Interaction between Diethyldithiocarbamate and Cu(II) on Gold in Non-Cyanide Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Preparation of AuNPs and DDTC-Metal Complex
2.2. Instrumentations and DFT Calculations
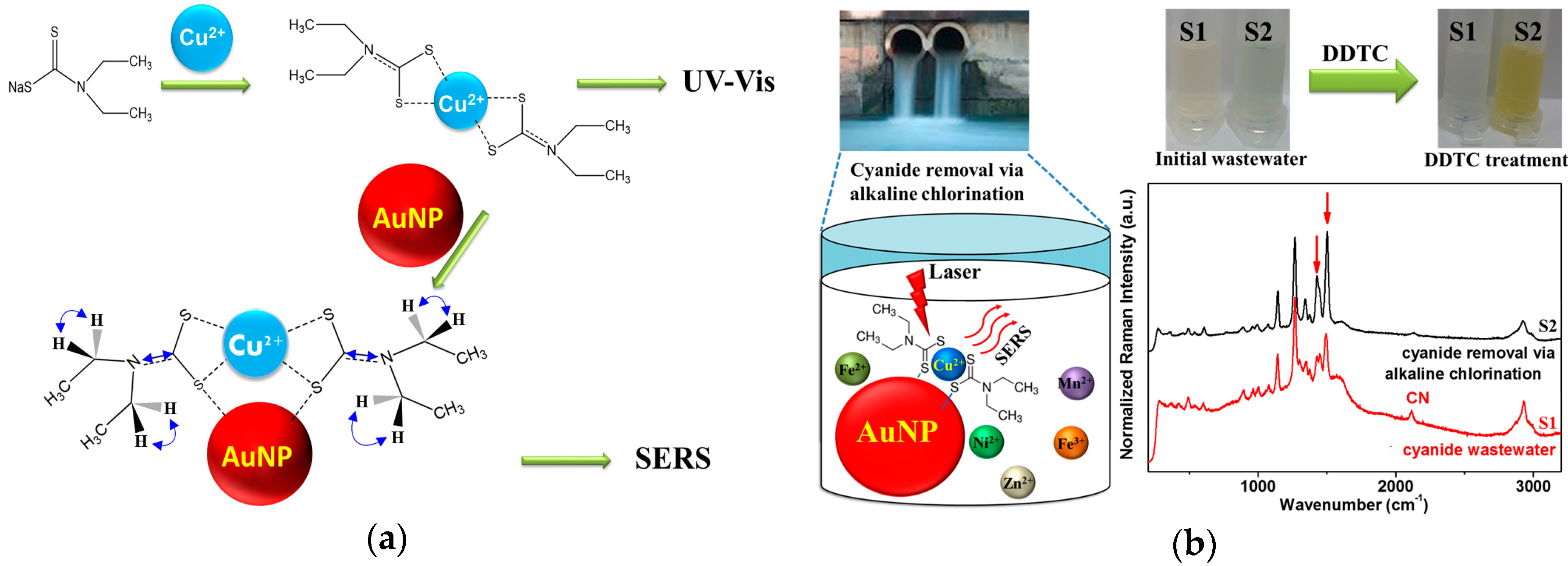
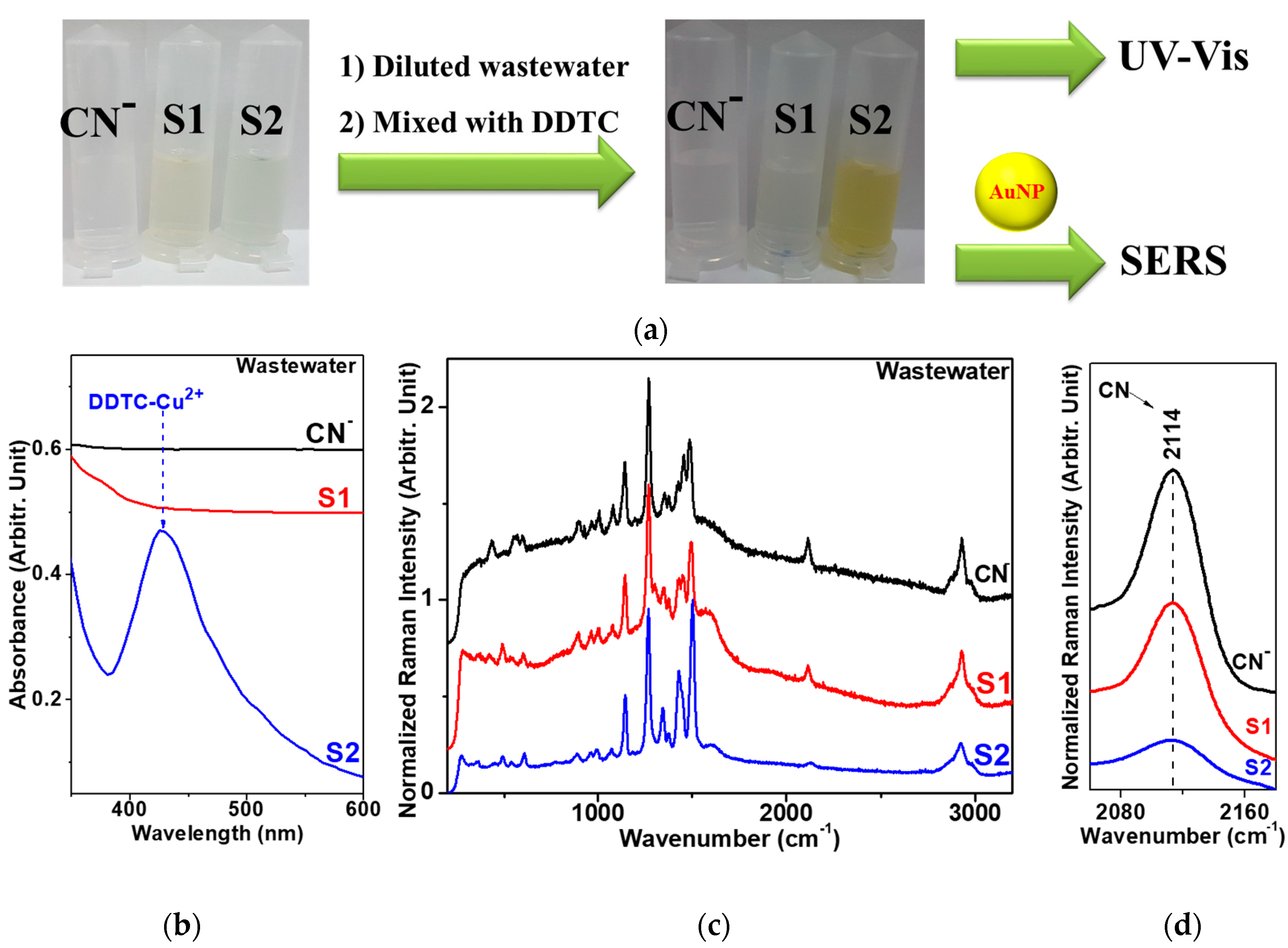
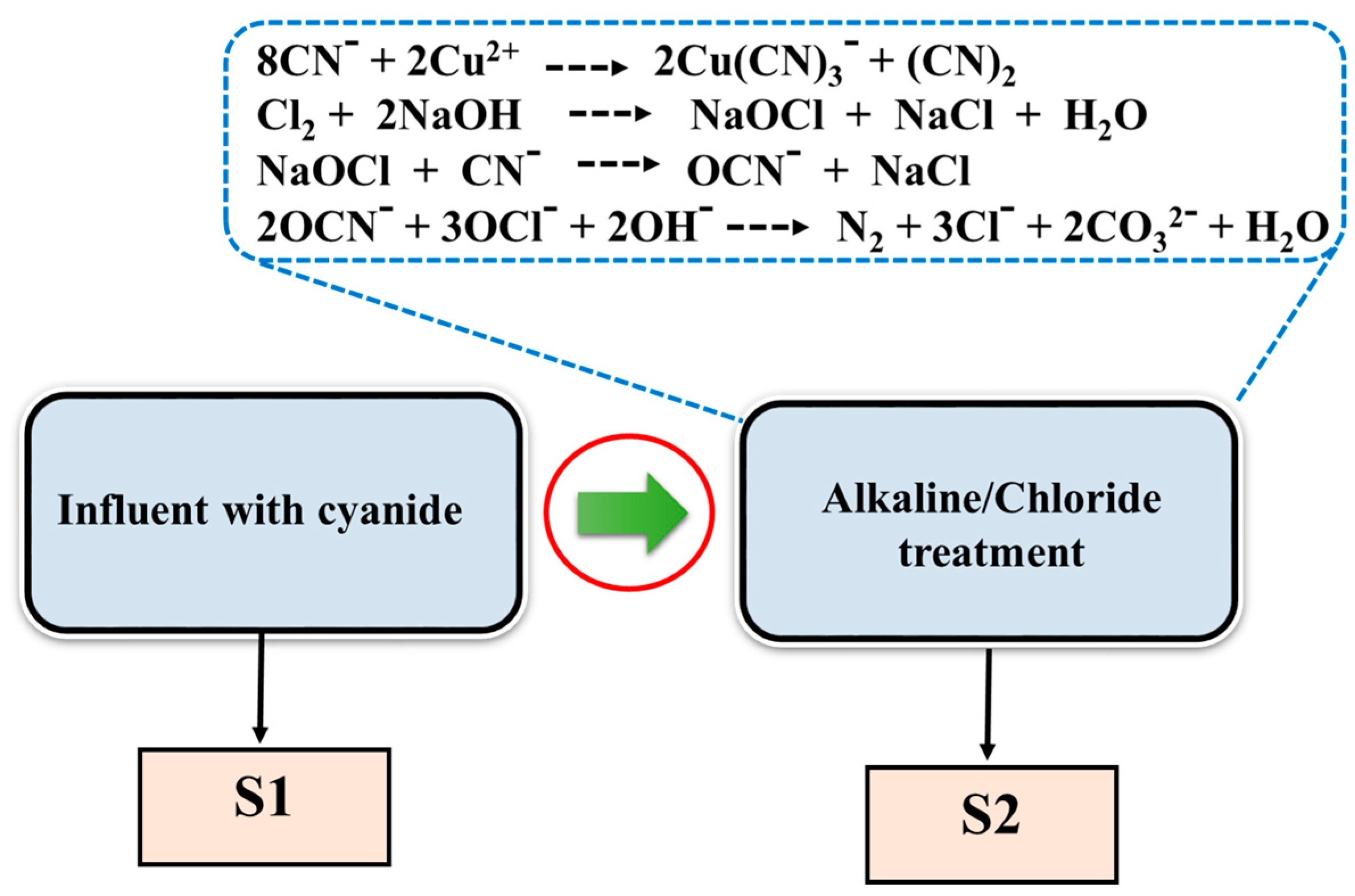
2.3. Preparation of Wastewater Samples and Removal of the Cyanide Species Using Alkaline Chlorination
3. Results and Discussion
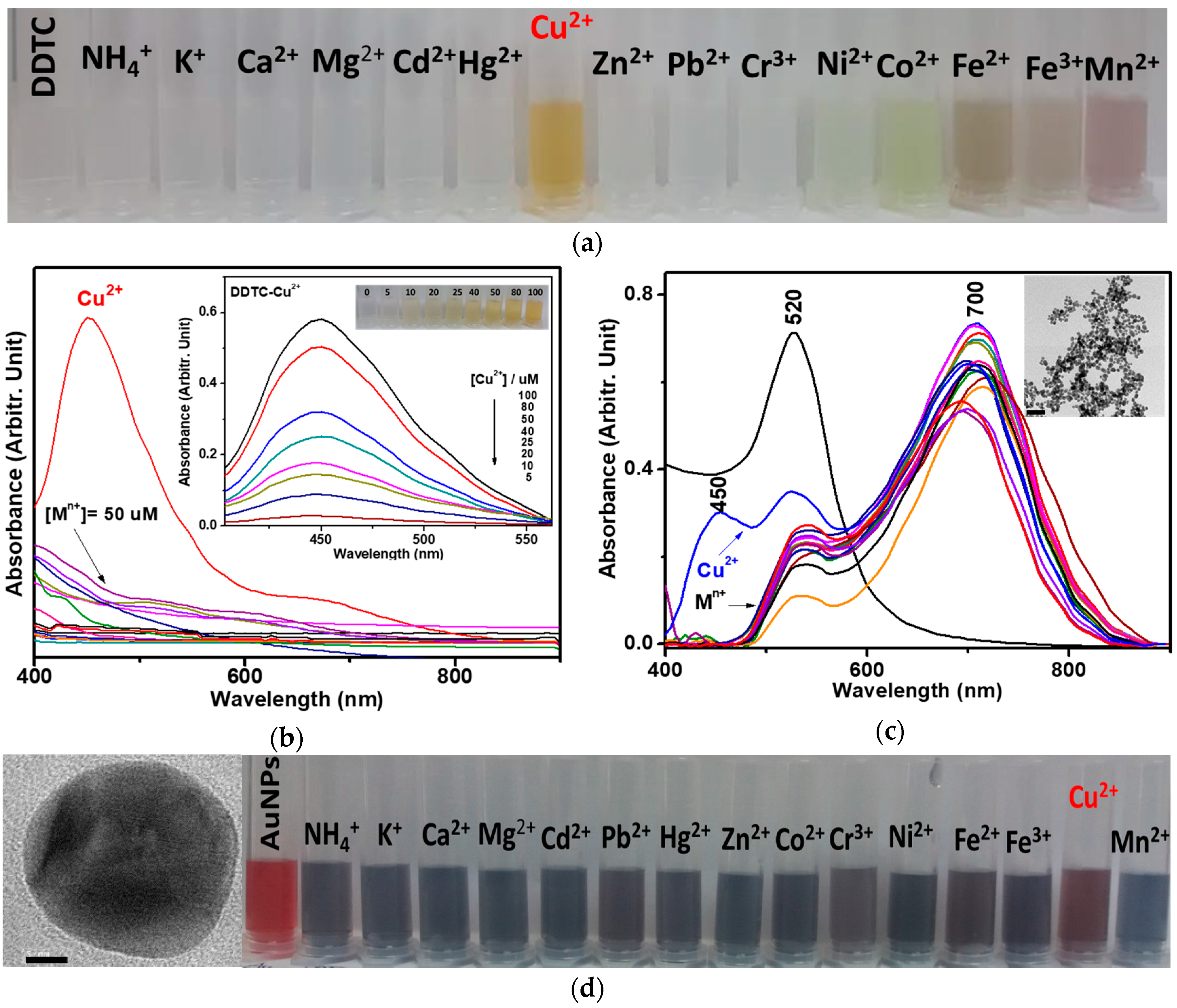
3.1. Adsorption of DDTC-Cu2+ on AuNPs
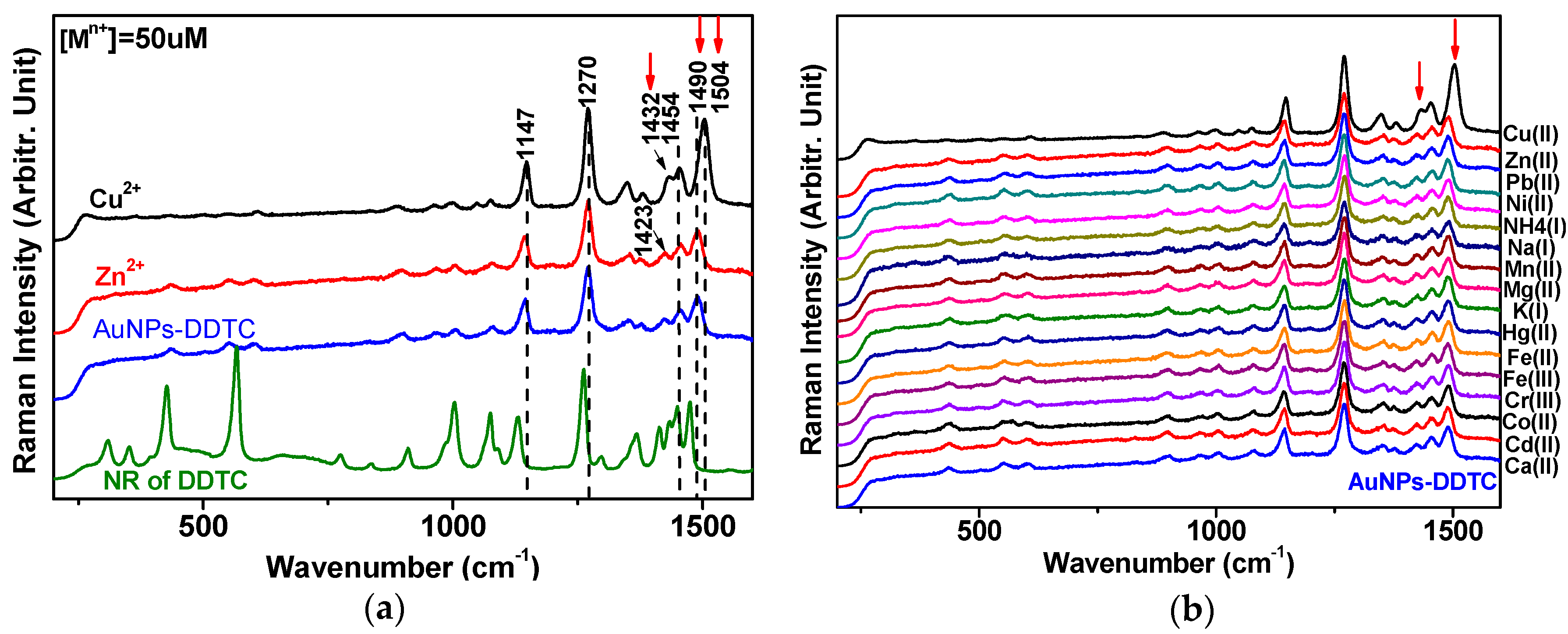
3.2. Raman Spectra of DDTC-Cu2+ on AuNPs
3.3. DFT Calculations of DDTC-Cu2+ on AuNPs
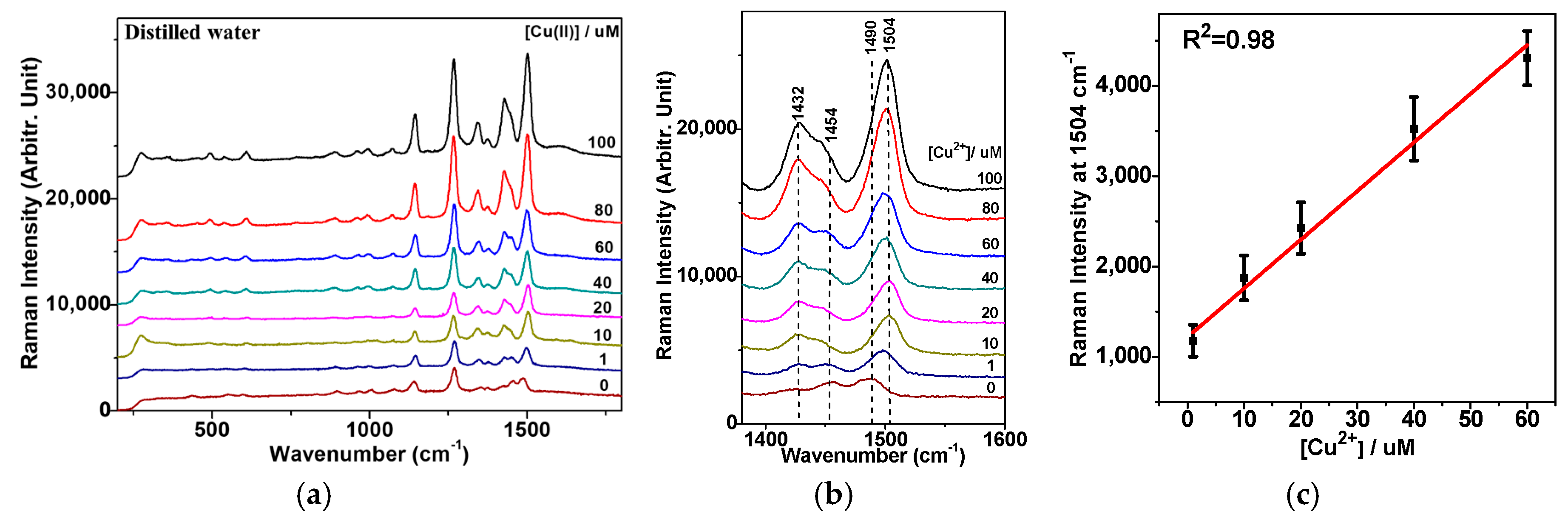
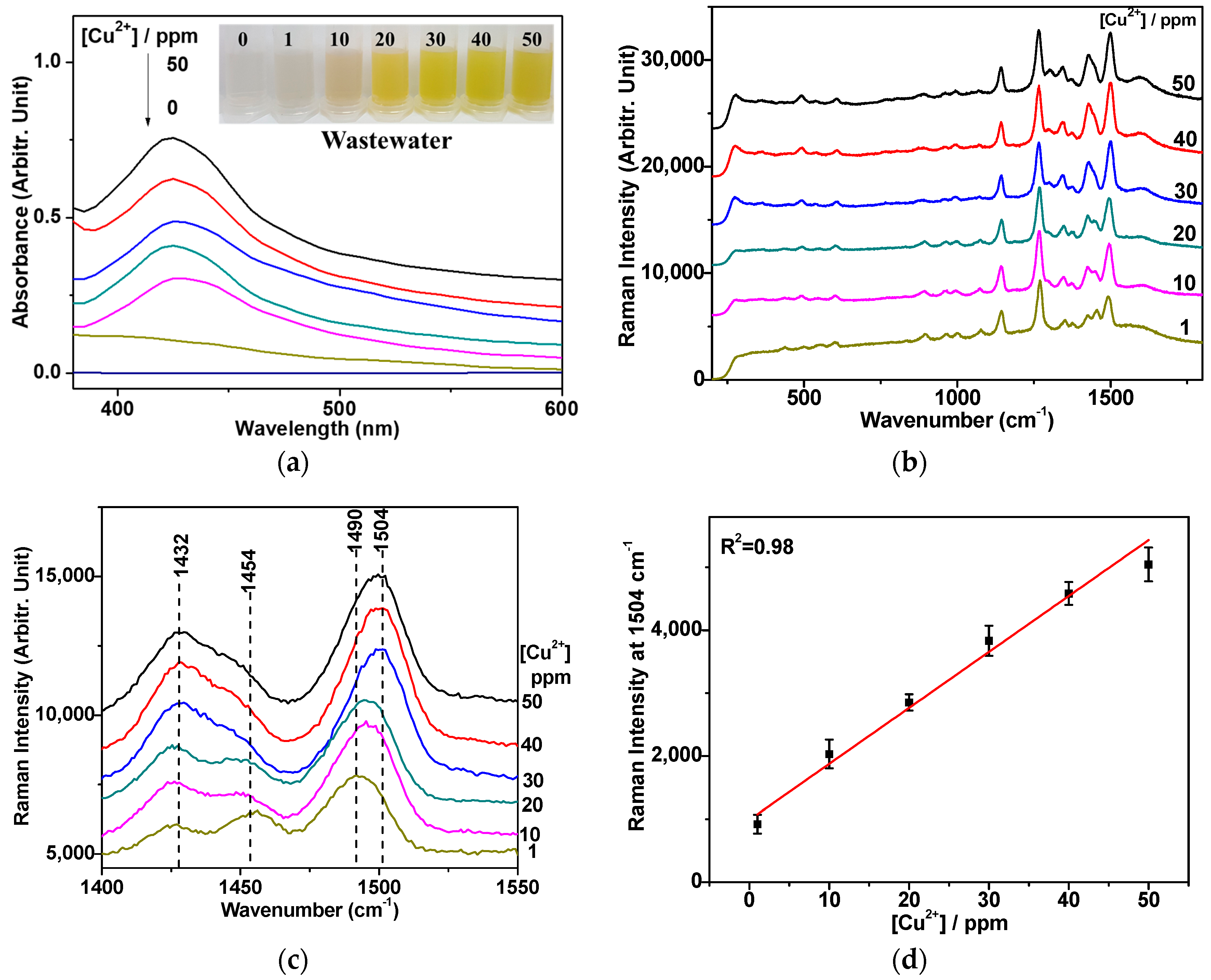
3.4. Quantification of the Cu2+ Ion on the Basis of Raman Spectra
3.5. Raman Spectroscopic Quantification of Cu2+ Ions in Electroplating Wastewater Samples
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chang, T.W.; Wang, X.; Mahigir, A.; Veronis, G.; Liu, G.L.; Gartia, M.R. Marangoni convection assisted single molecule detection with nanojet surface enhanced Raman spectroscopy. ACS Sens. 2017, 25, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Jiang, Z.; Wang, N.; Zhu, L.; Tang, H. Rapid surface enhanced Raman scattering (SERS) detection of sibutramine hydrochloride in pharmaceutical capsules with a β-cyclodextrin-Ag/polyvivnyl alcohol hydrogel substrate. Sensors 2017, 17, 1601. [Google Scholar] [CrossRef] [PubMed]
- Kosman, J.; Jatschka, J.; Csaki, A.; Fritzsche, W.; Juskowiak, B.; Stranik, O. A New Strategy for silver deposition on Au nanoparticles with the use of peroxidase-mimicking DNAzyme monitored via a localized surface plasmon resonance technique. Sensors 2017, 17, 849. [Google Scholar] [CrossRef] [PubMed]
- Koudelkova, Z.; Syrovy, T.; Ambrozova, P.; Moravec, Z.; Kubac, L.; Hynek, D.; Richtera, L.; Adam, V. Determination of zinc, cadmium, lead, copper and silver using a carbon paste electrode and a screen printed electrode modified with chromium(III) oxide. Sensors 2017, 17, 1832. [Google Scholar] [CrossRef] [PubMed]
- Biyani, M.; Biyani, R.; Tsuchihashi, T.; Takamura, Y.; Ushijima, H.; Tamiya, E.; Biyani, M. DEP-On-Go for simultaneous sensing of multiple heavy metals pollutants in environmental samples. Sensors 2017, 17, 849. [Google Scholar] [CrossRef] [PubMed]
- Futra, D.; Heng, L.Y.; Surif, S.; Ahmad, A.; Ling, T.L. Microencapsulated Aliivibrio fischeri in alginate microspheres for monitoring heavy metal toxicity in environmental waters. Sensors 2014, 14, 23248–23268. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C., Jr.; Ramos, J.M.; Tellez Soto, C.A.; Martin, A.A.; Raniero, L.; Ondar, G.F.; Versiane, O.; Moraes, L.S. Fourier Transform Infrared and Raman spectra, DFT: B3LYP/6–311G(d,p)calculations and structural properties of bis(diethyldithiocarbamate)copper(II). Spectrochim. Acta A 2013, 105, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Ricci, M.; Lofrumento, C.; Becucci, M.; Castellucci, E.M. The Raman and SERS spectra of indigo and indigo-Ag2 complex: DFT calculation and comparison with experiment. Spectrochim. Acta A 2017, 188, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Qian, S.; Wang, Y.; Lu, C.; Ji, X.; Lu, Z.; Lin, H. Single-indicator-based multidimensional sensing: Detection and identification of heavy metal ions and understanding the foundations from experiment to simulation. Sci. Rep. 2016, 6, 25354. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.K.; Zeng, X.; Li, J. Integrated bioleaching of copper metal from waste printed circuit board—A comprehensive review of approaches and challenges. Environ. Sci. Pollut. Res. Int. 2016, 23, 21141–21156. [Google Scholar] [CrossRef] [PubMed]
- Akcil, A.; Erust, C.; Gahan, C.S.; Ozgun, M.; Sahin, M.; Tuncuk, A. Precious metal recovery from waste printed circuit boards using cyanide and non-cyanide lixiviants—A review. Waste Manag. 2015, 45, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Lidia, S.; Francesco, Z.G.; Santosh, N.K.; Anna, M.P. Copper electrodeposition and oxidation of complex cyanide from wastewater in an electrochemical reactor with a Ti/Pt anode. Ind. Eng. Chem. Res. 2000, 39, 2132–2139. [Google Scholar]
- Dutra, A.J.; Rocha, G.P.; Pombo, F.R. Copper recovery and cyanide oxidation by electrowinning from a spent copper-cyanide electroplating electrolyte. J. Hazard. Mater. 2008, 152, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Lunn, G.; Sansone, E.B. Destruction of cyanogen bromide and inorganic cyanides. Anal. Biochem. 1985, 147, 245–250. [Google Scholar] [CrossRef]
- Wild, S.R.; Rudd, T.; Neller, A. Fate and effects of cyanide during wastewater treatment processes. Sci. Total Environ. 1994, 156, 93–107. [Google Scholar] [CrossRef]
- Mehta, V.N.; Anil Kumar, M.; Kailasa, S.K. Colorimetric detection of copper in water samples Using dopamine dithiocarbamate-functionalized Au nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 4414–4420. [Google Scholar] [CrossRef]
- Cvek, B.; Milacic, V.; Taraba, J.; Ping, D.Q. Ni(II), Cu(II), and Zn(II) diethyldithiocarbamate complexes show various activities against the proteasome in breast cancer cells. J. Med. Chem. 2008, 51, 6256–6258. [Google Scholar] [CrossRef] [PubMed]
- Sedlacek, J.; Martins, L.M.D.R.S.; Danek, P.; Pombeiro, A.J.L.; Cvek, B. Diethyldithiocarbamate complexes with metals used as food supplements show different effects in cancer cells. J. Appl. Biomed. 2014, 2, 301–308. [Google Scholar] [CrossRef]
- Han, J.; Liu, L.; Yue, X.; Chang, J.; Shi, W.; Hu, Y. A binuclear complex constituted by diethyldithiocarbamate and copper(I) functions as a proteasome activity inhibitor in pancreatic cancer cultures and xenografts. Toxicol. Appl. Pharmacol. 2013, 273, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Noll, C.A.; Betz, L.D. Determination of copper ion by modified sodium diethyldithiocarbamate procedure. Anal. Chem. 1952, 24, 1894–1895. [Google Scholar] [CrossRef]
- Uddin, M.N.; Salam, M.A.; Hossain, M.A. Spectrophotometric measurement of Cu(DDTC)2 for the simultaneous determination of zinc and copper. Chemosphere 2013, 90, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fu, Y.; Huang, T.; Liu, Y.; Wu, M.; Yuan, Y.; Li, S.; Li, C. Copper ion attenuated the antiproliferative activity of di-2-pyridylhydrazone dithiocarbamate derivative; However, there was a lack of correlation between ROS generation and antiproliferative activity. Molecules 2016, 21, 1088. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Abtahiabc, S.M.H.; Vikesland, P.J. Plasmonic colorimetric and SERS sensors for environmental analysis. Environ. Sci. Nano 2015, 2, 120–135. [Google Scholar] [CrossRef]
- Tellez, S.C.A.; Costa, A.C., Jr.; Ramos, J.M.; Vieira, L.S.; Rost, N.C.V.; Versiane, O.; Rangel, J.L.; Mondragon, M.A.; Raniero, L.; Martin, A.A. Surface enhanced Raman scattering, electronic spectrum, natural bond orbital, and mulliken charge distribution in the normal modes of diethyldithiocarbamate copper(II) complex, [Cu(DDTC)2]. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 116, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Anumol, T.; Daniels, K.D.; Wu, S.; Ziska, A.D.; Snyder, S.A. Predicting trace organic compound attenuation by ozone oxidation: Development of indicator and surrogate models. Water Res. 2017, 119, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Yao, B.; Lian, L.; Lu, X.; Snyder, S.A.; Li, R.; Song, W. Development of fluorescence surrogates to predict the photochemical transformation of pharmaceuticals in wastewater effluents. Environ. Sci. Technol. 2017, 51, 2738–2747. [Google Scholar] [CrossRef] [PubMed]
- Aulsebrook, M.L.; Biswas, S.; Leaver, F.M.; Grace, M.R.; Graham, B.; Barrios, A.M.; Tuck, K.L. A luminogenic lanthanide-based probe for the highly selective detection of nanomolar sulfide levels in aqueous samples. Chem. Commun. 2017, 53, 4911–4914. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys. Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- Ly, N.H.; Seo, C.; Joo, S.W. Detection of copper(II) ions using glycine on hydrazine-adsorbed gold nanoparticles via Raman spectroscopy. Sensors 2016, 16, 1785. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.B.; Nguyen, T.D.; Kim, S.; Joo, S.W. Vibrational fingerprints of N6-methyladenine and N6,N6-dimethyladenine in Raman spectra. Vib. Spectrosc. 2017, 90, 7–13. [Google Scholar] [CrossRef]
- Jamroz, M.H. Vibrational energy distribution analysis (VEDA): Scopes and limitations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 114, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Duke, F.R.; Courtney, W.G. Complexes in oxidation–reduction reactions. The copper(II)–cyanide reaction. J. Phys. Chem. 1952, 56, 19–21. [Google Scholar] [CrossRef]
- López-Garriga, J.J.; Babcock, G.T.; Harrison, J.F. Factors influencing the C=N stretching frequency in neutral and protonated Schiffs bases. J. Am. Chem. Soc. 1986, 108, 7241–7251. [Google Scholar] [CrossRef]
- Sánchez-Cortés, S.; Domingo, C.; García-Ramos, J.V.; Aznárez, J.A. Surface-enhanced vibrational study (SEIR and SERS) of dithiocarbamate pesticides on gold films. Langmuir 2001, 17, 1157–1162. [Google Scholar] [CrossRef]
| NR of DDTC | a DFT DDTC-Au6 | SERS on Au | a DFT Cu(DDTC)2-Au6 | SERS on Au | b Assignments Based on PED Calculations |
|---|---|---|---|---|---|
| --- | --- | --- | 268 | 268 | β(C–N–C) + β(N–C–S) |
| 350 | 329 | --- | 345 | 367 | ν(Cu–S) + β(S–C–S) |
| 426 | 415 | 435 | 407 | 434 | β(C–C–N) + ν(S–C) + γ(N–S–S–C) |
| 567 | 523 | 552 | 523 | 547 | ν(S–C) + γ(N–S–S–C) + β(C–N–C) |
| 775 | 756 | --- | 756 | --- | ν(N=C)(CH2) |
| 835 | 849 | --- | 841 | --- | δ(H–C–C–N) |
| 910 | 935 | 902 | 942 | 885 | ν(C–C) |
| 1003 | 997 | 1005 | 997 | 998 | ν(N=C)(CH2) + ν(S–C) + ν(C–C) |
| 1074 | 1051 | 1080 | 1043 | 1075 | ν(N=C)(CH2) + ν(C–C) |
| 1131 | 1144 | 1144 | 1152 | 1147 | δ(H–C–C–N) |
| 1261 | 1292 | 1270 | 1276 | 1270 | ν(N=C)(CS2) + δ(H–C–N–C) + β(H–C–C) |
| 1367 | 1354 | 1350 | 1354 | 1366 | δ(H–C–N–C) + β(H–C–H)(CH2) |
| 1412 | 1416 | 1423 | 1447 | 1432 | ν(N=C)(CS2) + β(H–C–H)(CH2) |
| 1449 | 1462 | 1454 | 1470 | 1454 | β(H–C–H)(CH2) + β(H–C–H)(CH3) |
| 1474 | 1493 | 1490 | 1517 | 1504 | ν(N=C)(CS2) + β(H–C–H)(CH2) |
| Sample | Cr | Mn | Fe | Ni | Cu | Zn |
|---|---|---|---|---|---|---|
| “S1” (cyanide wastewater) | ND * | ND | 342.95 | 703.85 | 84.69 | 2447.67 |
| “S2” (after alkaline chlorination) | ND | 2.38 | 468.28 | 667.66 | 77.06 | 2175.26 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ly, N.H.; Nguyen, T.D.; Zoh, K.-D.; Joo, S.-W. Interaction between Diethyldithiocarbamate and Cu(II) on Gold in Non-Cyanide Wastewater. Sensors 2017, 17, 2628. https://doi.org/10.3390/s17112628
Ly NH, Nguyen TD, Zoh K-D, Joo S-W. Interaction between Diethyldithiocarbamate and Cu(II) on Gold in Non-Cyanide Wastewater. Sensors. 2017; 17(11):2628. https://doi.org/10.3390/s17112628
Chicago/Turabian StyleLy, Nguyễn Hoàng, Thanh Danh Nguyen, Kyung-Duk Zoh, and Sang-Woo Joo. 2017. "Interaction between Diethyldithiocarbamate and Cu(II) on Gold in Non-Cyanide Wastewater" Sensors 17, no. 11: 2628. https://doi.org/10.3390/s17112628






