrossRef]
- Nam, S.-M.; Mori, N.; Kakemoto, H.; Wada, S.; Akedo, J.; Tsurumi, T. Alumina Thick Films as Integral Substrates Using Aerosol Deposition Method. Jpn. J. Appl. Phys. 2004, 43, 5414–5418. [Google Scholar] [CrossRef]
- Schubert, M.; Exner, J.; Moos, R. Influence of Carrier Gas Composition on the Stress of Al2O3 Coatings Prepared by the Aerosol Deposition Method. Materials 2014, 7, 5633–5642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Exner, J.; Schubert, M.; Hanft, D.; Kita, J.; Moos, R. How to treat powders for the room temperature aerosol deposition method to avoid porous, low strength ceramic films. J. Eur. Ceram. Soc. 2019, 39, 592–600. [Google Scholar] [CrossRef]
- Hanft, D.; Glosse, P.; Denneler, S.; Berthold, T.; Oomen, M.; Kauffmann-Weiss, S.; Weis, F.; Häßler, W.; Holzapfel, B.; Moos, R. The Aerosol Deposition Method: A Modified Aerosol Generation Unit to Improve Coating Quality. Materials 2018, 11, 1572. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.D.; Kub, F.J.; Eddy, C.R., Jr. ZnS/Diamond Composite Coatings for Infrared Transmission Applications Formed by the Aerosol Deposition Method. Proc. SPIE 2013, 8708, 87080T. [Google Scholar]
- Akedo, J. Aerosol Deposition of Ceramic Thick Films at Room Temperature: Densification Mechanism of Ceramic Layers. J. Am. Ceram. Soc. 2006, 89, 1834–1839. [Google Scholar] [CrossRef]
- Schubert, M.; Hahn, M.; Exner, J.; Kita, J.; Moos, R. Effect of substrate hardness and surface roughness on the film formation of aerosol-deposited ceramic films. Funct. Mater. Lett. 2017, 10, 1750045. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Ryu, J.; Choi, J.-J.; Hahn, B.-D.; Park, D.-S.; Yoon, W.-H.; Kim, K.-H. Fabrication and ferroelectric properties of highly dense lead-free piezoelectric (K0.5Na0.5)NbO3 thick films by aerosol deposition. Appl. Phys. Lett. 2007, 90, 152901. [Google Scholar] [CrossRef]
- Hoshina, T.; Furuta, T.; Kigoshi, Y.; Hatta, S.; Horiuchi, N.; Takeda, H.; Tsurumi, T. Size Effect of Nanograined BaTiO3 Ceramics Fabricated by Aerosol Deposition Method. Jpn. J. Appl. Phys. 2010, 49, 9. [Google Scholar] [CrossRef]
- Hoshina, T.; Furuta, T.; Yamazaki, T.; Takeda, H.; Tsurumi, T. Grain Size Effect on Dielectric Properties of Ba0.92Ca0.08TiO3 Ceramics. Jpn. J. Appl. Phys. 2013, 52, 9. [Google Scholar] [CrossRef]
- Akedo, J.; Lebedev, M. Microstructure and Electrical Properties of Lead Zirconate Titanate (Pb(Zr52/Ti48)O3) Thick Films Deposited by Aerosol Deposition Method. Jpn. J. Appl. Phys. 1999, 38, 5397–5401. [Google Scholar] [CrossRef]
- Akedo, J.; Lebedev, M. Powder Preparation in Aerosol Deposition Method for Lead Zirconate Titanate Thick Films. Jpn. J. Appl. Phys. 2002, 41, 6980–6984. [Google Scholar] [CrossRef]
- Ryu, J.; Priya, S.; Park, C.-S.; Kim, K.-Y.; Choi, J.-J.; Hahn, B.-D.; Yoon, W.H.; Lee, B.-K.; Park, D.-S.; Park, C. Enhanced domain contribution to ferroelectric properties in freestanding thick films. J. Appl. Phys. 2009, 106, 24108. [Google Scholar] [CrossRef] [Green Version]
- Wickham, D.G. Solid-phase equilibria in the system NiO-Mn2O3-O2. J. Inorg. Nucl. Chem. 1964, 26, 1369–1377. [Google Scholar] [CrossRef]
- Fang, D.-L.; Wang, Z.-B.; Yang, P.-H.; Liu, W.; Chen, C.-S.; Winnubst, A.J.A. Preparation of Ultra-Fine Nickel Manganite Powders and Ceramics by a Solid-State Coordination Reaction. J. Am. Ceram. Soc. 2006, 89, 230–235. [Google Scholar] [CrossRef]
- Feltz, A.; Töpfer, J.; Schirrmeister, F. Conductivity data and preparation routes for NiMn2O4 thermistor ceramics. J. Eur. Ceram. Soc. 1992, 9, 187–191. [Google Scholar] [CrossRef]
- Savic, S.M.; Nikolic, M.V.; Aleksic, O.S.; Slankamenac, M.; Nikolic, M.Z.P.M. Intrinsic resistivity of sintered Nickel Manganite vs. powder activation time and density. Sci. Sinter. 2008, 40, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Rousset, A.; Legros, R.; Lagrange, A. Recent progress in the fabrication of ceramic negative temperature coefficient thermistors. J. Eur. Ceram. Soc. 1994, 13, 185–195. [Google Scholar] [CrossRef]
- He, L.; Ling, Z.Y. Electrical conduction of intrinsic grain and grain boundary in Mn-Co-Ni-O thin film thermistors: Grain size influence. J. Appl. Phys. 2011, 110, 93708. [Google Scholar] [CrossRef]
- Brabers, V.A.M.; Terhell, J.C.J.M. Electrical Conductivity and Cation Valencies in Nickel Manganite. Phys. Status. Solidi A 1982, 1, 325–332. [Google Scholar] [CrossRef]
- Tang, X.-X.; Manthiram, A.; Goodenough, J.B. NiMn2O4 revisited. J. Less Common Met. 1989, 156, 357–368. [Google Scholar]
- Elbadraoui, E.; Baudour, J.L.; Leroux, C.; Fritsch, S.; Bouree, F.; Gillot, B.; Rousset, A. Cation Distribution, Short-Range Order and Small Polaron Hopping Conduction in Nickel Manganites, from a Neutron Diffraction Study. Phys. Status Solidi B 1999, 212, 129–139. [Google Scholar] [CrossRef]
- Feltz, A.; Töpfer, J. Redoxreaktionen in kondensierten Oxidsystemen. X Bildung von Defektspinellen und Phasenbeziehungen im System NixMn3−xO4. Z. Anorg. Allg. Chem. 1989, 576, 71–80. [Google Scholar] [CrossRef]
- Rao, G.V.S.; Wanklyn, B.M.; Rao, C.N.R. Electrical transport in rare earth ortho-chromites, -manganites and -ferrites. J. Phys. Chem. Solids 1971, 32, 345–358. [Google Scholar]
- Feltz, A.; Töpfer, J. Investigations on electronically conducting oxide systems XXVI. Preparation and properties of Ni6MnO8 and NiMnO3−δ (δ ≈ 0.02). J. Alloys Compd. 1993, 196, 75–79. [Google Scholar] [CrossRef]
- Gillot, B.; Baudour, J.L.; Bouree, F.; Metz, R.; Legros, R.; Rousset, A. Ionic configuration and cation distribution in cubic nickel manganite spinels NixMn3−xO4 (0.57 < x < 1) in relation with thermal histories. Solid State Ionics 1992, 58, 155–161. [Google Scholar]
- Cole, S.S., Jr. Oxidation and Reduction of Palladium in the Presence of Silver. J. Am. Ceram. Soc. 1985, 68, C-106–C-107. [Google Scholar] [CrossRef]
- Wang, S.F.; Dougherty, J.P.; Huebner, W.; Pepin, J.G. Silver-palladium thick-film conductors. J. Am. Ceram. Soc. 1994, 77, 3051–3072. [Google Scholar] [CrossRef]
- Wang, S.F.; Huebner, W.; Huang, C. Correlation of subsolidus phase relations in the Ag-Pd-O system to oxidation reduction kinetics and dilametric behavior. J. Am. Ceram. Soc. 1992, 75, 2232–2239. [Google Scholar] [CrossRef]
- Ortolino, D.; Kita, J.; Beart, K.; Wurm, R.; Kleinewig, S.; Pletsch, A.; Moos, R. Failure of electrical vias manufactured in thick-film technology when loaded with short high current pulses. Microelectron. Reliab. 2016, 56, 121–128. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schubert, M.; Münch, C.; Schuurman, S.; Poulain, V.; Kita, J.; Moos, R. Thermal Treatment of Aerosol Deposited NiMn2O4 NTC Thermistors for Improved Aging Stability. Sensors 2018, 18, 3982. https://doi.org/10.3390/s18113982
Schubert M, Münch C, Schuurman S, Poulain V, Kita J, Moos R. Thermal Treatment of Aerosol Deposited NiMn2O4 NTC Thermistors for Improved Aging Stability. Sensors. 2018; 18(11):3982. https://doi.org/10.3390/s18113982
Chicago/Turabian StyleSchubert, Michaela, Christian Münch, Sophie Schuurman, Véronique Poulain, Jaroslaw Kita, and Ralf Moos. 2018. "Thermal Treatment of Aerosol Deposited NiMn2O4 NTC Thermistors for Improved Aging Stability" Sensors 18, no. 11: 3982. https://doi.org/10.3390/s18113982




