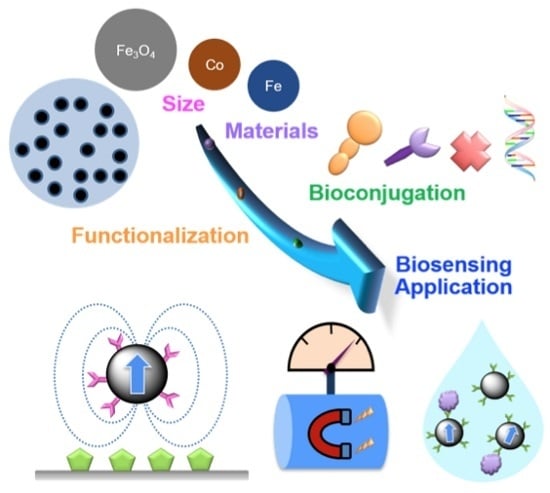
Biosensing Using Magnetic Particle Detection Techniques
Abstract
:1. Introduction
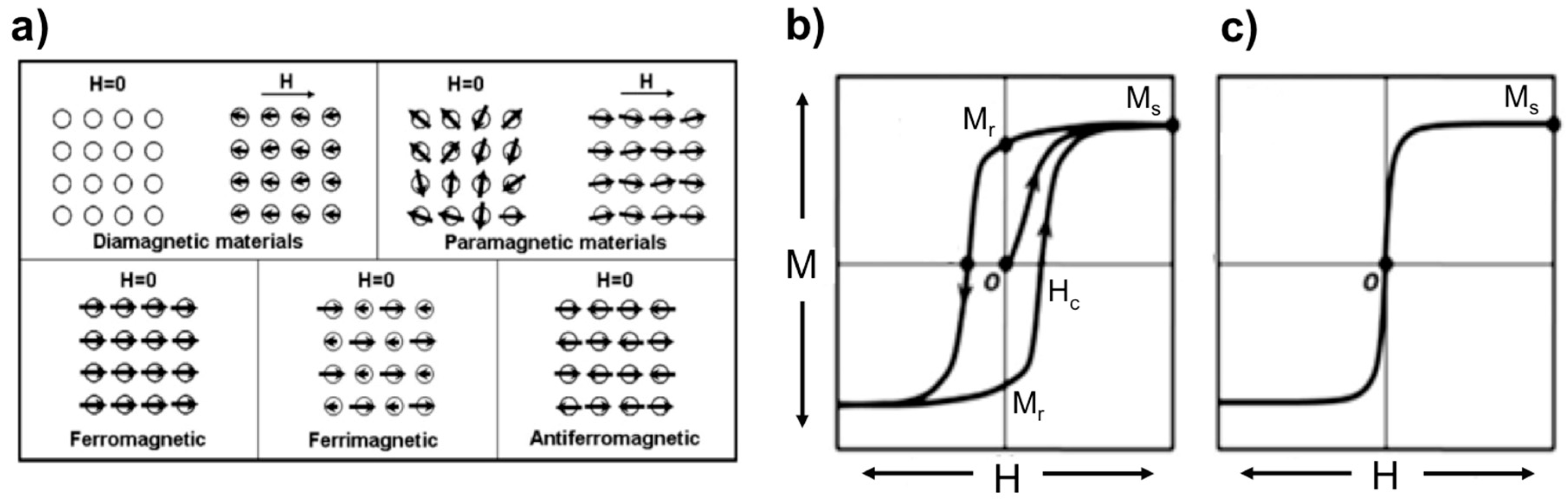
2. Basic Concepts in Magnetism and Use of Magnetic Particles in Biological Applications
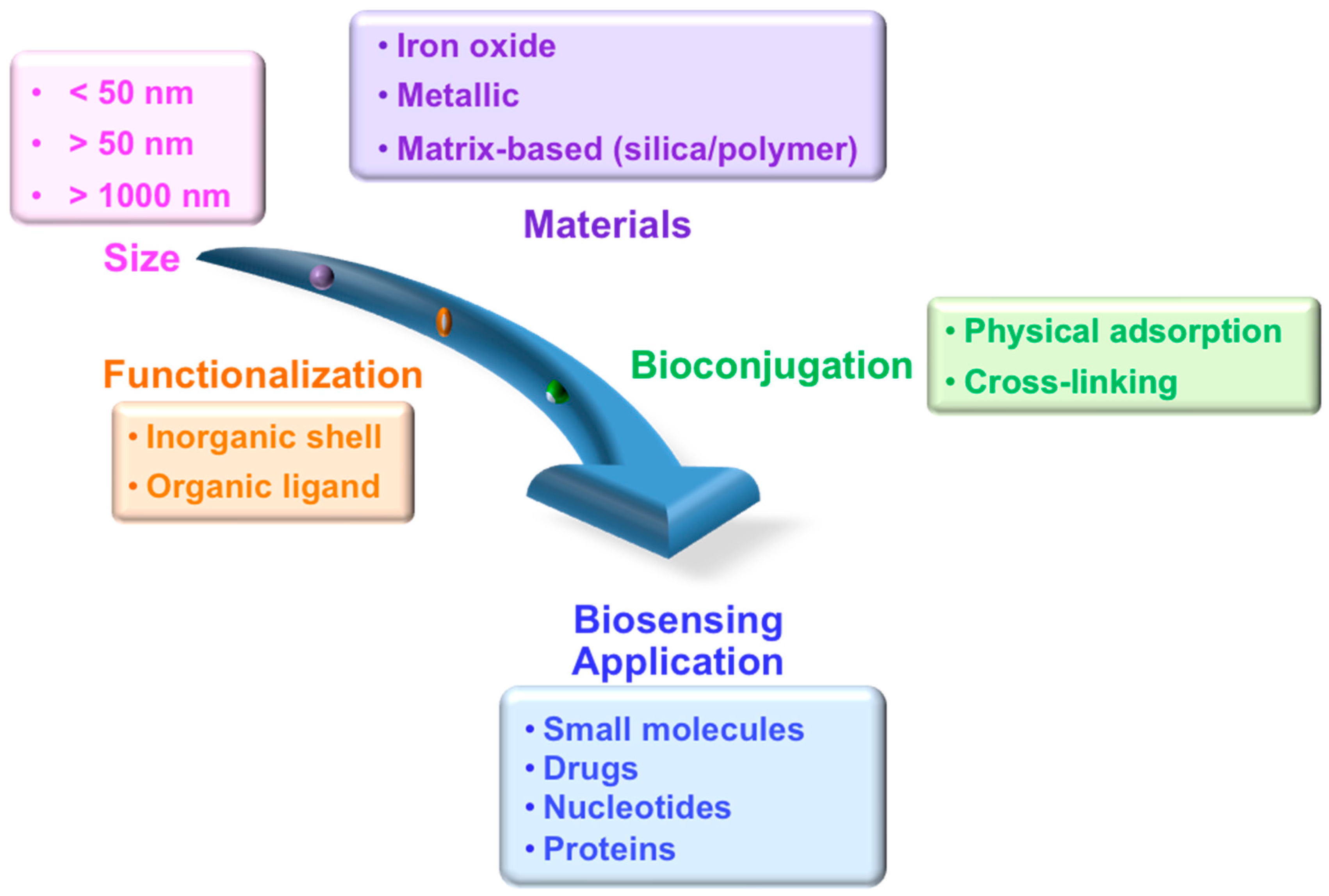
3. Synthesis, Functionalization, and Biological Conjugation of Magnetic Particles
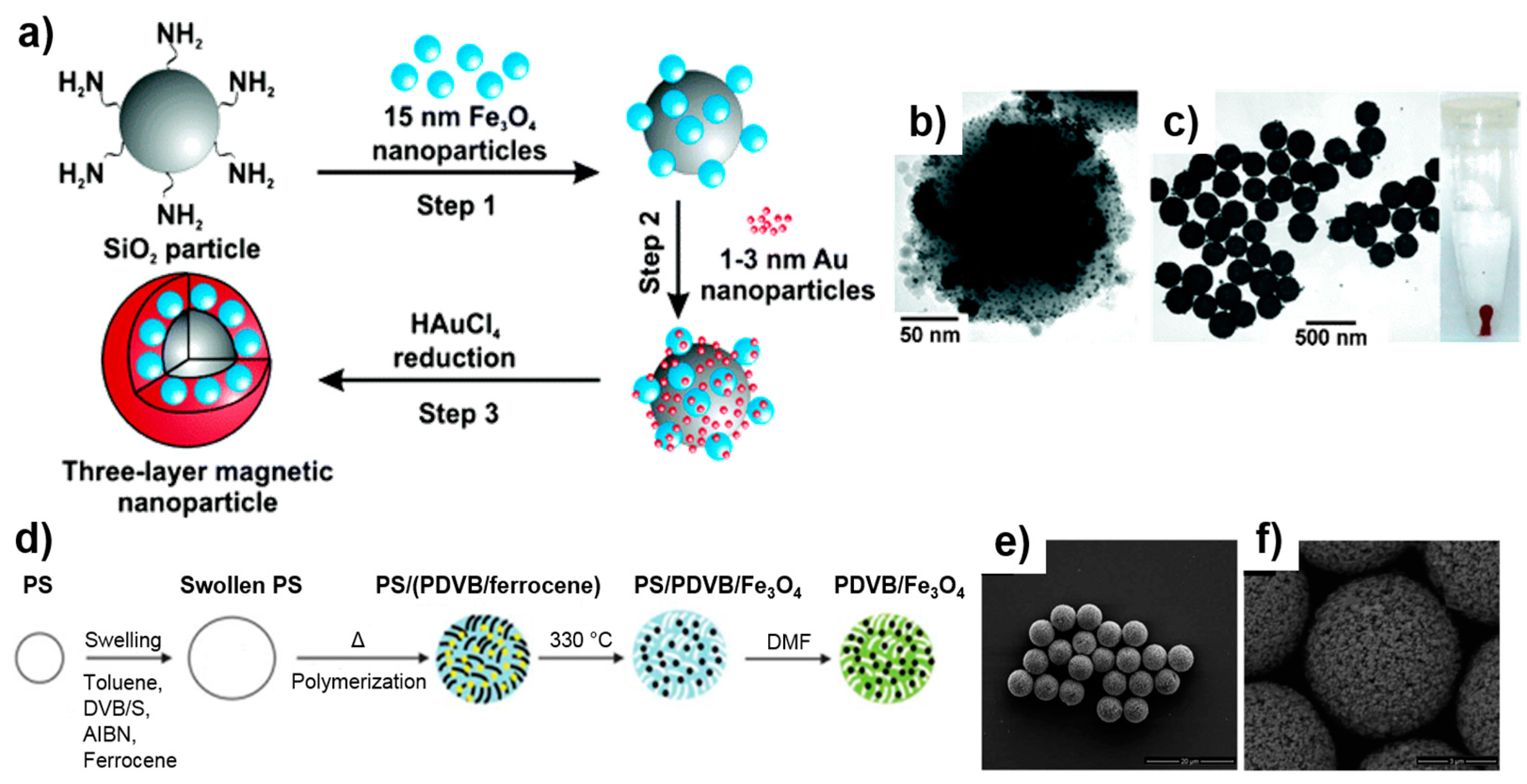
3.1. Synthesis of Magnetic Particles
3.1.1. Single-Core Magnetic Particles
3.1.2. Matrix-Dispersed Magnetic Particles
3.1.3. Toxicity
3.2. Functionalization of Magnetic Particles
3.2.1. Inorganic Shell Coatings: Silica and Gold
3.2.2. Functionalization with Organic Ligands
3.3. Biomolecular Conjugation
4. Detection Techniques and Their Applications
4.1. Spintronic Sensors: Giant Magnetoresistance (GMR), Tunneling Magnetoresistance (TMR), Planar Hall Effect (PHE)
4.2. Nuclear Magnetic Resonance (NMR)
4.3. Superconducting Quantum Interference Device (SQUID)
4.4. Atomic Magnetometer (AM)
4.5. Fluxgate Sensor
4.6. Frequency Mixing Magnetic Detection Techniques
4.7. Other Techniques
5. Summary and Perspectives
Acknowledgments
Conflicts of Interest
References
- Krishnan, K.M. Biomedical nanomagnetics: A spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans. Magn. 2010, 46, 2523–2558. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutierrez, L.; Morales, M.P.; Bohm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Shin, T.-H.; Cheon, J.; Weissleder, R. Recent developments in magnetic diagnostic systems. Chem. Rev. 2015, 115, 10690–10724. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.; Lee, J.-H.; Shin, T.-H.; Cheon, J. Theranostic magnetic nanoparticles. Acc. Chem. Res. 2011, 44, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhao, Y.; Liu, Y.; Chang, X.; Chen, C.; Zhao, Y. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small 2011, 7, 1322–1337. [Google Scholar] [CrossRef] [PubMed]
- Issadore, D.; Park, Y.I.; Shao, H.; Min, C.; Lee, K.; Liong, M.; Weissleder, R.; Lee, H. Magnetic sensing technology for molecular analyses. Lab Chip 2014, 14, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.A.; Peng, S.; Cheng, K.; Sun, S. Magnetic nanoparticles: Synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009, 38, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Gillis, P.; Koenig, S.H. Transverse relaxation of solvent protons induced by magnetized spheres: Application to ferritin, erythrocytes, and magnetite. Magn. Reson. Med. 1987, 5, 323–345. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.P.; Yan, Y.; Stacey, A.; Simpson, D.A.; Hall, L.T.; Maclaurin, D.; Prawer, S.; Mulvaney, P.; Wrachtrup, J.; Caruso, F.; et al. Quantum measurement and orientation tracking of fluorescent nanodiamonds inside living cells. Nat. Nanotechnol. 2011, 6, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Coey, J.M.D. Magnetism and Magnetic Materials; Cambridge University Press: Cambridge, UK, 2010; p. 614. [Google Scholar]
- Gaster, R.S.; Xu, L.; Han, S.-J.; Wilson, R.J.; Hall, D.A.; Osterfeld, S.J.; Yu, H.; Wang, S.X. Quantification of protein interactions and solution transport using high-density GMR sensor arrays. Nat. Nanotechnol. 2011, 6, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Castro, C.M.; Im, H.; Lee, H.; Weissleder, R. A magneto-DNA nanoparticle system for rapid detection and phenotyping of bacteria. Nat. Nanotechnol. 2013, 8, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yashchuk, V.V.; Donaldson, M.H.; Rochester, S.M.; Budker, D.; Pines, A. Magnetic resonance imaging with an optical atomic magnetometer. Proc. Natl. Acad. Sci. USA 2006, 103, 12668–12671. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Schrag, B.D.; Carter, M.J.; Xie, J.; Xu, C.; Sun, S.; Xiao, G. Detection of DNA labeled with magnetic nanoparticles using MgO-based magnetic tunnel junction sensors. J. Appl. Phys. 2008, 103, 07A306. [Google Scholar] [CrossRef]
- Ejsing, L.; Hansen, M.F.; Menon, A.K.; Ferreira, H.A.; Graham, D.L.; Freitas, P.P. Magnetic microbead detection using the planar Hall effect. J. Magn. Magn. Mater. 2005, 293, 677–684. [Google Scholar] [CrossRef]
- Ludwig, F.; Mäuselein, S.; Heim, E.; Schilling, M. Magnetorelaxometry of magnetic nanoparticles in magnetically unshielded environment utilizing a differential fluxgate arrangement. Rev. Sci. Instrum. 2005, 76, 106102. [Google Scholar] [CrossRef]
- Pellicer-Guridi, R.; Vogel, M.W.; Reutens, D.C.; Vegh, V. Towards ultimate low frequency air-core magnetometer sensitivity. Sci. Rep. 2017, 7, 2269. [Google Scholar] [CrossRef] [PubMed]
- Vavassori, P.; Metlushko, V.; Ilic, B.; Gobbi, M.; Donolato, M.; Cantoni, M.; Bertacco, R. Domain wall displacement in Py square ring for single nanometric magnetic bead detection. Appl. Phys. Lett. 2008, 93, 203502. [Google Scholar] [CrossRef]
- Liu, J.P. Nanoscale Magnetic Materials and Applications; Springer: New York, NY, USA, 2009; p. 719. [Google Scholar]
- Kenning, G.G.; Rodriguez, R.; Zotev, V.S.; Moslemi, A.; Wilson, S.; Hawel, L.; Byus, C.; Kovach, J.S. Detection of magnetically enhanced cancer tumors using SQUID magnetometry: A feasibility study. Rev. Sci. Instrum. 2005, 76, 014303. [Google Scholar] [CrossRef]
- Chemla, Y.R.; Grossman, H.L.; Poon, Y.; McDermott, R.; Stevens, R.; Alper, M.D.; Clarke, J. Ultrasensitive magnetic biosensor for homogeneous immunoassay. Proc. Natl. Acad. Sci. USA 2000, 97, 14268–14272. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Adolphi, N.L.; Butler, K.L.; Lovato, D.M.; Larson, R.; Schwindt, P.D.D.; Flynn, E.R. Magnetic relaxometry with an atomic magnetometer and SQUID sensors on targeted cancer cells. J. Magn. Magn. Mater. 2012, 324, 2613–2619. [Google Scholar] [CrossRef] [PubMed]
- Kotitz, R.; Matz, H.; Trahms, L.; Koch, H.; Weitschies, W.; Rheinlander, T.; Semmler, W.; Bunte, T. SQUID based remanence measurements for immunoassays. IEEE Trans. Appl. Supercond. 1997, 7, 3678–3681. [Google Scholar] [CrossRef]
- Yao, L.; Xu, S. Force-induced remnant magnetization spectroscopy for specific magnetic imaging of molecules. Angew. Chem. Int. Ed. 2011, 50, 4407–4409. [Google Scholar] [CrossRef] [PubMed]
- Krause, H.-J.; Wolters, N.; Zhang, Y.; Offenhäusser, A.; Miethe, P.; Meyer, M.H.F.; Hartmann, M.; Keusgen, M. Magnetic particle detection by frequency mixing for immunoassay applications. J. Magn. Magn. Mater. 2007, 311, 436–444. [Google Scholar] [CrossRef]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the magnetic properties of nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef] [PubMed]
- Jeong, U.; Teng, X.; Wang, Y.; Yang, H.; Xia, Y. Superparamagnetic colloids: Controlled synthesis and niche applications. Adv. Mater. 2007, 19, 33–60. [Google Scholar] [CrossRef]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; IEEE/Wiley: Hoboken, NJ, USA, 2009; p. 544. [Google Scholar]
- Neuman, K.C.; Nagy, A. Single-molecule force spectroscopy: Optical tweezers, magnetic tweezers and atomic force microscopy. Nat. Methods 2008, 5, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Lee, N.; Kim, H.; An, K.; Park, Y.I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S.G.; Na, H.B.; et al. Large-scale synthesis of uniform and extremely small-sized iron oxide nanoparticles for high-resolution T1 magnetic resonance imaging contrast agents. J. Am. Chem. Soc. 2011, 133, 12624–12631. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Insin, N.; Lee, J.; Han, H.-S.; Cordero, J.M.; Liu, W.; Bawendi, M.G. Compact zwitterion-coated iron oxide nanoparticles for biological applications. Nano Lett. 2012, 12, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bao, J.; Wang, L.; Zhang, F.; Li, Y. One-pot synthesis and bioapplication of amine-functionalized magnetite nanoparticles and hollow nanospheres. Chem. A Eur. J. 2006, 12, 6341–6347. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-C.; Chou, P.-H.; Chen, S.-H.; Liao, H.-K.; Wang, K.-Y.; Chen, Y.-J.; Lin, C.-C. Ethylene glycol-protected magnetic nanoparticles for a multiplexed immunoassay in human plasma. Small 2006, 2, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Leslie-Pelecky, D.L.; Rieke, R.D. Magnetic properties of nanostructured materials. Chem. Mater. 1996, 8, 1770–1783. [Google Scholar] [CrossRef]
- Fortin, J.-P.; Wilhelm, C.; Servais, J.; Ménager, C.; Bacri, J.-C.; Gazeau, F. Size-sorted anionic iron oxide nanomagnets as colloidal mediators for magnetic hyperthermia. J. Am. Chem. Soc. 2007, 129, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, M.; Hosseinkhani, H.; Hosseinkhani, M.; Boutry, S.; Simchi, A.; Journeay, W.S.; Subramani, K.; Laurent, S. Magnetic resonance imaging tracking of stem cells in vivo using iron oxide nanoparticles as a tool for the advancement of clinical regenerative medicine. Chem. Rev. 2011, 111, 253–280. [Google Scholar] [CrossRef] [PubMed]
- Jun, Y.-W.; Lee, J.-H.; Cheon, J. Chemical design of nanoparticle probes for high-performance magnetic resonance imaging. Angew. Chem. Int. Ed. 2008, 47, 5122–5135. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Perez, J.M.; Josephson, L.; O’Loughlin, T.; Hogemann, D.; Weissleder, R. Magnetic relaxation switches capable of sensing molecular interactions. Nat. Biotechnol. 2002, 20, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiao, S.Z.; Hu, Q.H.; Lu, G.Q. Magnetic nanocomposites with mesoporous structures: Synthesis and applications. Small 2011, 7, 425–443. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.L.C.; Pereira, G.A.; Voisin, P.; Kassem, J.; Bouchaud, V.; Etienne, L.; Peters, J.A.; Carlos, L.; Mornet, S.; Geraldes, C.F.G.C.; et al. Fine tuning of the relaxometry of γ-Fe2O3@SiO2 nanoparticles by tweaking the silica coating thickness. ACS Nano 2010, 4, 5339–5349. [Google Scholar] [CrossRef] [PubMed]
- Philipse, A.P.; van Bruggen, M.P.B.; Pathmamanoharan, C. Magnetic silica dispersions: Preparation and stability of surface-modified silica particles with a magnetic core. Langmuir 1994, 10, 92–99. [Google Scholar] [CrossRef]
- Yang, C.; Wang, G.; Lu, Z.; Sun, J.; Zhuang, J.; Yang, W. Effect of ultrasonic treatment on dispersibility of Fe3O4 nanoparticles and synthesis of multi-core Fe3O4/SiO2 core/shell nanoparticles. J. Mater. Chem. 2005, 15, 4252–4257. [Google Scholar] [CrossRef]
- Kouassi, G.K.; Irudayaraj, J. Magnetic and gold-coated magnetic nanoparticles as a DNA sensor. Anal. Chem. 2006, 78, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Caruntu, D.; Liu, H.; O’Connor, C.J. Water-dispersible iron oxide magnetic nanoparticles with versatile surface functionalities. Langmuir 2011, 27, 2271–2278. [Google Scholar] [CrossRef] [PubMed]
- Mazur, M.; Barras, A.; Kuncser, V.; Galatanu, A.; Zaitzev, V.; Turcheniuk, K.V.; Woisel, P.; Lyskawa, J.; Laure, W.; Siriwardena, A.; et al. Iron oxide magnetic nanoparticles with versatile surface functions based on dopamine anchors. Nanoscale 2013, 5, 2692–2702. [Google Scholar] [CrossRef] [PubMed]
- Kluchova, K.; Zboril, R.; Tucek, J.; Pecova, M.; Zajoncova, L.; Safarik, I.; Mashlan, M.; Markova, I.; Jancik, D.; Sebela, M.; et al. Superparamagnetic maghemite nanoparticles from solid-state synthesis—Their functionalization towards peroral MRI contrast agent and magnetic carrier for trypsin immobilization. Biomaterials 2009, 30, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Vreeland, E.C.; Watt, J.; Schober, G.B.; Hance, B.G.; Austin, M.J.; Price, A.D.; Fellows, B.D.; Monson, T.C.; Hudak, N.S.; Maldonado-Camargo, L.; et al. Enhanced nanoparticle size control by extending LaMer’s mechanism. Chem. Mater. 2015, 27, 6059–6066. [Google Scholar] [CrossRef]
- Rockenberger, J.; Scher, E.C.; Alivisatos, A.P. A new nonhydrolytic single-precursor approach to surfactant-capped nanocrystals of transition metal oxides. J. Am. Chem. Soc. 1999, 121, 11595–11596. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H.; Robinson, D.B.; Raoux, S.; Rice, P.M.; Wang, S.X.; Li, G. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2004, 126, 273–279. [Google Scholar] [CrossRef] [PubMed]
- De Palma, R.; Peeters, S.; Van Bael, M.J.; Van den Rul, H.; Bonroy, K.; Laureyn, W.; Mullens, J.; Borghs, G.; Maes, G. Silane ligand exchange to make hydrophobic superparamagnetic nanoparticles water-dispersible. Chem. Mater. 2007, 19, 1821–1831. [Google Scholar] [CrossRef]
- Silke, B.; Helmut, B.; Nina, M.; Angelika, G.; Eckhard, D.; Wilhelm, H.; Jens, B.; Svetlana, Z.; Natalie, P.; Josef, H.; et al. Surface engineering of Co and FeCo nanoparticles for biomedical application. J. Phys. Condens. Matter 2006, 18, S2543. [Google Scholar]
- Murray, C.B.; Sun, S.; Doyle, H.; Betley, T. Monodisperse 3d transition-metal (Co,Ni,Fe) nanoparticles and their assembly into nanoparticle superlattices. MRS Bull. 2001, 26, 985–991. [Google Scholar] [CrossRef]
- Huber, D.L. Synthesis, properties, and applications of iron nanoparticles. Small 2005, 1, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Sun, S. Recent advances in chemical synthesis, self-assembly, and applications of FePt nanoparticles. Adv. Mater. 2006, 18, 393–403. [Google Scholar] [CrossRef]
- Chaubey, G.S.; Barcena, C.; Poudyal, N.; Rong, C.; Gao, J.; Sun, S.; Liu, J.P. Synthesis and stabilization of FeCo nanoparticles. J. Am. Chem. Soc. 2007, 129, 7214–7215. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Anders, S.; Thomson, T.; Baglin, J.E.E.; Toney, M.F.; Hamann, H.F.; Murray, C.B.; Terris, B.D. Controlled synthesis and assembly of FePt nanoparticles. J. Phys. Chem. B 2003, 107, 5419–5425. [Google Scholar] [CrossRef]
- Chen, M.; Kim, J.; Liu, J.P.; Fan, H.; Sun, S. Synthesis of FePt nanocubes and their oriented self-assembly. J. Am. Chem. Soc. 2006, 128, 7132–7133. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hou, Y.; Kim, J.; Sun, S. A general strategy for synthesizing FePt nanowires and nanorods. Angew. Chem. Int. Ed. 2007, 46, 6333–6335. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Kakinuma, H.; Nagao, D.; Ando, Y.; Miyazaki, T.; Konno, M. Silica coating of Co–Pt alloy nanoparticles prepared in the presence of poly(vinylpyrrolidone). J. Nanopart. Res. 2009, 11, 1787–1794. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, J.; Peng, Q.; Li, Y. A general strategy for nanocrystal synthesis. Nature 2005, 437, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Hachani, R.; Lowdell, M.; Birchall, M.; Hervault, A.; Mertz, D.; Begin-Colin, S.; Thanh, N.T.K. Polyol synthesis, functionalisation, and biocompatibility studies of superparamagnetic iron oxide nanoparticles as potential MRI contrast agents. Nanoscale 2016, 8, 3278–3287. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Li, X.; Peng, Q.; Wang, X.; Chen, J.; Li, Y. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 2005, 44, 2782–2785. [Google Scholar] [CrossRef] [PubMed]
- Xuan, S.; Wang, Y.-X.J.; Yu, J.C.; Cham-Fai Leung, K. Tuning the grain size and particle size of superparamagnetic Fe3O4 microparticles. Chem. Mater. 2009, 21, 5079–5087. [Google Scholar] [CrossRef]
- Yang, H.-H.; Zhang, S.-Q.; Chen, X.-L.; Zhuang, Z.-X.; Xu, J.-G.; Wang, X.-R. Magnetite-containing spherical silica nanoparticles for biocatalysis and bioseparations. Anal. Chem. 2004, 76, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-J.; Kauzlarich, S.M.; Olamit, J.; Liu, K.; Grandjean, F.; Rebbouh, L.; Long, G.J. Characterization and magnetic properties of core/shell structured Fe/Au nanoparticles. J. Appl. Phys. 2004, 95, 6804–6806. [Google Scholar] [CrossRef]
- Liu, C.; Zou, B.; Rondinone, A.J.; Zhang, Z.J. Reverse micelle synthesis and characterization of superparamagnetic MnFe2O4 spinel ferrite nanocrystallites. J. Phys. Chem. B 2000, 104, 1141–1145. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Bae, C.J.; Park, J.G.; Noh, H.J.; Park, J.H.; Hyeon, T. Large-scale synthesis of uniform and crystalline magnetite nanoparticles using reverse micelles as nanoreactors under reflux conditions. Adv. Funct. Mater. 2005, 15, 503–509. [Google Scholar] [CrossRef]
- Salgueiriño-Maceira, V.; Spasova, M.; Farle, M. Water-stable, magnetic silica–cobalt/cobalt oxide–silica multishell submicrometer spheres. Adv. Funct. Mater. 2005, 15, 1036–1040. [Google Scholar] [CrossRef]
- Wang, J.; Loh, K.P.; Zhong, Y.L.; Lin, M.; Ding, J.; Foo, Y.L. Bifunctional FePt core-shell and hollow spheres: Sonochemical preparation and self-assembly. Chem. Mater. 2007, 19, 2566–2572. [Google Scholar] [CrossRef]
- Stoeva, S.I.; Huo, F.; Lee, J.-S.; Mirkin, C.A. Three-layer composite magnetic nanoparticle probes for DNA. J. Am. Chem. Soc. 2005, 127, 15362–15363. [Google Scholar] [CrossRef] [PubMed]
- Amara, D.; Margel, S. Synthesis and characterization of superparamagnetic core-shell micrometre-sized particles of narrow size distribution by a swelling process. J. Mater. Chem. 2012, 22, 9268–9276. [Google Scholar] [CrossRef]
- Qiao, R.; Yang, C.; Gao, M. Superparamagnetic iron oxide nanoparticles: From preparations to in vivo MRI applications. J. Mater. Chem. 2009, 19, 6274–6293. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cui, L.; Tong, N.; Gu, H. Development of high magnetization Fe3O4/polystyrene/silica nanospheres via combined miniemulsion/emulsion polymerization. J. Am. Chem. Soc. 2006, 128, 15582–15583. [Google Scholar] [CrossRef] [PubMed]
- Stappert, S.; Rellinghaus, B.; Acet, M.; Wassermann, E.F. Gas-phase preparation of L10 ordered FePt nanoparticles. J. Cryst. Growth 2003, 252, 440–450. [Google Scholar] [CrossRef]
- Wei, X.-W.; Zhu, G.-X.; Liu, Y.-J.; Ni, Y.-H.; Song, Y.; Xu, Z. Large-scale controlled synthesis of FeCo nanocubes and microcages by wet chemistry. Chem. Mater. 2008, 20, 6248–6253. [Google Scholar] [CrossRef]
- Kolhatkar, A.G.; Nekrashevich, I.; Litvinov, D.; Willson, R.C.; Lee, T.R. Cubic silica-coated and amine-functionalized FeCo nanoparticles with high saturation magnetization. Chem. Mater. 2013, 25, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, X.; Klemmer, T.; Shukla, N.; Yang, X.; Weller, D.; Roy, A.G.; Tanase, M.; Laughlin, D. Polyol process synthesis of monodispersed FePt nanoparticles. J. Phys. Chem. B 2004, 108, 6121–6123. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.-H.; Salabas, E.L.; Schüth, F. Magnetic nanoparticles: Synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Bruce, I.J.; Taylor, J.; Todd, M.; Davies, M.J.; Borioni, E.; Sangregorio, C.; Sen, T. Synthesis, characterisation and application of silica-magnetite nanocomposites. J. Magn. Magn. Mater. 2004, 284, 145–160. [Google Scholar] [CrossRef]
- Li, G.; Joshi, V.; White, R.L.; Wang, S.X.; Kemp, J.T.; Webb, C.; Davis, R.W.; Sun, S. Detection of single micron-sized magnetic bead and magnetic nanoparticles using spin valve sensors for biological applications. J. Appl. Phys. 2003, 93, 7557–7559. [Google Scholar] [CrossRef]
- Shubayev, V.I.; Pisanic, T.R., II; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Jenkins, G.J.S.; Asadi, R.; Doak, S.H. Potential toxicity of superparamagnetic iron oxide nanoparticles (spion). Nano Rev. 2010, 1, 5358. [Google Scholar] [CrossRef] [PubMed]
- Seo, W.S.; Lee, J.H.; Sun, X.; Suzuki, Y.; Mann, D.; Liu, Z.; Terashima, M.; Yang, P.C.; McConnell, M.V.; Nishimura, D.G.; et al. FeCo/graphitic-shell nanocrystals as advanced magnetic-resonance-imaging and near-infrared agents. Nat. Mater. 2006, 5, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Markides, H.; Rotherham, M.; El Haj, A.J. Biocompatibility and toxicity of magnetic nanoparticles in regenerative medicine. J. Nanomater. 2012, 2012, 11. [Google Scholar] [CrossRef]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Chin, S.F.; Iyer, K.S.; Raston, C.L. Facile and green approach to fabricate gold and silver coated superparamagnetic nanoparticles. Cryst. Growth Des. 2009, 9, 2685–2689. [Google Scholar] [CrossRef]
- Li, W.; Deng, Y.; Wu, Z.; Qian, X.; Yang, J.; Wang, Y.; Gu, D.; Zhang, F.; Tu, B.; Zhao, D. Hydrothermal etching assisted crystallization: A facile route to functional yolk-shell titanate microspheres with ultrathin nanosheets-assembled double shells. J. Am. Chem. Soc. 2011, 133, 15830–15833. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Yuan, R.; Chai, Y. Magnetic core-shell Fe3O4@Ag nanoparticles coated carbon paste interface for studies of carcinoembryonic antigen in clinical immunoassay. J. Phys. Chem. B 2006, 110, 11640–11646. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/shell nanoparticles: Classes, properties, synthesis mechanisms, characterization, and applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yin, Y.; Mayers, B.T.; Xia, Y. Modifying the surface properties of superparamagnetic iron oxide nanoparticles through a sol-gel approach. Nano Lett. 2002, 2, 183–186. [Google Scholar] [CrossRef]
- Hui, C.; Shen, C.; Tian, J.; Bao, L.; Ding, H.; Li, C.; Tian, Y.; Shi, X.; Gao, H.-J. Core-shell Fe3O4@SiO2 nanoparticles synthesized with well-dispersed hydrophilic Fe3O4 seeds. Nanoscale 2011, 3, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Cui, P.; Lee, J.-K. A simple method to synthesize multifunctional silica nanocomposites, NPs@SiO2, using polyvinylpyrrolidone (PVP) as a mediator. J. Mater. Chem. 2010, 20, 5533–5537. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Horie, M.; Konno, M.; Rodríguez-González, B.; Liz-Marzán, L.M. Preparation and properties of silica-coated cobalt nanoparticles. J. Phys. Chem. B 2003, 107, 7420–7425. [Google Scholar] [CrossRef]
- Nagao, D.; Yokoyama, M.; Yamauchi, N.; Matsumoto, H.; Kobayashi, Y.; Konno, M. Synthesis of highly monodisperse particles composed of a magnetic core and fluorescent shell. Langmuir 2008, 24, 9804–9808. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.L.; Zhang, Y.X.; Wang, S.; Xu, J.M.; Xu, S.C.; Li, G.H. Fe3O4@SiO2 core/shell nanoparticles: The silica coating regulations with a single core for different core sizes and shell thicknesses. Chem. Mater. 2012, 24, 4572–4580. [Google Scholar] [CrossRef]
- Yue, Q.; Li, J.; Luo, W.; Zhang, Y.; Elzatahry, A.A.; Wang, X.; Wang, C.; Li, W.; Cheng, X.; Alghamdi, A.; et al. An interface coassembly in biliquid phase: Toward core–shell magnetic mesoporous silica microspheres with tunable pore size. J. Am. Chem. Soc. 2015, 137, 13282–13289. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Sun, Y.; Tang, X.; Ren, Q.; Yang, W. Tumor-targeting multifunctional rattle-type theranostic nanoparticles for MRI/NIRF bimodal imaging and delivery of hydrophobic drugs. Small 2015, 11, 1962–1974. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-P.; Liao, P.-Y.; Su, C.-H.; Yeh, C.-S. Formation of oligonucleotide-gated silica shell-coated Fe3O4-Au core-shell nanotrisoctahedra for magnetically targeted and near-infrared light-responsive theranostic platform. J. Am. Chem. Soc. 2014, 136, 10062–10075. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-W.; Zhu, X.-M.; Lee, S.-F.; Chan, H.-M.; Li, H.-W.; Kong, S.K.; Yu, J.C.; Cheng, C.H.K.; Wang, Y.-X.J.; Leung, K.C.-F. Folate-conjugated Fe3O4@SiO2@gold nanorods@mesoporous SiO2 hybrid nanomaterial: A theranostic agent for magnetic resonance imaging and photothermal therapy. J. Mater. Chem. B 2013, 1, 2934–2942. [Google Scholar] [CrossRef]
- Wang, L.; Luo, J.; Maye, M.M.; Fan, Q.; Rendeng, Q.; Engelhard, M.H.; Wang, C.; Lin, Y.; Zhong, C.-J. Iron oxide-gold core-shell nanoparticles and thin film assembly. J. Mater. Chem. 2005, 15, 1821–1832. [Google Scholar] [CrossRef]
- Ji, X.; Shao, R.; Elliott, A.M.; Stafford, R.J.; Esparza-Coss, E.; Bankson, J.A.; Liang, G.; Luo, Z.-P.; Park, K.; Markert, J.T.; et al. Bifunctional gold nanoshells with a superparamagnetic iron oxide-silica core suitable for both MR imaging and photothermal therapy. J. Phys. Chem. C 2007, 111, 6245–6251. [Google Scholar] [CrossRef] [PubMed]
- Melancon, M.P.; Lu, W.; Li, C. Gold-based magneto/optical nanostructures: Challenges for in vivo applications in cancer diagnostics and therapy. MRS Bull. 2009, 34, 415–421. [Google Scholar] [CrossRef]
- Xu, Z.; Li, C.; Kang, X.; Yang, D.; Yang, P.; Hou, Z.; Lin, J. Synthesis of a multifunctional nanocomposite with magnetic, mesoporous, and near-IR absorption properties. J. Phys. Chem. C 2010, 114, 16343–16350. [Google Scholar] [CrossRef]
- Deng, Y.; Cai, Y.; Sun, Z.; Liu, J.; Liu, C.; Wei, J.; Li, W.; Liu, C.; Wang, Y.; Zhao, D. Multifunctional mesoporous composite microspheres with well-designed nanostructure: A highly integrated catalyst system. J. Am. Chem. Soc. 2010, 132, 8466–8473. [Google Scholar] [CrossRef] [PubMed]
- Krüger, S.; Stener, M.; Rösch, N. Relativistic density functional study of gold coated magnetic nickel clusters. J. Chem. Phys. 2001, 114, 5207–5215. [Google Scholar] [CrossRef]
- Huang, X.; Zhuang, J.; Chen, D.; Liu, H.; Tang, F.; Yan, X.; Meng, X.; Zhang, L.; Ren, J. General strategy for designing functionalized magnetic microspheres for different bioapplications. Langmuir 2009, 25, 11657–11663. [Google Scholar] [CrossRef] [PubMed]
- Shultz, M.D.; Reveles, J.U.; Khanna, S.N.; Carpenter, E.E. Reactive nature of dopamine as a surface functionalization agent in iron oxide nanoparticles. J. Am. Chem. Soc. 2007, 129, 2482–2487. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Schmucker, A.; Dyke, J.; Hall, S.M.; Retrum, J.; Stein, B.; Remmes, N.; Baxter, D.V.; Dragnea, B.; Bronstein, L.M. Magnetic nanoparticles with functional silanes: Evolution of well-defined shells from anhydride containing silane. J. Mater. Chem. 2009, 19, 4231–4239. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Teoh, W.Y.; Gooding, J.J.; Selomulya, C.; Amal, R. Functionalization strategies for protease immobilization on magnetic nanoparticles. Adv. Funct. Mater. 2010, 20, 1767–1777. [Google Scholar] [CrossRef]
- Yoon, T.-J.; Yu, K.N.; Kim, E.; Kim, J.S.; Kim, B.G.; Yun, S.-H.; Sohn, B.-H.; Cho, M.-H.; Lee, J.-K.; Park, S.B. Specific targeting, cell sorting, and bioimaging with smart magnetic silica core–shell nanomaterials. Small 2006, 2, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, R.; Chen, W.; Perez-Torres, C.; Bartels, M.; Huschka, R.M.; Zhao, L.L.; Morosan, E.; Pautler, R.G.; Joshi, A.; Halas, N.J. Nanoshells with targeted simultaneous enhancement of magnetic and optical imaging and photothermal therapeutic response. Adv. Funct. Mater. 2009, 19, 3901–3909. [Google Scholar] [CrossRef]
- Sharma, R.; Xu, Y.; Kim, S.W.; Schueller, M.J.; Alexoff, D.; Smith, S.D.; Wang, W.; Schlyer, D. Carbon-11 radiolabeling of iron-oxide nanoparticles for dual-modality PET/MR imaging. Nanoscale 2013, 5, 7476–7483. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Bhattarai, N.; Sun, C.; Zhang, M. Functionalized nanoparticles with long-term stability in biological media. Small 2009, 5, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, K.; Gu, H.; Zheng, R.; Liu, H.; Zhang, X.; Guo, Z.; Xu, B. Dopamine as a robust anchor to immobilize functional molecules on the iron oxide shell of magnetic nanoparticles. J. Am. Chem. Soc. 2004, 126, 9938–9939. [Google Scholar] [CrossRef] [PubMed]
- White, M.A.; Johnson, J.A.; Koberstein, J.T.; Turro, N.J. Toward the syntheses of universal ligands for metal oxide surfaces: Controlling surface functionality through click chemistry. J. Am. Chem. Soc. 2006, 128, 11356–11357. [Google Scholar] [CrossRef] [PubMed]
- Boyer, C.; Priyanto, P.; Davis, T.P.; Pissuwan, D.; Bulmus, V.; Kavallaris, M.; Teoh, W.Y.; Amal, R.; Carroll, M.; Woodward, R.; et al. Anti-fouling magnetic nanoparticles for siRNA delivery. J. Mater. Chem. 2010, 20, 255–265. [Google Scholar] [CrossRef]
- Boyer, C.; Bulmus, V.; Priyanto, P.; Teoh, W.Y.; Amal, R.; Davis, T.P. The stabilization and bio-functionalization of iron oxide nanoparticles using heterotelechelic polymers. J. Mater. Chem. 2009, 19, 111–123. [Google Scholar] [CrossRef]
- N’Guyen, T.T.T.; Duong, H.T.T.; Basuki, J.; Montembault, V.; Pascual, S.; Guibert, C.; Fresnais, J.; Boyer, C.; Whittaker, M.R.; Davis, T.P.; et al. Functional iron oxide magnetic nanoparticles with hyperthermia-induced drug release ability by using a combination of orthogonal click reactions. Angew. Chem. Int. Ed. 2013, 52, 14152–14156. [Google Scholar] [CrossRef] [PubMed]
- Manasmita, D.; Debasish, M.; Maiti, T.K.; Basak, A.; Pramanik, P. Bio-functionalization of magnetite nanoparticles using an aminophosphonic acid coupling agent: New, ultradispersed, iron-oxide folate nanoconjugates for cancer-specific targeting. Nanotechnology 2008, 19, 415101. [Google Scholar]
- Hatakeyama, M.; Kishi, H.; Kita, Y.; Imai, K.; Nishio, K.; Karasawa, S.; Masaike, Y.; Sakamoto, S.; Sandhu, A.; Tanimoto, A.; et al. A two-step ligand exchange reaction generates highly water-dispersed magnetic nanoparticles for biomedical applications. J. Mater. Chem. 2011, 21, 5959–5966. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, T.; Wu, C.; Qiu, L.; Hu, R.; Li, J.; Cansiz, S.; Zhang, L.; Cui, C.; Zhu, G.; et al. Facile surface functionalization of hydrophobic magnetic nanoparticles. J. Am. Chem. Soc. 2014, 136, 12552–12555. [Google Scholar] [CrossRef] [PubMed]
- Thanh, N.T.K.; Green, L.A.W. Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Josephson, L.; Tung, C.-H.; Moore, A.; Weissleder, R. High-efficiency intracellular magnetic labeling with novel superparamagnetic-Tat peptide conjugates. Bioconjug. Chem. 1999, 10, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qin, Y.; Palchoudhury, S.; Bao, Y. Water-soluble iron oxide nanoparticles with high stability and selective surface functionality. Langmuir 2011, 27, 8990–8997. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, F.; Liu, A.; Wang, L.; Wang, J.-C.; Ren, L.; Liu, W.; Tu, Q.; Li, L.; Wang, J. Cancer stem cell labeling using poly(l-lysine)-modified iron oxide nanoparticles. Biomaterials 2012, 33, 3719–3732. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Lee, H.; Hong, K.S.; Cho, M.Y.; Sung, M.-H.; Poo, H.; Lim, Y.T. Synthesis and high performance of magnetofluorescent polyelectrolyte nanocomposites as MR/near-infrared multimodal cellular imaging nanoprobes. ACS Nano 2011, 5, 8230–8240. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Han, Y.; Qiao, R.; Zeng, J.; Jia, Q.; Wang, Y.; Gao, M. Investigations on the interactions between plasma proteins and magnetic iron oxide nanoparticles with different surface modifications. J. Phys. Chem. C 2010, 114, 21270–21276. [Google Scholar] [CrossRef]
- Lutz, J.-F.; Stiller, S.; Hoth, A.; Kaufner, L.; Pison, U.; Cartier, R. One-pot synthesis of PEGylated ultrasmall iron-oxide nanoparticles and their in vivo evaluation as magnetic resonance imaging contrast agents. Biomacromolecules 2006, 7, 3132–3138. [Google Scholar] [CrossRef] [PubMed]
- Le Droumaguet, B.; Nicolas, J.; Brambilla, D.; Mura, S.; Maksimenko, A.; De Kimpe, L.; Salvati, E.; Zona, C.; Airoldi, C.; Canovi, M.; et al. Versatile and efficient targeting using a single nanoparticulate platform: Application to cancer and Alzheimer’s disease. ACS Nano 2012, 6, 5866–5879. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Du, K.; Fang, C.; Bhattarai, N.; Veiseh, O.; Kievit, F.; Stephen, Z.; Lee, D.; Ellenbogen, R.G.; Ratner, B.; et al. PEG-mediated synthesis of highly dispersive multifunctional superparamagnetic nanoparticles: Their physicochemical properties and function in vivo. ACS Nano 2010, 4, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
- Amstad, E.; Zurcher, S.; Mashaghi, A.; Wong, J.Y.; Textor, M.; Reimhult, E. Surface functionalization of single superparamagnetic iron oxide nanoparticles for targeted magnetic resonance imaging. Small 2009, 5, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chow, G.M. Carboxyl group (-CO2H) functionalized ferrimagnetic iron oxide nanoparticles for potential bio-applications. J. Mater. Chem. 2004, 14, 2781–2786. [Google Scholar] [CrossRef]
- Chen, H.; Deng, C.; Zhang, X. Synthesis of Fe3O4@SiO2@PMMA core–shell–shell magnetic microspheres for highly efficient enrichment of peptides and proteins for MALDI-ToF MS analysis. Angew. Chem. Int. Ed. 2010, 49, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wu, X.; Duan, H.; Wang, Y.A.; Wang, L.; Zhang, M.; Mao, H. Biocompatible polysiloxane-containing diblock copolymer PEO-b-PγMPS for coating magnetic nanoparticles. ACS Appl. Mater. Interfaces 2009, 1, 2134–2140. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-S.; Cong, Z.-X.; Cao, J.-B.; Ke, K.-M.; Peng, Q.-L.; Gao, J.; Yang, H.-H.; Liu, G.; Chen, X. Multifunctional Fe3O4@polydopamine core–shell nanocomposites for intracellular mRNA detection and imaging-guided photothermal therapy. ACS Nano 2014, 8, 3876–3883. [Google Scholar] [CrossRef] [PubMed]
- Li, G.L.; Mohwald, H.; Shchukin, D.G. Precipitation polymerization for fabrication of complex core-shell hybrid particles and hollow structures. Chem. Soc. Rev. 2013, 42, 3628–3646. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.-F.; Wu, K.-Y.; Tang, J.; Li, D.; Wei, C.; Guo, J.; Wang, S.-L.; Wang, C.-C. Magnetic drug carrier with a smart pH-responsive polymer network shell for controlled delivery of doxorubicin. J. Mater. Chem. 2012, 22, 15206–15214. [Google Scholar] [CrossRef]
- Ma, W.; Xu, S.; Li, J.; Guo, J.; Lin, Y.; Wang, C. Hydrophilic dual-responsive magnetite/PMAA core/shell microspheres with high magnetic susceptibility and pH sensitivity via distillation-precipitation polymerization. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 2725–2733. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, W.; Li, D.; Yu, M.; Guo, J.; Wang, C. Benzoboroxole-functionalized magnetic core/shell microspheres for highly specific enrichment of glycoproteins under physiological conditions. Small 2014, 10, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Y.; Ma, W.; Guo, J.; Lin, Y.; Wang, C. Uniform magnetic core/shell microspheres functionalized with Ni2+–iminodiacetic acid for one step purification and immobilization of his-tagged enzymes. ACS Appl. Mater. Interfaces 2013, 5, 2626–2633. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Yi, G.; Zhao, S.; Chen, D.; Guo, L.-H.; Cheng, J. Synthesis and characterization of multi-functional nanoparticles possessing magnetic, up-conversion fluorescence and bio-affinity properties. J. Mater. Chem. 2004, 14, 1336–1341. [Google Scholar] [CrossRef]
- Shinkai, M. Functional magnetic particles for medical application. J. Biosci. Bioeng. 2002, 94, 606–613. [Google Scholar] [CrossRef]
- Arsianti, M.; Lim, M.; Lou, S.N.; Goon, I.Y.; Marquis, C.P.; Amal, R. Bi-functional gold-coated magnetite composites with improved biocompatibility. J. Colloid Interface Sci. 2011, 354, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Weissleder, R.; Kelly, K.; Sun, E.Y.; Shtatland, T.; Josephson, L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat. Biotechnol. 2005, 23, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.L.; Ferreira, H.A.; Feliciano, N.; Freitas, P.P.; Clarke, L.A.; Amaral, M.D. Magnetic field-assisted DNA hybridisation and simultaneous detection using micron-sized spin-valve sensors and magnetic nanoparticles. Sens. Actuators B Chem. 2005, 107, 936–944. [Google Scholar] [CrossRef]
- Liang, Y.-C.; Chang, L.; Qiu, W.; Kolhatkar, A.G.; Vu, B.; Kourentzi, K.; Lee, T.R.; Zu, Y.; Willson, R.; Litvinov, D. Ultrasensitive magnetic nanoparticle detector for biosensor applications. Sensors 2017, 17, 1296. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Chang, L.; Liang, Y.-C.; Litvinov, J.; Guo, J.; Chen, Y.-T.; Vu, B.; Kourenzi, K.; Xu, S.; Lee, T.R.; et al. Spin-valve based magnetoresistive nanoparticle detector for applications in biosensing. Sens. Actuators A Phys. 2017, 265, 174–180. [Google Scholar] [CrossRef]
- Freitas, P.P.; Cardoso, F.A.; Martins, V.C.; Martins, S.A.M.; Loureiro, J.; Amaral, J.; Chaves, R.C.; Cardoso, S.; Fonseca, L.P.; Sebastiao, A.M.; et al. Spintronic platforms for biomedical applications. Lab Chip 2012, 12, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.A.; Gaster, R.S.; Makinwa, K.A.A.; Wang, S.X.; Murmann, B. A 256 pixel magnetoresistive biosensor microarray in 0.18 μm CMOS. IEEE J. Solid-State Circuits 2013, 48, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Koh, I.; Josephson, L. Magnetic nanoparticle sensors. Sensors 2009, 9, 8130–8145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, L.; Xu, S.J. Detection of magnetic nanomaterials in molecular imaging and diagnosis applications. Nanotechnol. Rev. 2014, 3, 247–268. [Google Scholar] [CrossRef]
- Osterfeld, S.J.; Yu, H.; Gaster, R.S.; Caramuta, S.; Xu, L.; Han, S.-J.; Hall, D.A.; Wilson, R.J.; Sun, S.; White, R.L.; et al. Multiplex protein assays based on real-time magnetic nanotag sensing. Proc. Natl. Acad. Sci. USA 2008, 105, 20637–20640. [Google Scholar] [CrossRef] [PubMed]
- De Palma, R.; Reekmans, G.; Liu, C.; Wirix-Speetjens, R.; Laureyn, W.; Nilsson, O.; Lagae, L. Magnetic bead sensing platform for the detection of proteins. Anal. Chem. 2007, 79, 8669–8677. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Srinivasan, B.; Jing, Y.; Yao, X.; Hugger, M.A.; Wang, J.-P.; Xing, C. Nanomagnetic competition assay for low-abundance protein biomarker quantification in unprocessed human sera. J. Am. Chem. Soc. 2010, 132, 4388–4392. [Google Scholar] [CrossRef] [PubMed]
- Carregal-Romero, S.; Caballero-Díaz, E.; Beqa, L.; Abdelmonem, A.M.; Ochs, M.; Hühn, D.; Suau, B.S.; Valcarcel, M.; Parak, W.J. Multiplexed sensing and imaging with colloidal nano- and microparticles. Annu. Rev. Anal. Chem. 2013, 6, 53–81. [Google Scholar] [CrossRef] [PubMed]
- Baselt, D.R.; Lee, G.U.; Natesan, M.; Metzger, S.W.; Sheehan, P.E.; Colton, R.J. A biosensor based on magnetoresistance technology. Biosens. Bioelectron. 1998, 13, 731–739. [Google Scholar] [CrossRef]
- Miller, M.M.; Sheehan, P.E.; Edelstein, R.L.; Tamanaha, C.R.; Zhong, L.; Bounnak, S.; Whitman, L.J.; Colton, R.J. A DNA array sensor utilizing magnetic microbeads and magnetoelectronic detection. J. Magn. Magn. Mater. 2001, 225, 138–144. [Google Scholar] [CrossRef]
- Schotter, J.; Kamp, P.B.; Becker, A.; Pühler, A.; Reiss, G.; Brückl, H. Comparison of a prototype magnetoresistive biosensor to standard fluorescent DNA detection. Biosens. Bioelectron. 2004, 19, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Hu, W.; Xu, L.; Wilson, R.J.; Yu, H.; Osterfeld, S.J.; Gambhir, S.S.; Wang, S.X. Protein-functionalized synthetic antiferromagnetic nanoparticles for biomolecule detection and magnetic manipulation. Angew. Chem. Int. Ed. 2009, 48, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.A.; Gaster, R.S.; Lin, T.; Osterfeld, S.J.; Han, S.; Murmann, B.; Wang, S.X. GMR biosensor arrays: A system perspective. Biosens. Bioelectron. 2010, 25, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, G.; Østerberg, F.W.; Henriksen, A.D.; Dufva, M.; Hansen, M.F. On-chip magnetic bead-based DNA melting curve analysis using a magnetoresistive sensor. J. Magn. Magn. Mater. 2015, 380, 215–220. [Google Scholar] [CrossRef]
- Dias, T.M.; Cardoso, F.A.; Martins, S.A.M.; Martins, V.C.; Cardoso, S.; Gaspar, J.F.; Monteiro, G.; Freitas, P.P. Implementing a strategy for on-chip detection of cell-free DNA fragments using GMR sensors: A translational application in cancer diagnostics using ALU elements. Anal. Methods 2016, 8, 119–128. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Tu, L.; Klein, T.; Feng, Y.L.; Wang, J.P. Surface modification for protein and DNA immobilization onto GMR biosensor. IEEE Trans. Magn. 2013, 49, 296–299. [Google Scholar] [CrossRef]
- Srinivasan, B.; Li, Y.; Jing, Y.; Xing, C.; Slaton, J.; Wang, J.-P. A three-layer competition-based giant magnetoresistive assay for direct quantification of endoglin from human urine. Anal. Chem. 2011, 83, 2996–3002. [Google Scholar] [CrossRef] [PubMed]
- Chaves, R.C.; Bensimon, D.; Freitas, P.P. Single molecule actuation and detection on a lab-on-a-chip magnetoresistive platform. J. Appl. Phys. 2011, 109, 064702. [Google Scholar] [CrossRef]
- Grancharov, S.G.; Zeng, H.; Sun, S.; Wang, S.X.; O’Brien, S.; Murray, C.B.; Kirtley, J.R.; Held, G.A. Bio-functionalization of monodisperse magnetic nanoparticles and their use as biomolecular labels in a magnetic tunnel junction based sensor. J. Phys. Chem. B 2005, 109, 13030–13035. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.Q.; Li, L.; Li, G.J.; Leung, C.W.; Shi, J.; Wong, C.M.; Lo, K.C.; Chan, W.K.; Mak, C.S.K.; Chan, S.B.; et al. Liver cancer immunoassay with magnetic nanoparticles and MgO-based magnetic tunnel junction sensors. J. Appl. Phys. 2012, 111, 07E505. [Google Scholar] [CrossRef] [Green Version]
- Albon, C.; Weddemann, A.; Auge, A.; Rott, K.; Hütten, A. Tunneling magnetoresistance sensors for high resolutive particle detection. Appl. Phys. Lett. 2009, 95, 023101. [Google Scholar] [CrossRef]
- Cousins, A.; Balalis, G.L.; Thompson, S.K.; Forero Morales, D.; Mohtar, A.; Wedding, A.B.; Thierry, B. Novel handheld magnetometer probe based on magnetic tunnelling junction sensors for intraoperative sentinel lymph node identification. Sci. Rep. 2015, 5, 10842. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.P.; Albisetti, E.; Massetti, M.; Scolari, M.; La Torre, C.; Monticelli, M.; Leone, M.; Damin, F.; Gervasoni, G.; Ferrari, G.; et al. Integrated platform for detecting pathogenic DNA via magnetic tunneling junction-based biosensors. Sens. Actuators B Chem. 2017, 242, 280–287. [Google Scholar] [CrossRef]
- Ejsing, L.; Hansen, M.F.; Menon, A.K.; Ferreira, H.A.; Graham, D.L.; Freitas, P.P. Planar Hall effect sensor for magnetic micro- and nanobead detection. Appl. Phys. Lett. 2004, 84, 4729–4731. [Google Scholar] [CrossRef] [Green Version]
- Damsgaard, C.D.; Dalslet, B.T.; Freitas, S.C.; Freitas, P.P.; Hansen, M.F. Temperature effects in exchange-biased planar Hall sensors for bioapplications. Sens. Actuators A Phys. 2009, 156, 103–108. [Google Scholar] [CrossRef]
- O/sterberg, F.W.; Dalslet, B.T.; Snakenborg, D.; Johansson, C.; Hansen, M.F. Chip-based measurements of Brownian relaxation of magnetic beads using a planar Hall effect magnetic field sensor. AIP Conf. Proc. 2010, 1311, 176–183. [Google Scholar]
- Dalslet, B.T.; Damsgaard, C.D.; Donolato, M.; Stromme, M.; Stromberg, M.; Svedlindh, P.; Hansen, M.F. Bead magnetorelaxometry with an on-chip magnetoresistive sensor. Lab Chip 2011, 11, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.Q.; Oh, S.; Jeong, J.-R.; Kim, C. Spin-valve planar Hall sensor for single bead detection. Sens. Actuators A Phys. 2010, 157, 42–46. [Google Scholar] [CrossRef]
- Volmer, M.; Avram, M. Microbeads detection using spin-valve planar Hall effect sensors. J. Nanosci. Nanotechnol. 2012, 12, 7456–7459. [Google Scholar] [CrossRef] [PubMed]
- Østerberg, F.W.; Rizzi, G.; Zardán Gómez de la Torre, T.; Strömberg, M.; Strømme, M.; Svedlindh, P.; Hansen, M.F. Measurements of Brownian relaxation of magnetic nanobeads using planar Hall effect bridge sensors. Biosens. Bioelectron. 2013, 40, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Østerberg, F.W.; Rizzi, G.; Henriksen, A.D.; Hansen, M.F. Planar Hall effect bridge geometries optimized for magnetic bead detection. J. Appl. Phys. 2014, 115, 184505. [Google Scholar] [CrossRef] [Green Version]
- Enpuku, K.; Soejima, K.; Nishimoto, T.; Matsuda, T.; Tokumitsu, H.; Tanaka, T.; Yoshinaga, K.; Kuma, H.; Hamasaki, N. Biological immunoassays without bound/free separation utilizing magnetic marker and HTS SQUID. IEEE Trans. Appl. Supercond. 2007, 17, 816–819. [Google Scholar] [CrossRef]
- Grossman, H.L.; Myers, W.R.; Vreeland, V.J.; Bruehl, R.; Alper, M.D.; Bertozzi, C.R.; Clarke, J. Detection of bacteria in suspension by using a superconducting quantum interference device. Proc. Natl. Acad. Sci. USA 2004, 101, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Enpuku, K.; Minotani, T.; Hotta, M.; Nakahodo, A. Application of high Tc SQUID magnetometer to biological immunoassays. IEEE Trans. Appl. Supercond. 2001, 11, 661–664. [Google Scholar] [CrossRef]
- Adolphi, N.L.; Butler, K.S.; Lovato, D.M.; Tessier, T.E.; Trujillo, J.E.; Hathaway, H.J.; Fegan, D.L.; Monson, T.C.; Stevens, T.E.; Huber, D.L.; et al. Imaging of Her2-targeted magnetic nanoparticles for breast cancer detection: Comparison of SQUID-detected magnetic relaxometry and MRI. Contrast Media Mol. Imaging 2012, 7, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Kawagishi, K.; Itozaki, H.; Kondo, T.; Komori, K.; Kotitz, R. Detection of fine magnetic particles coated on a thread using an HTS-SQUID. Physics C 2004, 412, 1491–1495. [Google Scholar] [CrossRef]
- Yang, C.-C.; Yang, S.-Y.; Chieh, J.-J.; Horng, H.-E.; Hong, C.-Y.; Yang, H.-C.; Chen, K.H.; Shih, B.Y.; Chen, T.-F.; Chiu, M.-J. Biofunctionalized magnetic nanoparticles for specifically detecting biomarkers of Alzheimer’s disease in vitro. ACS Chem. Neurosci. 2011, 2, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Ota, H.; Kondo, Y.; Tamaki, Y.; Kobayashi, S.; Noguchi, S. Detection of magnetic nanoparticles in lymph nodes of rat by high Tc SQUID. IEEE Trans. Appl. Supercond. 2003, 13, 377–380. [Google Scholar] [CrossRef]
- Shinji, K.; Takao, Y.; Ken, H.; Akira, M.; Saburo, T. Development of a new detection method for DNA molecules. Supercond. Sci. Technol. 2001, 14, 1131–1134. [Google Scholar]
- Bhuiya, A.K.; Asai, M.; Watanabe, H.; Hirata, T.; Higuchi, Y.; Yoshida, T.; Enpuku, K. Characterization of magnetic markers and sensors for liquid-phase immunoassays using Brownian relaxation. IEEE Trans. Magn. 2012, 48, 2838–2841. [Google Scholar] [CrossRef]
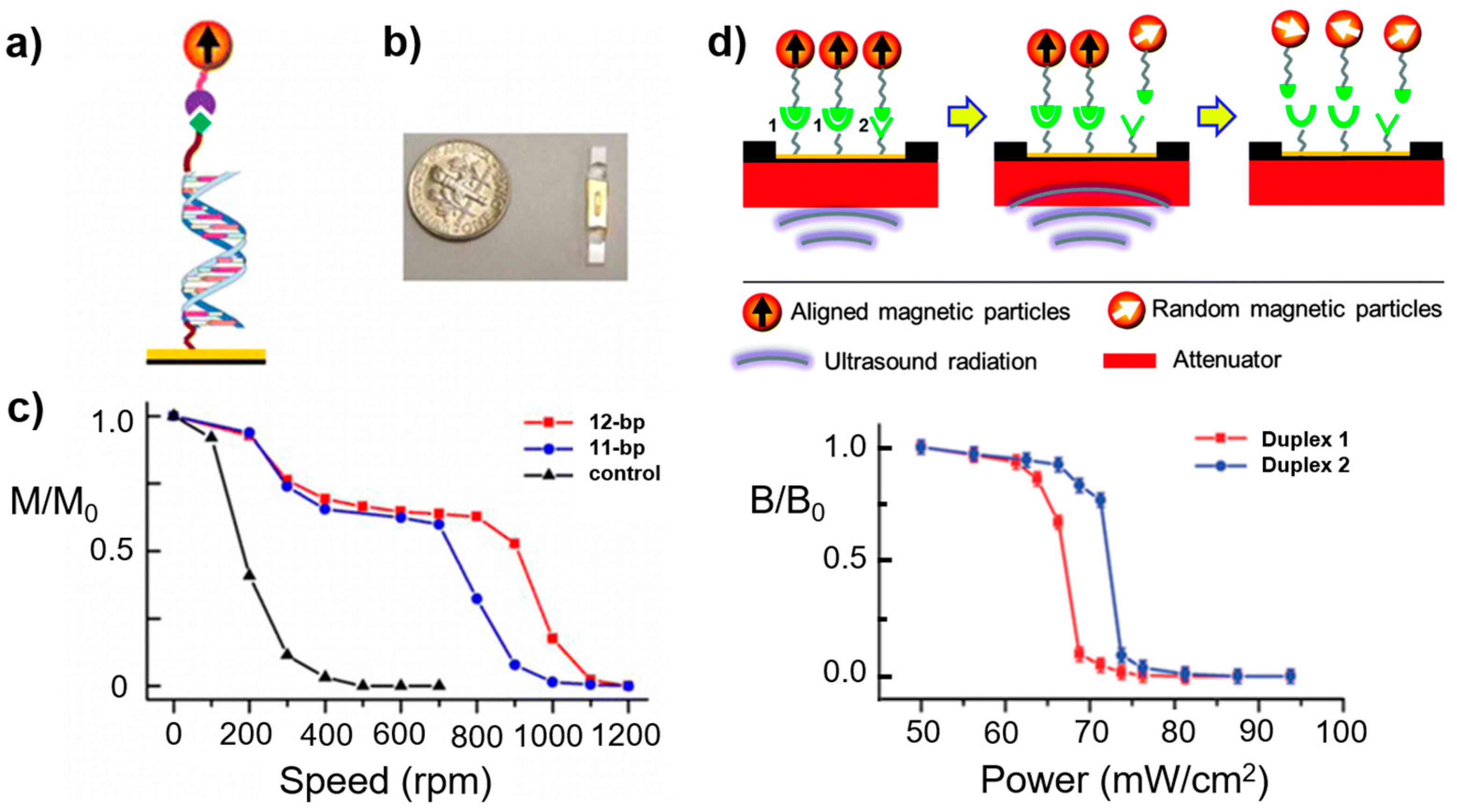
- De Silva, L.; Yao, L.; Xu, S. Mechanically resolving noncovalent bonds using acoustic radiation force. Chem. Commun. 2014, 50, 10786–10789. [Google Scholar] [CrossRef] [PubMed]
- De Silva, L.; Yao, L.; Wang, Y.; Xu, S. Well-defined and sequence-specific noncovalent binding forces of DNA. J. Phys. Chem. B 2013, 117, 7554–7558. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Xu, S. Force-induced selective dissociation of noncovalent antibody—Antigen bonds. J. Phys. Chem. B 2012, 116, 9944–9948. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-T.; Jamison, A.C.; Lee, T.R.; Xu, S. Quantitatively resolving ligand–receptor bonds on cell surfaces using force-induced remnant magnetization spectroscopy. ACS Cent. Sci. 2016, 2, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Li, Y.; Tsai, T.-W.; Xu, S.; Wang, Y. Noninvasive measurement of the mechanical force generated by motor protein Ef-G during ribosome translocation. Angew. Chem. Int. Ed. 2013, 52, 14041–14044. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Wang, Y.; Xu, S. Label-free microRNA detection based on exchange-induced remnant magnetization. Chem. Commun. 2013, 49, 5183–5185. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.; Lei, C.; Wang, T.; Yang, Z.; Zhou, Y. Detection of targeted carcinoembryonic antigens using a micro-fluxgate-based biosensor. Appl. Phys. Lett. 2013, 103, 203705. [Google Scholar] [CrossRef]
- Lei, J.; Lei, C.; Wang, T.; Yang, Z.; Zhou, Y. Investigation of targeted biomolecules in a micro-fluxgate-based bio-sensing system. Biomed. Microdevices 2014, 16, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Heim, E.; Ludwig, F.; Schilling, M. Binding assays with streptavidin-functionalized superparamagnetic nanoparticles and biotinylated analytes using fluxgate magnetorelaxometry. J. Magn. Magn. Mater. 2009, 321, 1628–1631. [Google Scholar] [CrossRef]
- Tu, L.; Jing, Y.; Li, Y.; Wang, J.-P. Real-time measurement of Brownian relaxation of magnetic nanoparticles by a mixing-frequency method. Appl. Phys. Lett. 2011, 98, 213702. [Google Scholar] [CrossRef]
- Nikitin, P.I.; Vetoshko, P.M.; Ksenevich, T.I. New type of biosensor based on magnetic nanoparticle detection. J. Magn. Magn. Mater. 2007, 311, 445–449. [Google Scholar] [CrossRef]
- Hong, H.B.; Krause, H.J.; Nam, I.H.; Choi, C.J.; Shin, S.W. Magnetic immunoassay based on frequency mixing magnetic detection and magnetic particles of different magnetic properties. Anal. Methods 2014, 6, 8055–8058. [Google Scholar] [CrossRef]
- Nahrendorf, M.; Zhang, H.; Hembrador, S.; Panizzi, P.; Sosnovik, D.E.; Aikawa, E.; Libby, P.; Swirski, F.K.; Weissleder, R. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation 2008, 117, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Cheon, J.; Lee, J.-H. Synergistically integrated nanoparticles as multimodal probes for nanobiotechnology. Acc. Chem. Res. 2008, 41, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Castro, C.M.; Ghazani, A.A.; Chung, J.; Shao, H.; Issadore, D.; Yoon, T.-J.; Weissleder, R.; Lee, H. Miniaturized nuclear magnetic resonance platform for detection and profiling of circulating tumor cells. Lab Chip 2014, 14, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Danieli, E.; Perlo, J.; Blümich, B.; Casanova, F. Small magnets for portable NMR spectrometers. Angew. Chem. Int. Ed. 2010, 49, 4133–4135. [Google Scholar] [CrossRef] [PubMed]
- Zalesskiy, S.S.; Danieli, E.; Blümich, B.; Ananikov, V.P. Miniaturization of NMR systems: Desktop spectrometers, microcoil spectroscopy, and “NMR on a chip” for chemistry, biochemistry, and industry. Chem. Rev. 2014, 114, 5641–5694. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Jon, S. Magnetic nanoparticle-based theranostics. Theranostics 2012, 2, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Ghazani, A.A.; Pectasides, M.; Sharma, A.; Castro, C.M.; Mino-Kenudson, M.; Lee, H.; Shepard, J.-A.O.; Weissleder, R. Molecular characterization of scant lung tumor cells using iron-oxide nanoparticles and micro-nuclear magnetic resonance. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Reiner, T.; Budin, G.; Min, C.; Liong, M.; Issadore, D.; Lee, H.; Weissleder, R. Ubiquitous detection of gram-positive bacteria with bioorthogonal magnetofluorescent nanoparticles. ACS Nano 2011, 5, 8834–8841. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Chung, J.; Balaj, L.; Charest, A.; Bigner, D.D.; Carter, B.S.; Hochberg, F.H.; Breakefield, X.O.; Weissleder, R.; Lee, H. Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat. Med. 2012, 18, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Drung, D.; Assmann, C.; Beyer, J.; Kirste, A.; Peters, M.; Ruede, F.; Schurig, T. Highly sensitive and easy-to-use SQUID sensors. IEEE Trans. Appl. Supercond. 2007, 17, 699–704. [Google Scholar] [CrossRef]
- Ryhänen, T.; Seppä, H.; Ilmoniemi, R.; Knuutila, J. SQUID magnetometers for low-frequency applications. J. Low Temp. Phys. 1989, 76, 287–386. [Google Scholar] [CrossRef]
- Song, G.; Xiangyang, S.; James, R.B., Jr.; Mark, M.B.H.; Bradford, G.O. Development of a remanence measurement-based SQUID system with in-depth resolution for nanoparticle imaging. Phys. Med. Biol. 2009, 54, N177–N188. [Google Scholar]
- Cohen, D. Ferromagnetic contamination in the lungs and other organs of the human body. Science 1973, 180, 745–748. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Myers, W.R.; Grossman, H.L.; Cho, H.-M.; Chemla, Y.R.; Clarke, J. Magnetic gradiometer based on a high-transition temperature superconducting quantum interference device for improved sensitivity of a biosensor. Appl. Phys. Lett. 2002, 81, 3094–3096. [Google Scholar] [CrossRef]
- Matz, H.; Drung, D.; Hartwig, S.; Groß, H.; Kötitz, R.; Müller, W.; Vass, A.; Weitschies, W.; Trahms, L. A SQUID measurement system for immunoassays. Appl. Supercond. 1999, 6, 577–583. [Google Scholar] [CrossRef]
- Eberbeck, D.; Wiekhorst, F.; Steinhoff, U.; Schwarz, K.O.; Kummrow, A.; Kammel, M.; Neukammer, J.; Trahms, L. Specific binding of magnetic nanoparticle probes to platelets in whole blood detected by magnetorelaxometry. J. Magn. Magn. Mater. 2009, 321, 1617–1620. [Google Scholar] [CrossRef]
- Tsukamoto, A.; Saitoh, K.; Suzuki, D.; Sugita, N.; Seki, Y.; Kandori, A.; Tsukada, K.; Sugiura, Y.; Hamaoka, S.; Kuma, H.; et al. Development of multisample biological immunoassay system using HTS SQUID and magnetic nanoparticles. IEEE Trans. Appl. Supercond. 2005, 15, 656–659. [Google Scholar] [CrossRef]
- Sarangi, S.; Tan, I.C.; Brazdeikis, A. Magnetic imaging method based on magnetic relaxation of magnetic nanoparticles. J. Appl. Phys. 2009, 105, 093926. [Google Scholar] [CrossRef]
- Liao, S.H.; Yang, H.-C.; Horng, H.E.; Liu, C.W.; Chen, H.H.; Chen, M.J.; Chen, K.L.; Liu, C.I.; Wang, L.M. Characterizing the field-dependent T1-relaxation and imaging of ferrofluids using high-Tc superconducting quantum interference device magnetometer in low magnetic fields. J. Appl. Phys. 2012, 112, 123908. [Google Scholar] [CrossRef]
- Jia, W.; Xu, G.; Sclabassi, R.J.; Zhu, J.-G.; Bagic, A.; Sun, M. Detection of magnetic nanoparticles with magnetoencephalography. J. Magn. Magn. Mater. 2008, 320, 1472–1478. [Google Scholar] [CrossRef]
- Budker, D.; Romalis, M. Optical magnetometry. Nat. Phys. 2007, 3, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Kominis, I.K.; Kornack, T.W.; Allred, J.C.; Romalis, M.V. A subfemtoTesla multichannel atomic magnetometer. Nature 2003, 422, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Maser, D.; Pandey, S.; Ring, H.; Ledbetter, M.P.; Knappe, S.; Kitching, J.; Budker, D. Note: Detection of a single cobalt microparticle with a microfabricated atomic magnetometer. Rev. Sci. Instrum. 2011, 82, 086112. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Xu, S. Long-range, high-resolution magnetic imaging of nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 5679–5682. [Google Scholar] [CrossRef] [PubMed]
- Garcia, N.C.; Yu, D.; Yao, L.; Xu, S. Optical atomic magnetometer at body temperature for magnetic particle imaging and nuclear magnetic resonance. Opt. Lett. 2010, 35, 661–663. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Xu, S. Sequence and chiral selectivity of drug–DNA interactions revealed by force spectroscopy. Angew. Chem. Int. Ed. 2014, 53, 14135–14138. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, F.; Heim, E.; Mäuselein, S.; Eberbeck, D.; Schilling, M. Magnetorelaxometry of magnetic nanoparticles with fluxgate magnetometers for the analysis of biological targets. J. Magn. Magn. Mater. 2005, 293, 690–695. [Google Scholar] [CrossRef]
- Ludwig, F.; Heim, E.; Menzel, D.; Schilling, M. Investigation of superparamagnetic Fe3O4 nanoparticles by fluxgate magnetorelaxometry for use in magnetic relaxation immunoassays. J. Appl. Phys. 2006, 99, 08P106. [Google Scholar] [CrossRef]
- Heim, E.; Harling, S.; Pöhlig, K.; Ludwig, F.; Menzel, H.; Schilling, M. Fluxgate magnetorelaxometry of superparamagnetic nanoparticles for hydrogel characterization. J. Magn. Magn. Mater. 2007, 311, 150–154. [Google Scholar] [CrossRef]
- Remmer, H.; Roeben, E.; Schmidt, A.M.; Schilling, M.; Ludwig, F. Dynamics of magnetic nanoparticles in viscoelastic media. J. Magn. Magn. Mater. 2017, 427, 331–335. [Google Scholar] [CrossRef]
- Ludwig, F.; Heim, E.; Schilling, M. Characterization of superparamagnetic nanoparticles by analyzing the magnetization and relaxation dynamics using fluxgate magnetometers. J. Appl. Phys. 2007, 101, 113909. [Google Scholar] [CrossRef]
- Ludwig, F.; Heim, E.; Eberbeck, D.; Schwarz, K.; Trahms, L.; Schilling, M. Comparison and calibration of fluxgate and SQUID magnetorelaxometry techniques for the characterization of magnetic core-shell nanoparticles. IEEE Trans. Magn. 2009, 45, 4857–4860. [Google Scholar] [CrossRef]
- Meyer, M.H.F.; Hartmann, M.; Krause, H.-J.; Blankenstein, G.; Mueller-Chorus, B.; Oster, J.; Miethe, P.; Keusgen, M. CRP determination based on a novel magnetic biosensor. Biosens. Bioelectron. 2007, 22, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.H.F.; Krause, H.-J.; Hartmann, M.; Miethe, P.; Oster, J.; Keusgen, M. Francisella tularensis detection using magnetic labels and a magnetic biosensor based on frequency mixing. J. Magn. Magn. Mater. 2007, 311, 259–263. [Google Scholar] [CrossRef]
- Meyer, M.H.F.; Stehr, M.; Bhuju, S.; Krause, H.-J.; Hartmann, M.; Miethe, P.; Singh, M.; Keusgen, M. Magnetic biosensor for the detection of Yersinia pestis. J. Microbiol. Methods 2007, 68, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Rettcher, S.; Jungk, F.; Kühn, C.; Krause, H.-J.; Nölke, G.; Commandeur, U.; Fischer, R.; Schillberg, S.; Schröper, F. Simple and portable magnetic immunoassay for rapid detection and sensitive quantification of plant viruses. Appl. Environ. Microbiol. 2015, 81, 3039–3048. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.-B.; Krause, H.-J.; Song, K.-B.; Choi, C.-J.; Chung, M.-A.; Son, S.-W.; Offenhäusser, A. Detection of two different influenza A viruses using a nitrocellulose membrane and a magnetic biosensor. J. Immunol. Methods 2011, 365, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-B.; Lim, E.-G.; Shin, S.W.; Krause, H.J.; Hong, H. Magnetic immunoassay platform based on the planar frequency mixing magnetic technique. Biosens. Bioelectron. 2016, 83, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Payet, B.; Vincent, D.; Delaunay, L.; Noyel, G. Influence of particle size distribution on the initial susceptibility of magnetic fluids in the Brown relaxation range. J. Magn. Magn. Mater. 1998, 186, 168–174. [Google Scholar] [CrossRef]
- Prieto Astalan, A.; Jonasson, C.; Petersson, K.; Blomgren, J.; Ilver, D.; Krozer, A.; Johansson, C. Magnetic response of thermally blocked magnetic nanoparticles in a pulsed magnetic field. J. Magn. Magn. Mater. 2007, 311, 166–170. [Google Scholar] [CrossRef]
- Kriz, K.; Gehrke, J.; Kriz, D. Advancements toward magneto immunoassays. Biosens. Bioelectron. 1998, 13, 817–823. [Google Scholar] [CrossRef]
- Barbic, M.; ElBidweihy, H. Effect of magnetic nanoparticle shape on flux amplification in inductive coil magnetic resonance detection. J. Appl. Phys. 2016, 120, 104506. [Google Scholar] [CrossRef]
- Donolato, M.; Vavassori, P.; Gobbi, M.; Deryabina, M.; Hansen, M.F.; Metlushko, V.; Ilic, B.; Cantoni, M.; Petti, D.; Brivio, S.; et al. On-chip manipulation of protein-coated magnetic beads via domain-wall conduits. Adv. Mater. 2010, 22, 2706–2710. [Google Scholar] [CrossRef] [PubMed]
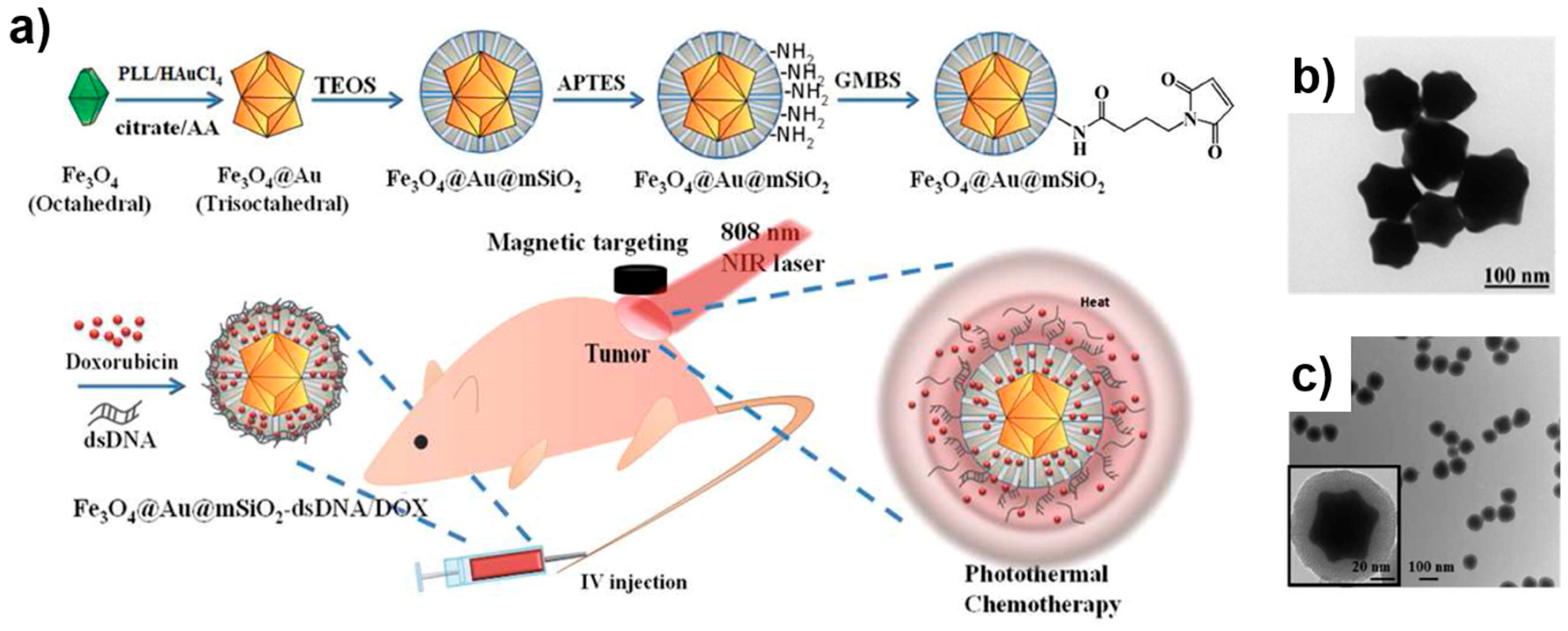
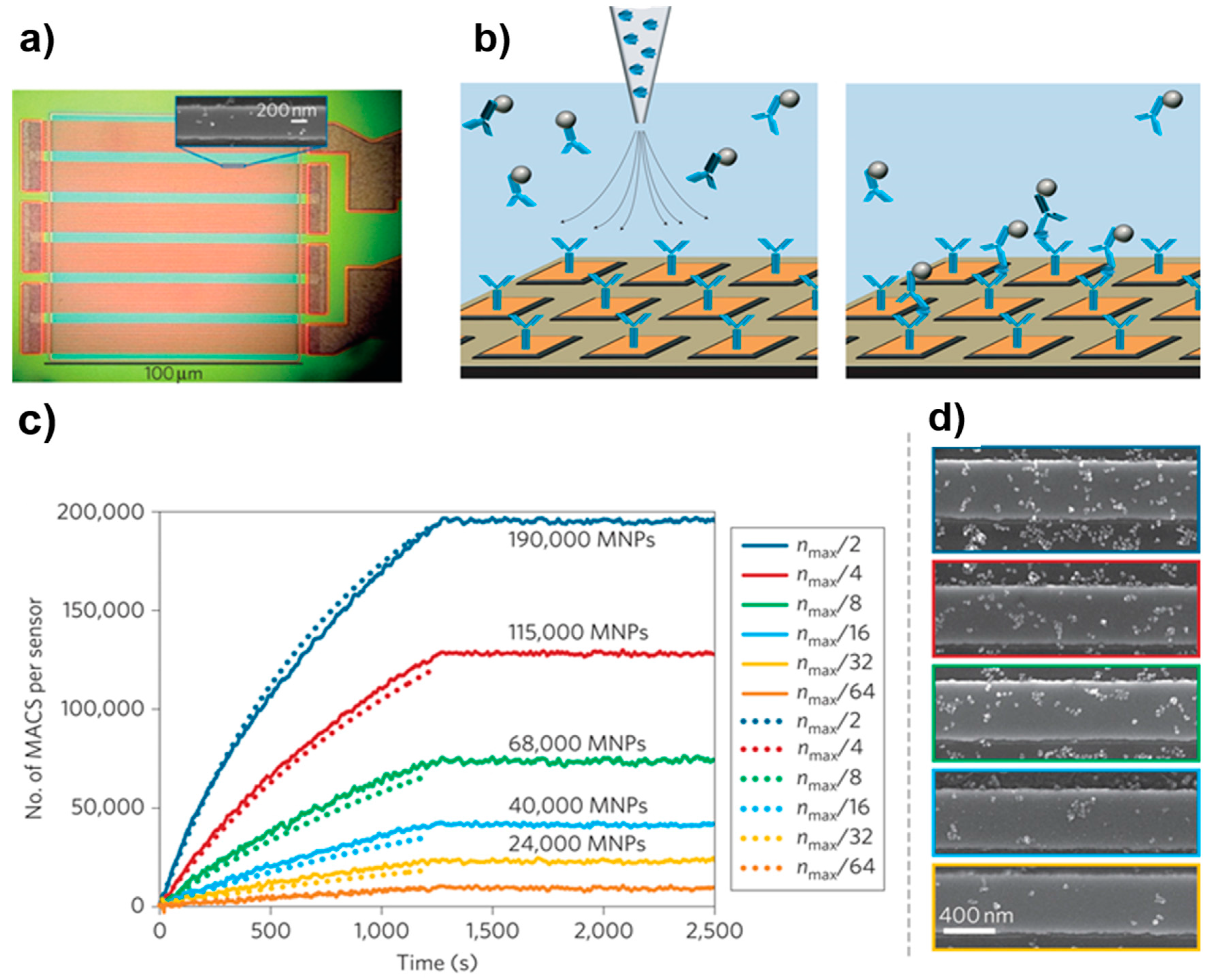
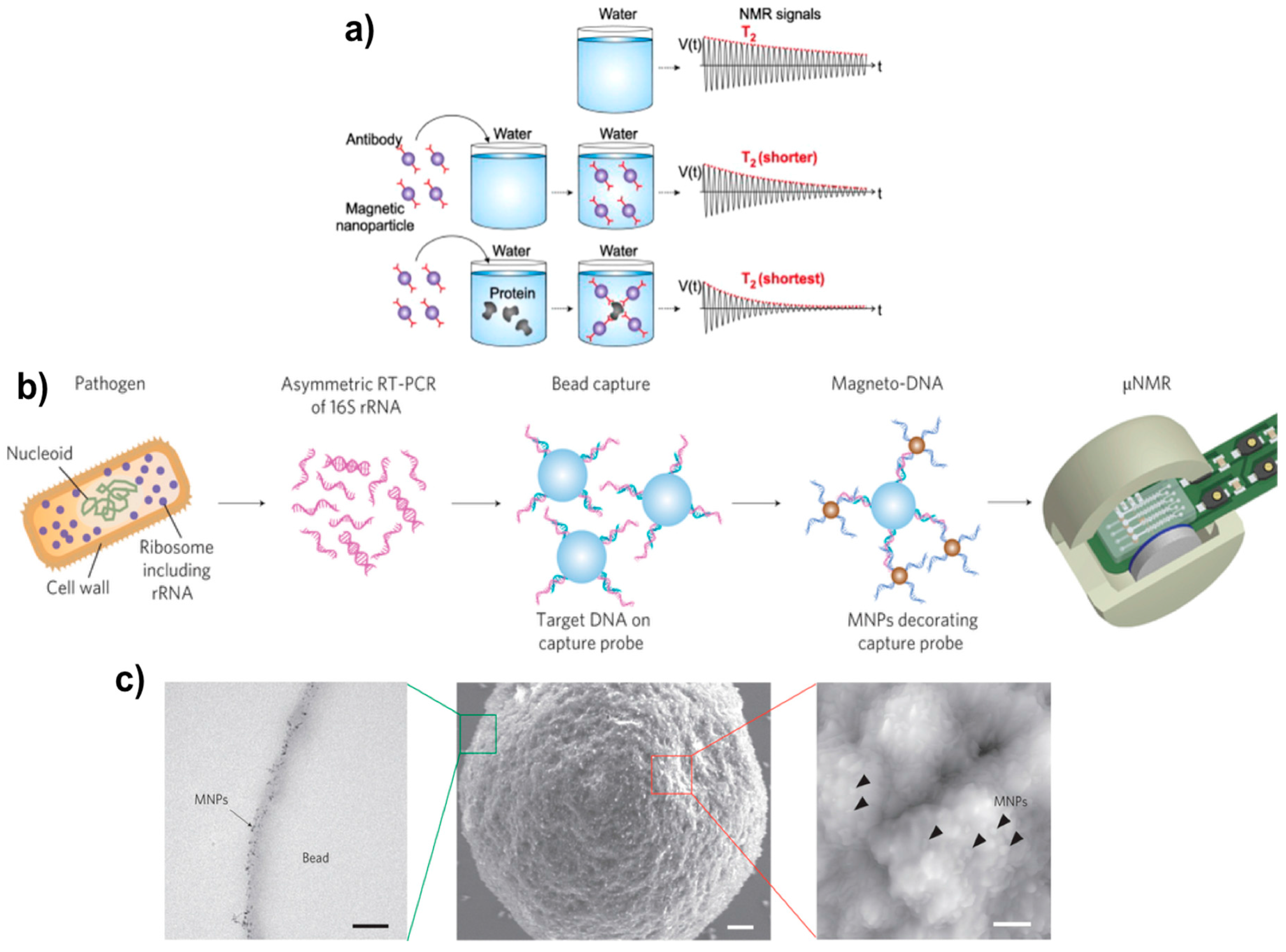
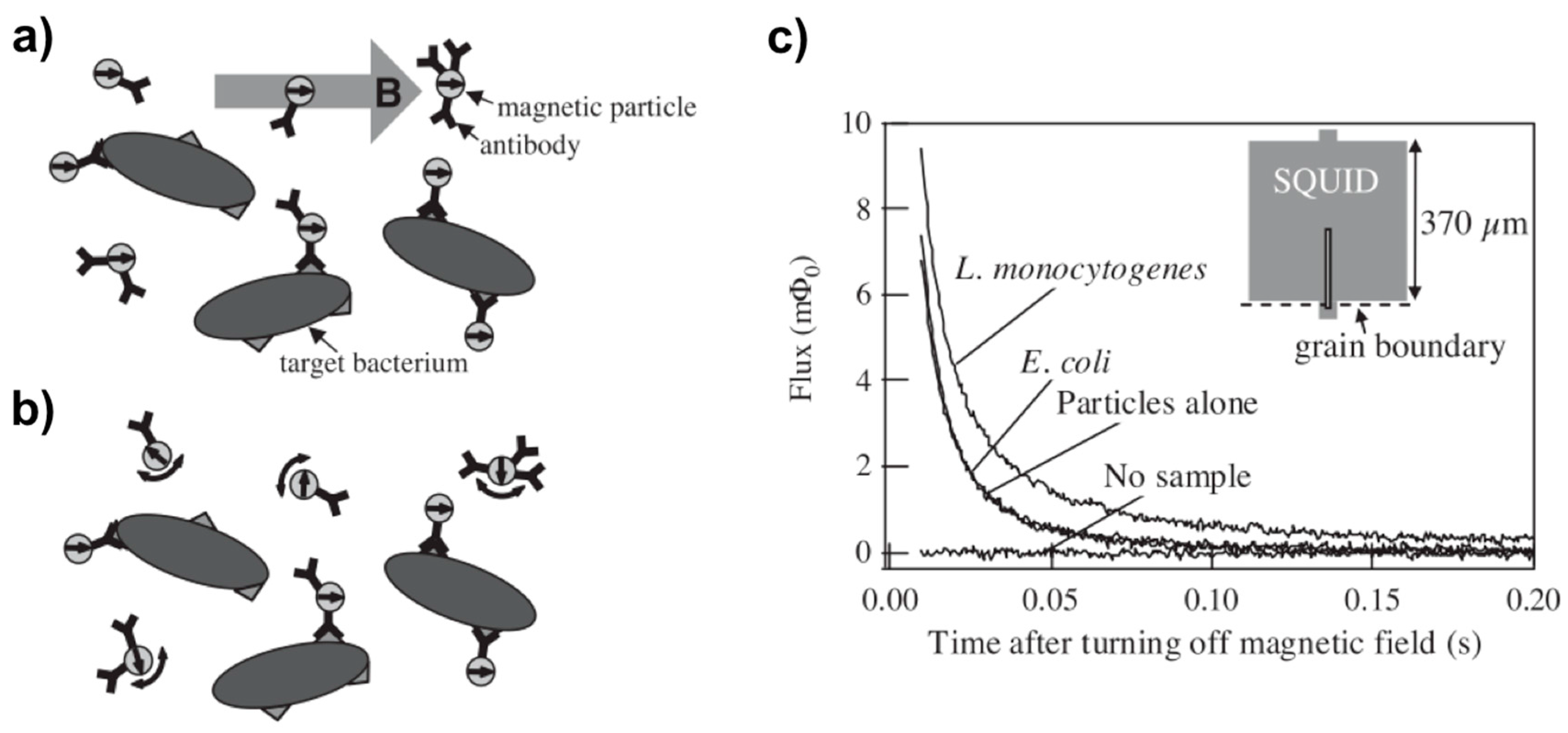
| Method | Material | Size (nm) | Reference |
|---|---|---|---|
Co-precipitation | Fe3O4, Fe2O3 | 4–43 | [42,43,44,45,46,47] |
Thermal Decomposition | MFe2O4 (M = Fe, Co, Ni, Zn) | 2–150 | [48,49,50,51,52] |
| Fe, Co, Ni | [53,54,55] | ||
| FePt, FeCo, CoPt | [56,57,58,59,60,61] | ||
Hydrothermal | MFe2O4 (M = Fe, Co) | 9–12 | [62] |
Polyol | Fe3O4, FePt | 3–10 | [60,63] |
| MFe2O4 (M = Fe, Co, Ni, Mn, Zn) | 50–800 | [64,65] | |
Emulsion/microemulsion/reverse micelles (self-assembly) | Fe3O4, MnFe2O4, Fe3O4/SiO2 | 2–200 | [66,67,68,69] |
| SiO2/Fe3O4 SiO2/Co SiO2/FePt | 50–200 | [66,70,71,72] | |
| Polymer/Fe3O4 | 1000–6000 | [73] |
| Functional Ligands on MP | Reactive Molecules | Conjugated Product |
|---|---|---|
| Carboxyl group | ||
| Carbodiimide coupling | ||
 |  |  |
| Amino group | ||
| Homobifunctional cross-linking | a) Glutaraldehyde as a linker | |
 |  | b) Disuccinimidyl suberate (DSS) as a linker |
| Maleimide coupling | ||
 |  |  |
| Epoxide group | ||
| Direct reaction | ||
 |  |  |
| Thiol group | ||
| Maleimide coupling | ||
 |  |  |
| Aldehyde group | ||
| Schiff-Base Condensation | ||
1) | 1) | 1) |
| Alkyne/azide group | ||
| Click reaction | ||
1) | 1) | 1) |
2) | 2) | 2) |
| Method | Role of MPs | Type of MPs | Size | Biological Studies | Reference |
|---|---|---|---|---|---|
| GMR | Label, magneto-resistive effect on surface | Streptavidin-coated M270 and M280 (Dynal) * | 2.8 μm | DNA | [84,162,163] |
| Dynabeads Myone streptavidin | 1 μm | Protein | [159] | ||
| Bio-Adembeads Streptavidin | 300 nm | Protein | |||
| Iron oxide (Bangs, CM021N) | 350, 860 nm | DNA | [164] | ||
| FeCo | 100 nm | DNA | [165] | ||
| Iron oxide MPs § | 30 nm | DNA, antibody | [169] | ||
| Streptavidin-AF488-modified Cubic FeCo Nanoparticle | 12.8 nm | Interleukin-6 | [160,170] | ||
| Streptavidin-coated superpara-magnetic beads (Micromod) | 250 nm | DNA (cfDNA) fragments | [168] | ||
| MACS † | 50 nm | DNA, protein, biotin-streptavidin | [12,158,166] | ||
| TMR | MnFe2O4 | 12 nm | DNA | [172] | |
| MACS ** | 50 nm | DNA | [15] | ||
| Fe3O4 | 16 nm | ||||
| Fe3O4 (Ocean Nanotech) *** | 20 nm | Protein | [173] | ||
| Micromod Nanomag-D | 250 nm | DNA | [176] | ||
| PHE | Amine-functionalized beads (Micromod) | 50 nm | DNA | [183] | |
| NMR | Label, relaxation | Dextran-coated iron oxide | 21, 45 nm | DNA, protein | [185] |
| SQUID | Contrast agent, label, signal | Magnetite core (Quantum Magnetics, Madison, CT) | 35 nm | Protein | [22] |
| MACS † | 50 nm | Antibody-antigen, bacteria | [21,186,187] | ||
| SHP-20 nanoparticles§ | 20 nm | Antibody | [188] | ||
| Streptavidin-modified dextran-coated MPs (Meito Sangyo) | 60 nm | DNA | [189] | ||
| Polystyrene-coated particles (Dynal) | 2.8 μm | DNA | |||
| Dextran-coated Fe3O4 (MagQu) | 55–50 nm | Protein | [190] | ||
| Fe3O4-coated with dextran (Meito Sangyo Co. Ltd.) | 11 nm | Lymph node | [191,192] | ||
| Self-made MPs | 278, 322 nm | Biotin | [193] | ||
| Ocean Nano-Technology | 54 nm | Biotin | |||
| Bayer Schering Pharmacy, Resovist | 58 nm | Biotin | |||
| R&D Systems, Mag-Cellect | 112 nm | Biotin | |||
| Micromod, Nanomag D130 | 126 nm | Biotin | |||
| Micromod, Nanomag D250 | 150 nm | Biotin | |||
| AM | Label | Ocean (SHP-28-50) | 24 nm | Cell | [23] |
| Label, signal, force transducer | Streptavidin-coated M280 (Dynal) * | 2.8 μm | DNA, antibody-antigen | [25,194,195,196,197,198,199] | |
| Flux-gate | Label and relaxation | Dynabead MyOne | 1 μm | Antibody-antigen | [200,201] |
| Streptavidin-coated MPs (Chemicell GmbH, Germany) | 100 nm | Streptavidin-biotin BSA | [202] | ||
| Frequency mixing | Label and relaxation | Ocean SHP-35 nanoparticles | 35 nm | Antibody-antigen | [203] |
| Dynabead MyOne | 1 μm | Antibody-antigen | [204] | ||
| Dynabead M-280 | 2.8 μm | ||||
| Signal | Nanomag®-D-spio-biotin | 20 nm | Biotin-avidin | [205] | |
| Label, Separation | Micromod | 500 nm |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-T.; Kolhatkar, A.G.; Zenasni, O.; Xu, S.; Lee, T.R. Biosensing Using Magnetic Particle Detection Techniques. Sensors 2017, 17, 2300. https://doi.org/10.3390/s17102300
Chen Y-T, Kolhatkar AG, Zenasni O, Xu S, Lee TR. Biosensing Using Magnetic Particle Detection Techniques. Sensors. 2017; 17(10):2300. https://doi.org/10.3390/s17102300
Chicago/Turabian StyleChen, Yi-Ting, Arati G. Kolhatkar, Oussama Zenasni, Shoujun Xu, and T. Randall Lee. 2017. "Biosensing Using Magnetic Particle Detection Techniques" Sensors 17, no. 10: 2300. https://doi.org/10.3390/s17102300