Amperometric Detection of Conformational Change of Proteins Using Immobilized-Liposome Sensor System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Electrolyte-Encapsulated Liposomes
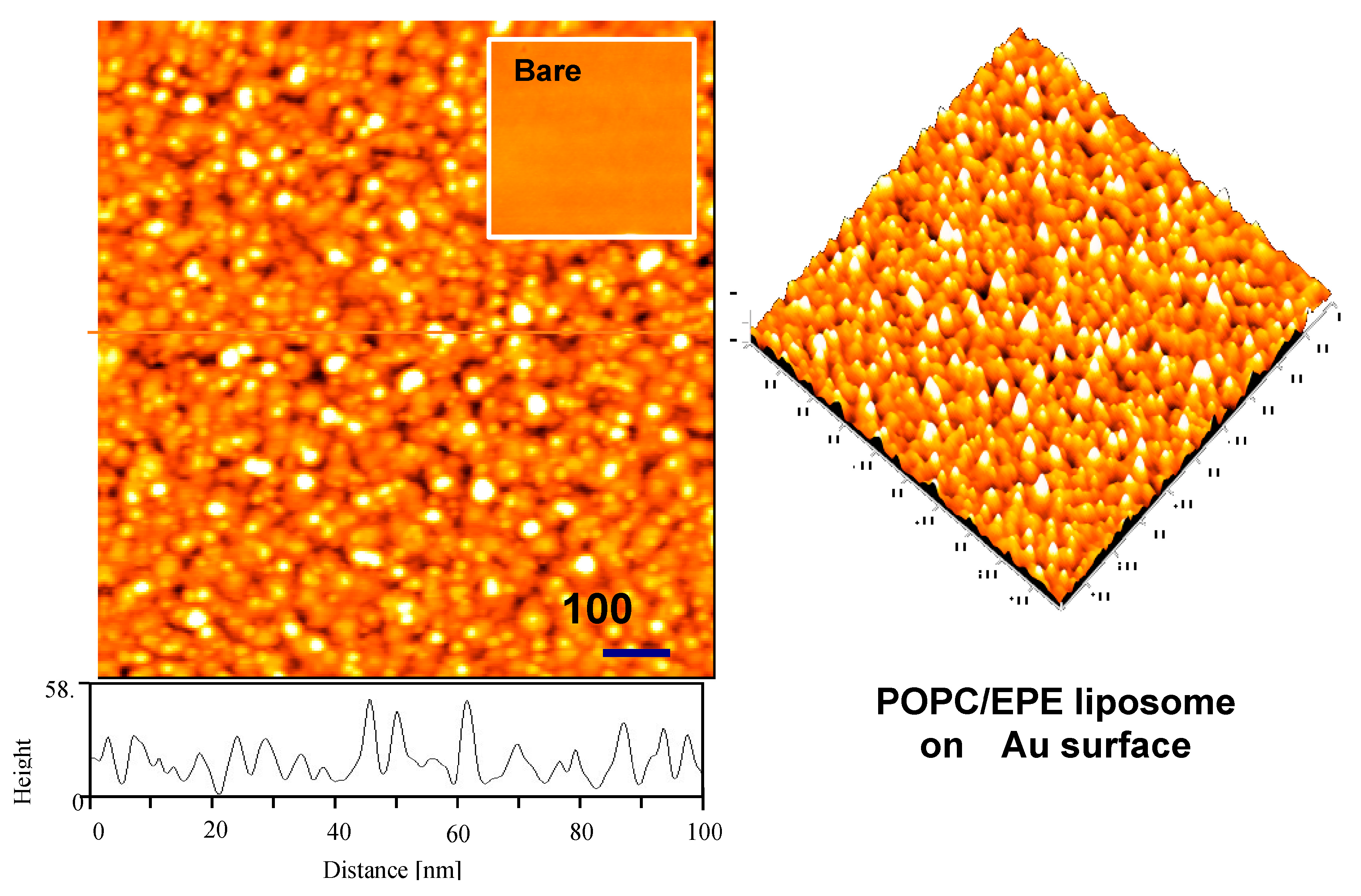
2.3. Tapping-Mode Atomic Force Microscopy (TM-AFM)
2.4. Preparation of Immobilized Liposome Electrode (ILE)
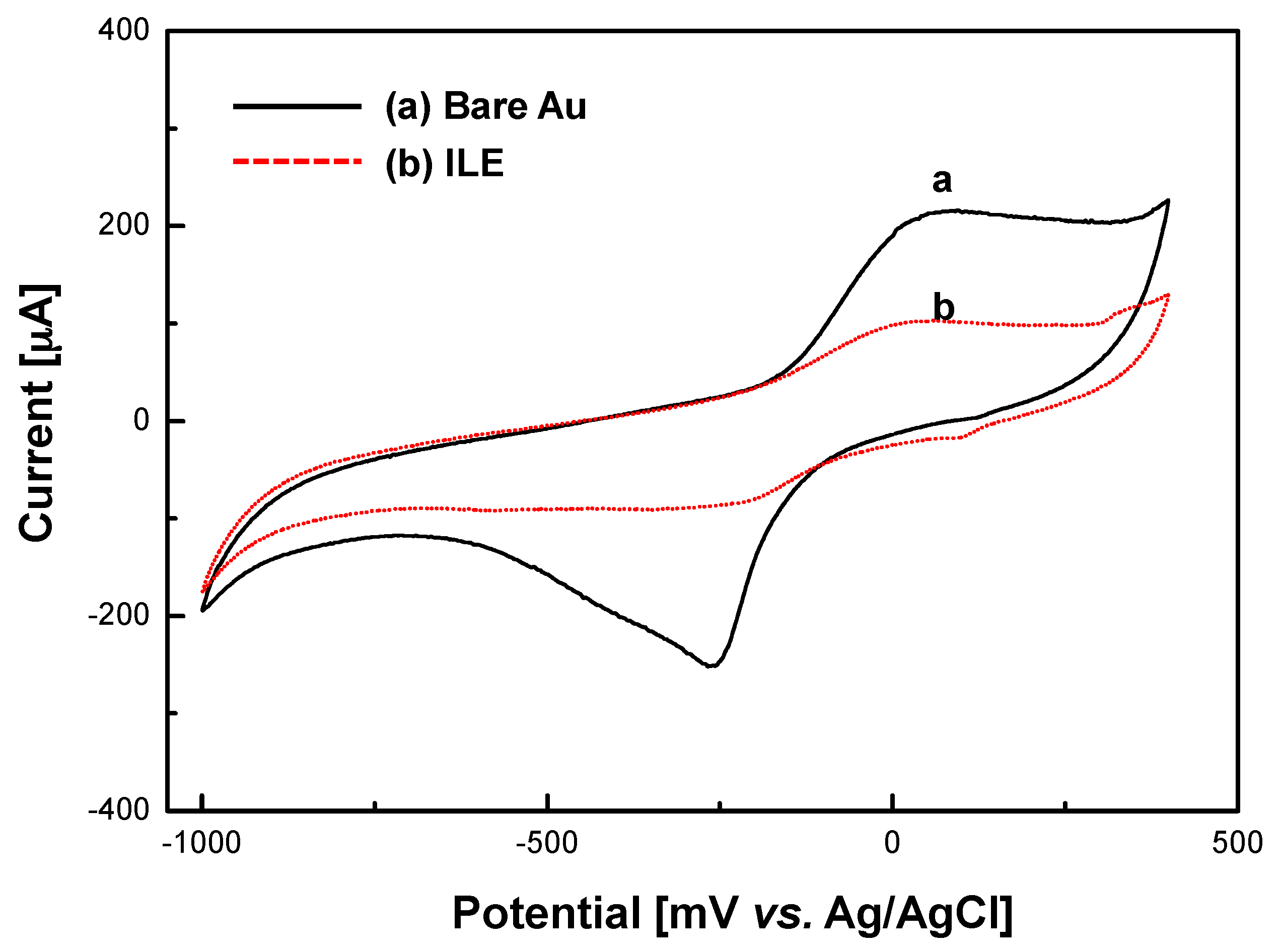
2.5. Electrochemical Measurement
2.6. Membrane Permeability
3. Results and Discussion
3.1. Characterization of ILE
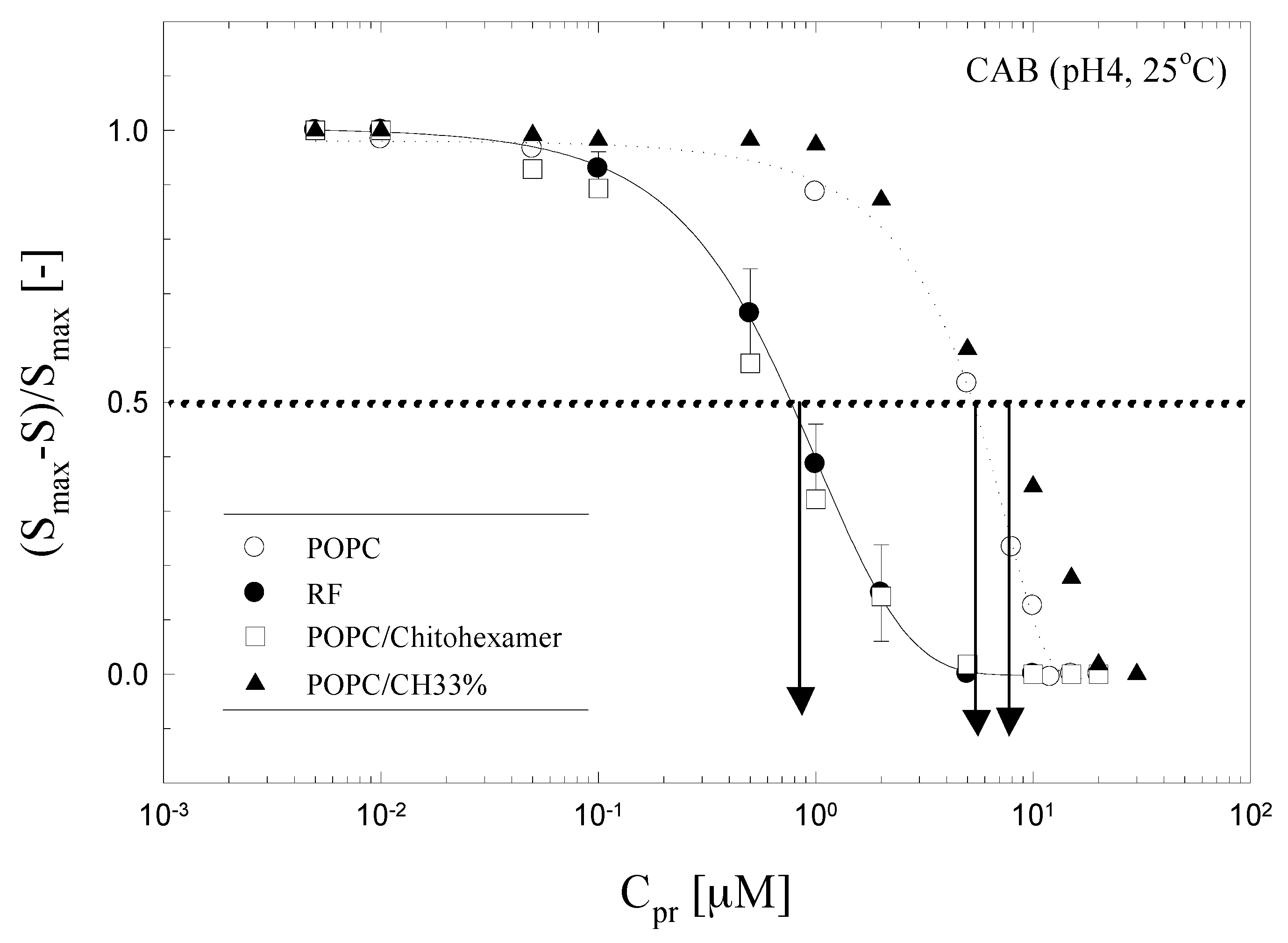
3.2. Response Behavior of Various Composition of ILE
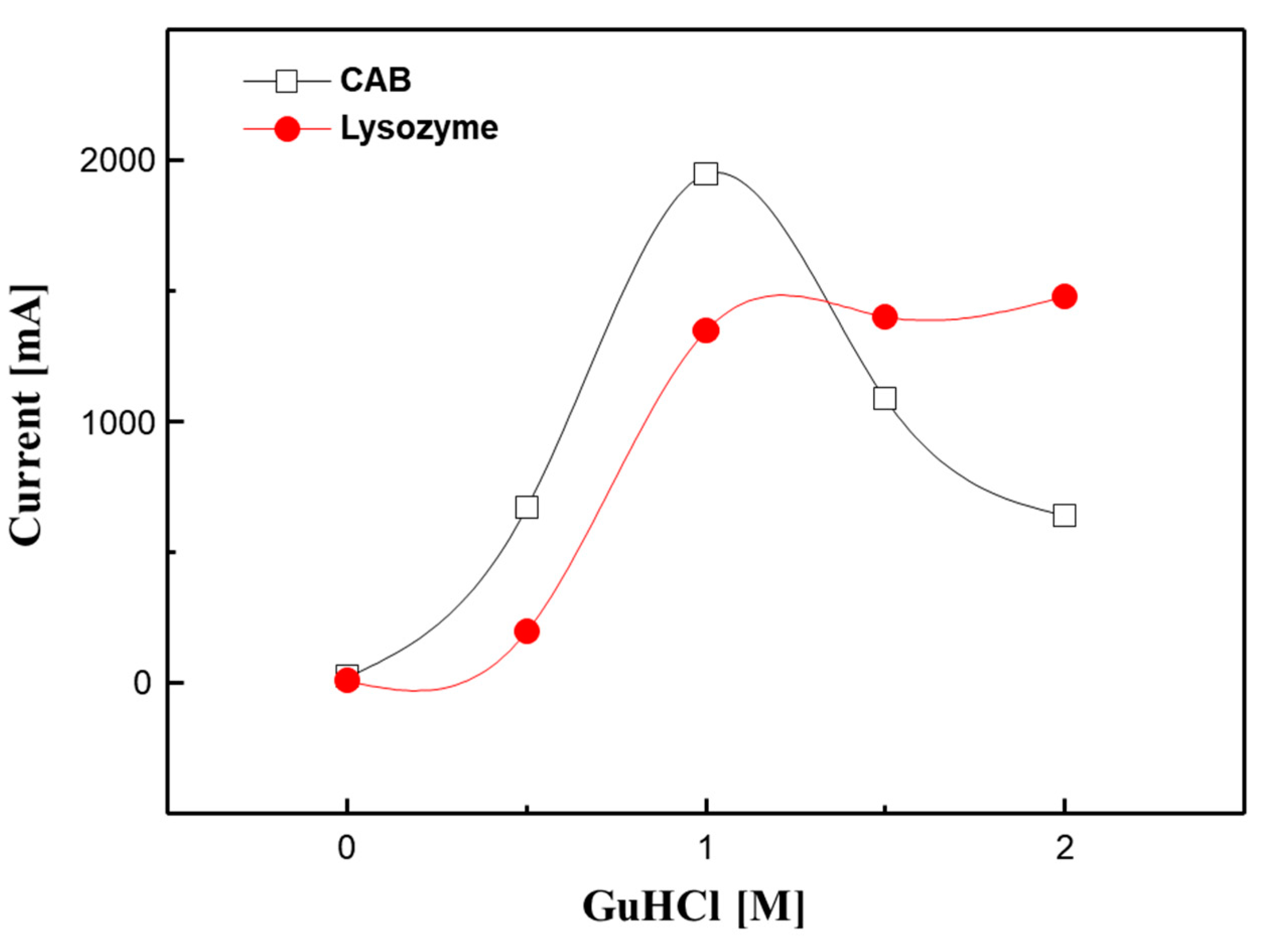
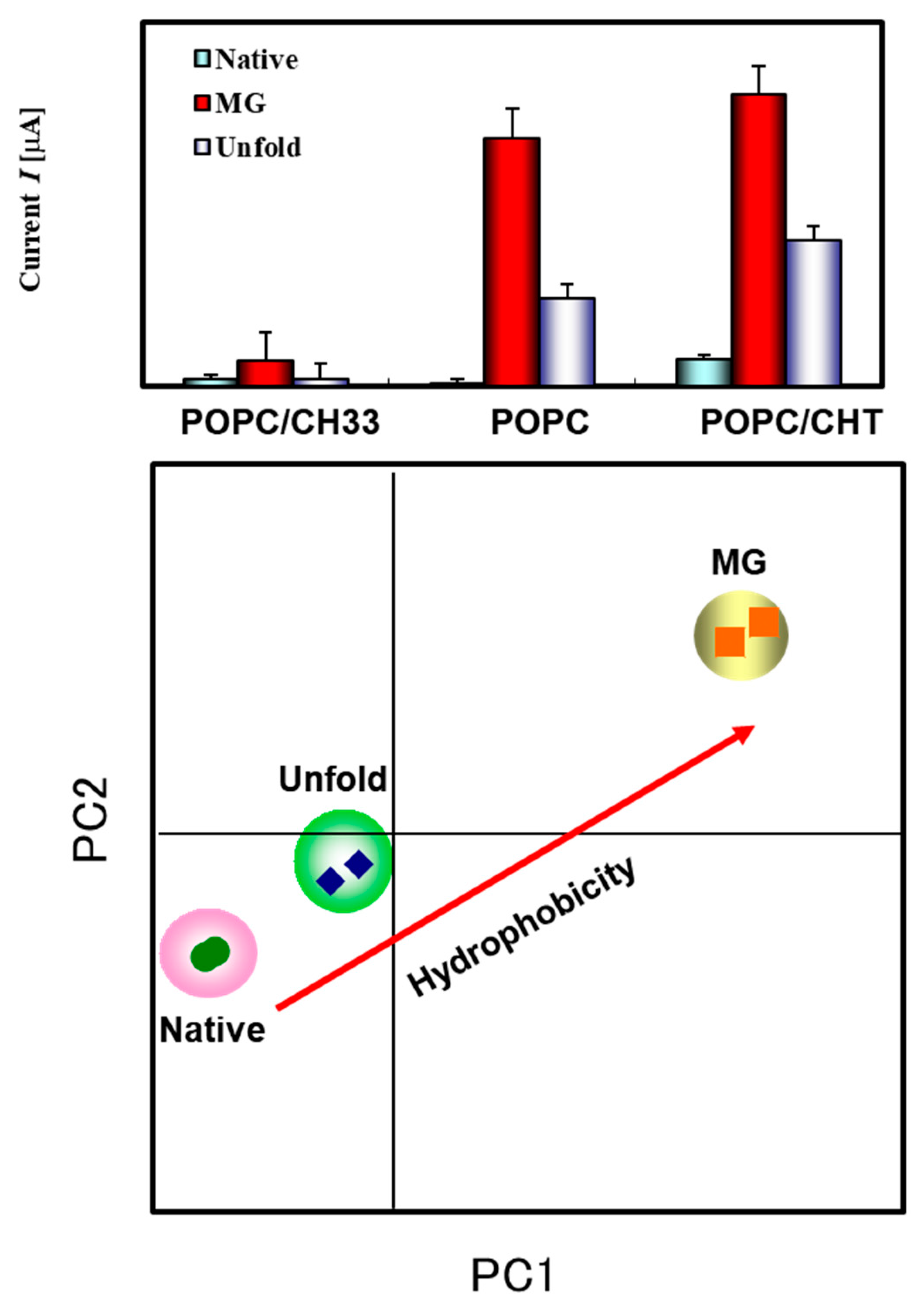
3.3. Response Behavior of the ILE against Proteins Structural Conditions
3.4. Principle Component Analysis for Discrimination of Proteins and Its Conditions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Memoli, A.; Annesini, M.C.; Mascini, M.; Papale, S.; Petralito, S. A comparison between different immobilised glucoseoxidase-based electrodes. J. Pharm. Biomed. Anal. 2002, 29, 1045–1052. [Google Scholar] [CrossRef]
- Bäumner, A.J.; Schmid, R.D. Development of a new immunosensor for pesticide detection: A disposable system with liposome-enhancement and amperometric detection. Biosens. Bioelectron. 1998, 13, 519–529. [Google Scholar] [CrossRef]
- Alkasrawi, M.; Nandakumar, R.; Margesin, R.; Schinner, F.; Mattiasson, B. A microbial biosensor based on yarrowia lipolytica for the off-line determination of middle-chain alkanes. Biosens. Bioelectron. 1999, 14, 723–727. [Google Scholar] [CrossRef]
- Bayram, E.; Akyilmaz, E. Development of a new microbial biosensor based on conductive polymer/multiwalled carbon nanotube and its application to paracetamol determination. Sens. Actuators B: Chem. 2016, 233, 409–418. [Google Scholar] [CrossRef]
- Ji, J.-H.; Shin, K.-S.; Kang, S.; Lee, S.H.; Kang, J.Y.; Kim, S.; Jun, S.C. Fundamental monomeric biomaterial diagnostics by radio frequency signal analysis. Biosens. Bioelectron. 2016, 82, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Sun, R.; Zhang, X.; Feng, X.; Jiang, L. Oxygen-rich enzyme biosensor based on superhydrophobic electrode. Adv. Mater. 2016, 28, 1477–1481. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, L.; Zhang, R.; Chen, Z.; Zhu, L. Rgd peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer. J. Control. Release 2014, 196, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Pan, H.; Li, P.; Wang, H.; Wang, X.; Pan, W.; Yuan, Y. The potential use of novel chitosan-coated deformable liposomes in an ocular drug delivery system. Colloids Surf. B: Biointerfaces 2016, 143, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi-Badiki, T.; Alipour, E.; Hamishehkar, H.; Golabi, S.M. Dopamine-loaded liposome and its application in electrochemical DNA biosensor. J. Biomater. Appl. 2016, 31, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Soh, J.H.; Lin, Y.; Rana, S.; Ying, J.Y.; Stevens, M.M. Colorimetric detection of small molecules in complex matrixes via target-mediated growth of aptamer-functionalized gold nanoparticles. Anal. Chem. 2015, 87, 7644–7652. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Mo, R.; Bellotti, A.; Zhou, J.; Gu, Z. Gel–liposome-mediated co-delivery of anticancer membrane-associated proteins and small-molecule drugs for enhanced therapeutic efficacy. Adv. Funct. Mater. 2014, 24, 2295–2304. [Google Scholar] [CrossRef]
- Jung, H.-S.; Park, K.-M.; Kang, D.H.; Kwak, M.K.; Lim, S.; Chang, P.-S.; Kim, K. Gas-sensing array application for on-line monitoring in a heat-responsive bioprocess of streptomyces griseus hut 6037. Food Sci. Biotechnol. 2015, 24, 875–881. [Google Scholar] [CrossRef]
- Bañuelos, S.; Muga, A. Interaction of native and partially folded conformations of α-lactalbumin with lipid bilayers: Characterization of two membrane-bound states. FEBS Lett. 1996, 386, 21–25. [Google Scholar] [CrossRef]
- Török, Z.; Horváth, I.; Goloubinoff, P.; Kovács, E.; Glatz, A.; Balogh, G.; Vígh, L. Evidence for a lipochaperonin: Association of active proteinfolding groesl oligomers with lipids can stabilize membranes under heat shock conditions. Proc. Natl. Acad. Sci. USA 1997, 94, 2192–2197. [Google Scholar] [CrossRef] [PubMed]
- Lala, A.K.; Kaul, P.; Ratnam, P.B. Membrane-protein interaction and the molten globule state: Interaction of α-lactalbumin with membranes. J. Protein Chem. 1995, 14, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Ptitsyn, O.B.; Pain, R.H.; Semisotnov, G.V.; Zerovnik, E.; Razgulyaev, O.I. Evidence for a molten globule state as a general intermediate in protein folding. FEBS Lett. 1990, 262, 20–24. [Google Scholar] [CrossRef]
- Uversky, V.N.; Ptitsyn, O.B. Further evidence on the equilibrium “pre-molten globule state”: Four-state guanidinium chloride-induced unfolding of carbonic anhydrase b at low temperature. J. Mol. Biol. 1996, 255, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Jagannadham, M.V.; Balasubramanian, D. The molten globular intermediate form in the folding pathway of human carbonic anhydrase b. FEBS Lett. 1985, 188, 326–330. [Google Scholar] [CrossRef]
- Tscheliessnig, A.L.; Konrath, J.; Bates, R.; Jungbauer, A. Host cell protein analysis in therapeutic protein bioprocessing—Methods and applications. Biotechnol. J. 2013, 8, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Lu, Y.; Chang, N.; Yang, J.; Zhang, S.; Liu, Y. Multidimensional colorimetric sensor array for discrimination of proteins. Biosens. Bioelectron. 2016, 86, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Truong, K.; Ikura, M. The use of fret imaging microscopy to detect protein–protein interactions and protein conformational changes in vivo. Curr. Opin. Struct. Biol. 2001, 11, 573–578. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2007, 1, 2876–2890. [Google Scholar] [CrossRef] [PubMed]
- Haga, M.; Sugawara, S.; Itagaki, H. Drug sensor: Liposome immunosensor for theophylline. Anal. Biochem. 1981, 118, 286–293. [Google Scholar] [CrossRef]
- Yoshimoto, M.; Kuboi, R.; Yang, Q.; Miyake, J. Immobilized liposome chromatography for studies of protein–membrane interactions and refolding of denatured bovine carbonic anhydrase. J. Chromatogr. B: Biomed. Sci. Appl. 1998, 712, 59–71. [Google Scholar] [CrossRef]
- FilipoviĆ-GrČIĆ, J.; ŠKalko-Basnet, N.; JalŠIenjak, I. Mucoadhesive chitosan-coated liposomes: Characteristics and stability. J. Microencapsul. 2001, 18, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Thanou, M.; Verhoef, J.C.; Junginger, H.E. Chitosan and its derivatives as intestinal absorption enhancers. Adv. Drug Deliv. Rev. 2001, 50, S91–S101. [Google Scholar] [CrossRef]
- Sato, T.; Ishii, T.; Okahata, Y. In vitro gene delivery mediated by chitosan. Effect of ph, serum and molecular mass of chitosan on the transfection efficiency. Biomaterials 2001, 22, 2075–2080. [Google Scholar] [CrossRef]
- Henriksen, I.; Våagen, S.R.; Sande, S.A.; Smistad, G.; Karlsen, J. Interactions between liposomes and chitosan ii: Effect of selected parameters on aggregation and leakage. Int. J. Pharm. 1997, 146, 193–203. [Google Scholar] [CrossRef]
- Yoshimoto, N.; Yoshimoto, M.; Yasuhara, K.; Shimanouchi, T.; Umakoshi, H.; Kuboi, R. Evaluation of temperature and guanidine hydrochloride-induced protein–liposome interactions by using immobilized liposome chromatography. Biochem. Eng. J. 2006, 29, 174–181. [Google Scholar] [CrossRef]
- Kuboi, R.; Shimanouchi, T.; Yoshimoto, M.; Umakoshi, H. Detection of protein conformation under stress conditions using liposomes as sensor materials. Sens. Mater. 2004, 16, 241–254. [Google Scholar]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.; Son, Y.H.; Kim, H.-J.; Kim, K.; Chang, P.-S.; Jung, H.-S. Amperometric Detection of Conformational Change of Proteins Using Immobilized-Liposome Sensor System. Sensors 2018, 18, 136. https://doi.org/10.3390/s18010136
Yu H, Son YH, Kim H-J, Kim K, Chang P-S, Jung H-S. Amperometric Detection of Conformational Change of Proteins Using Immobilized-Liposome Sensor System. Sensors. 2018; 18(1):136. https://doi.org/10.3390/s18010136
Chicago/Turabian StyleYu, Hyunjong, Young Hwan Son, Hak-Jin Kim, Keesung Kim, Pahn-Shick Chang, and Ho-Sup Jung. 2018. "Amperometric Detection of Conformational Change of Proteins Using Immobilized-Liposome Sensor System" Sensors 18, no. 1: 136. https://doi.org/10.3390/s18010136
APA StyleYu, H., Son, Y. H., Kim, H.-J., Kim, K., Chang, P.-S., & Jung, H.-S. (2018). Amperometric Detection of Conformational Change of Proteins Using Immobilized-Liposome Sensor System. Sensors, 18(1), 136. https://doi.org/10.3390/s18010136





