Penetration Depth Measurement of Near-Infrared Hyperspectral Imaging Light for Milk Powder
Abstract
:1. Introduction
2. Experimental Section
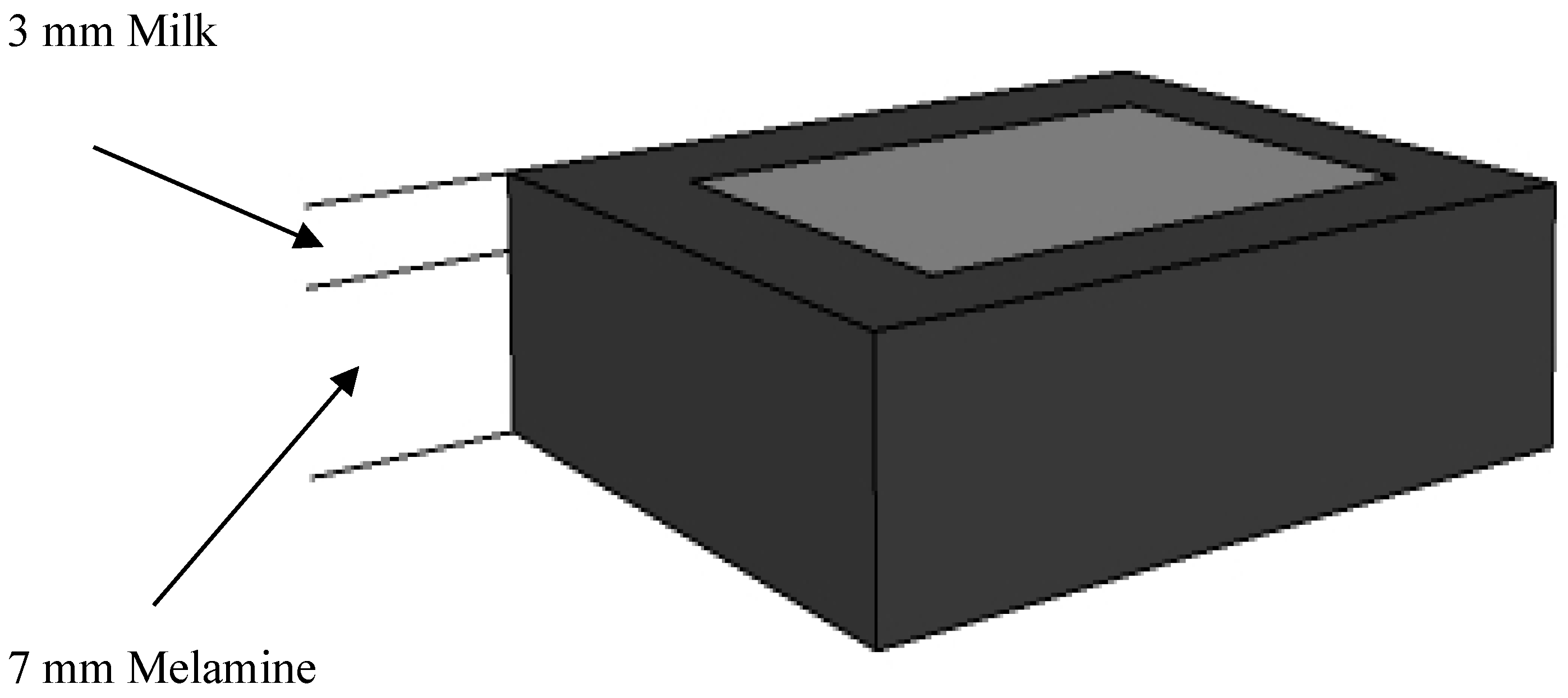
2.1. Sample Preparation
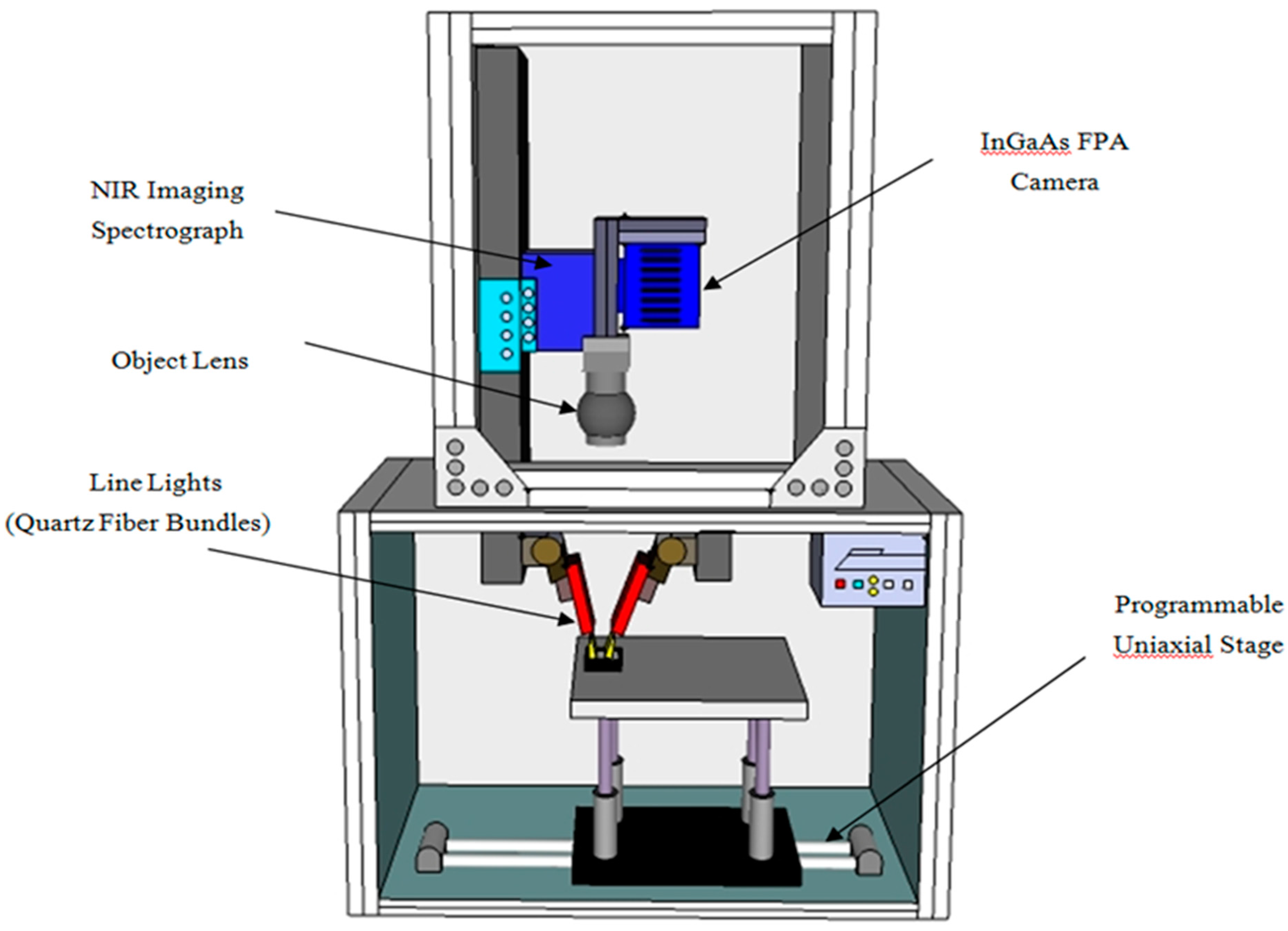
2.2. Instrument and Experiment
2.3. Hyperspectral Data Analysis
2.3.1. Image Preprocessing
2.3.2. Spectral Preprocessing
2.3.3. Development of the Classification Model
3. Results and Discussion
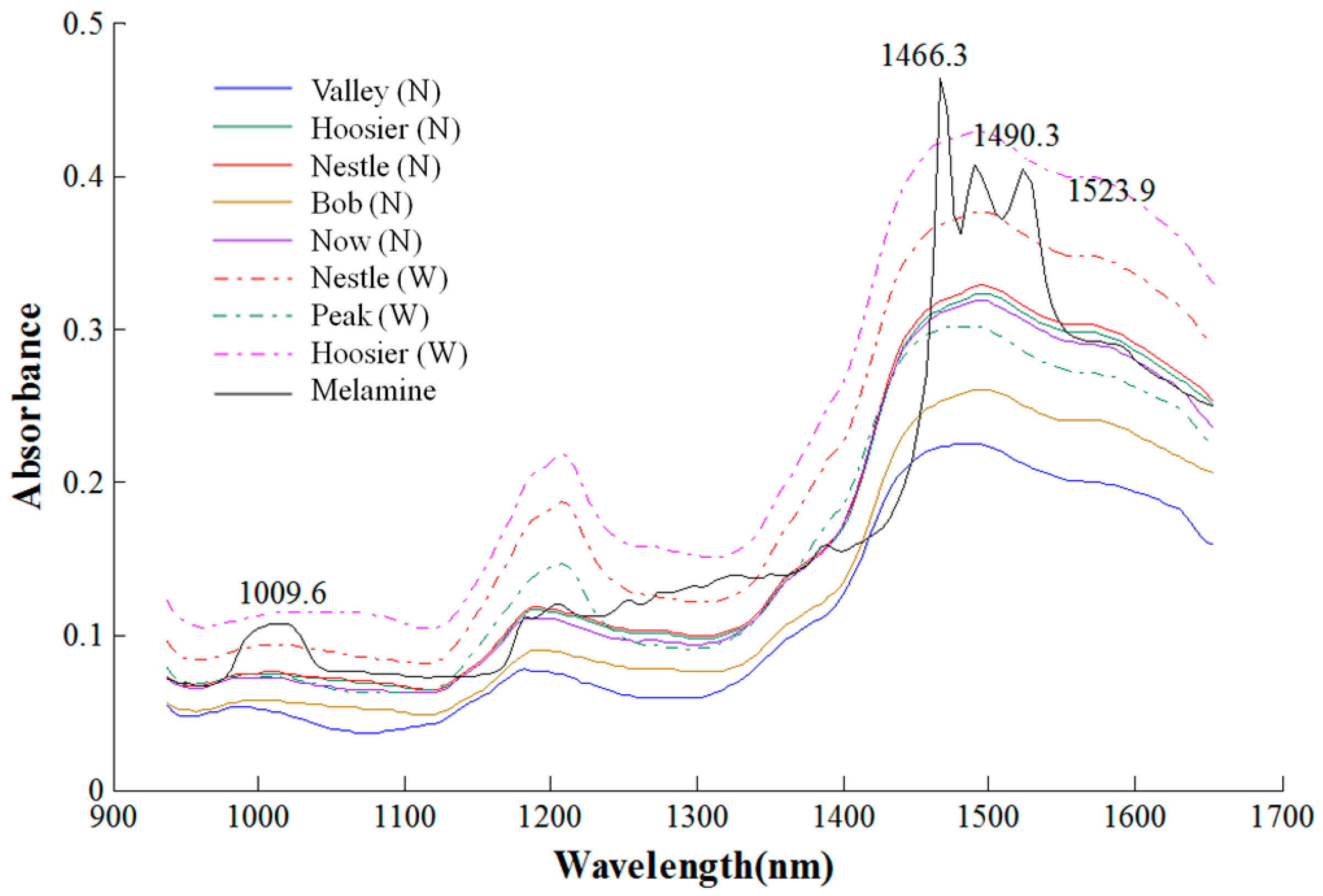
3.1. Hyperspectral Spectra
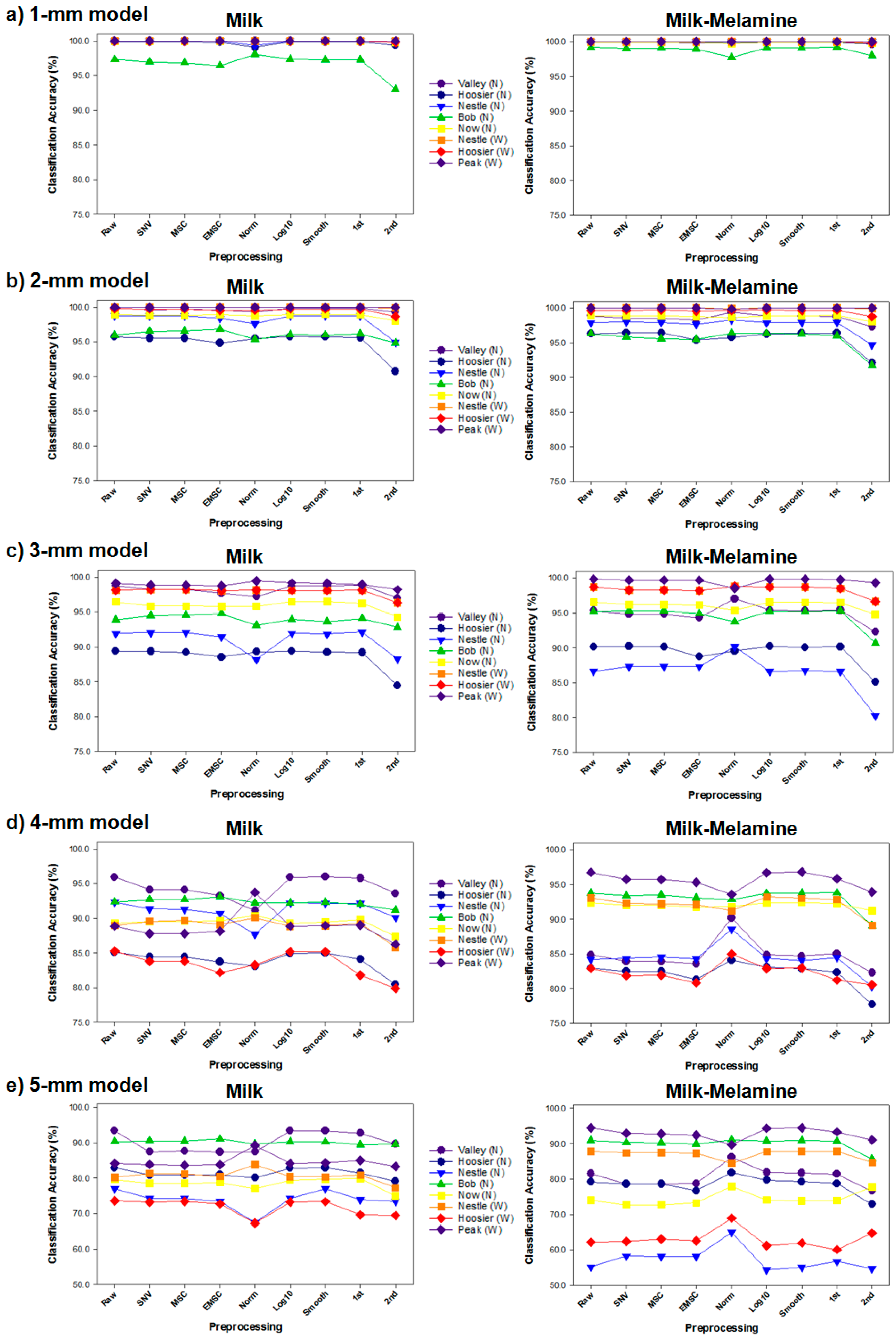
3.2. Discriminant Models for Milk Depth Classification
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Filigenzi, M.S.; Puschner, B.; Aston, L.S.; Poppenga, R.H. Diagnostic determination of melamine and related compounds in kidney tissue by liquid chromatography/tandem mass spectrometry. J. Agric. Food. Chem. 2008, 56, 7593–7599. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ren, Y.; Zhu, Y.; Cai, Z.; Han, J.; Huang, B.; Zhu, Y. Direct determination of melamine in dairy products by gas chromatography/mass spectrometry with coupled column separation. Anal. Chim. Acta 2009, 650, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Mauer, L.J.; Chernyshova, A.A.; Hiatt, A.; Deering, A.; Davis, R. Melamine detection in infant formula powder using near- and mid infrared spectroscopy. J. Agric. Food. Chem. 2009, 57, 3974–3980. [Google Scholar] [CrossRef] [PubMed]
- Balabin, R.M.; Smirnov, S.V. Melamine detection by mid-and near-infrared (MIR/NIR) spectroscopy: A quick and sensitive method for dairy products analysis including liquid milk, infant formula, and milk powder. Talanta 2011, 85, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Sun, D. Advanced applications of hyperspectral imaging technology for food quality and safety analysis and assessment: A review—Part I: Fundamentals. Innovative Food Sci. Emerg. Technol. 2013, 19, 1–14. [Google Scholar] [CrossRef]
- Cogdill, R.; Hurburgh, C.; Rippke, G. Single-kernel maize analysis by near-infrared hyperspectral imaging. Trans. ASAE 2004, 47, 311–320. [Google Scholar] [CrossRef]
- Qin, J.; Lu, R. Detection of pits in tart cherries by hyperspectral transmission imaging. Trans. ASAE 2005, 48, 1963–1970. [Google Scholar] [CrossRef]
- Coelho, P.A.; Soto, M.E.; Torres, S.N.; Sbarbaro, D.G.; Pezoa, J.E. Hyperspectral transmittance imaging of the shell-free cooked clam Mulinia edulis for parasite detection. J. Food Eng. 2013, 117, 408–416. [Google Scholar] [CrossRef]
- Huang, M.; Wan, X.; Zhang, M.; Zhu, Q. Detection of insect-damaged vegetable soybeans using hyperspectral transmittance image. J. Food Eng. 2013, 116, 45–49. [Google Scholar] [CrossRef]
- Huang, M.; Lu, R. Apple mealiness detection using hyperspectral scattering technique. Postharvest Biol. Technol. 2010, 58, 168–175. [Google Scholar] [CrossRef]
- Lu, R.; Peng, Y. Hyperspectral scattering for assessing peach fruit firmness. Biosystems Eng. 2006, 93, 161–171. [Google Scholar] [CrossRef]
- Gowen, A.A.; Taghizadeh, M.; O’donnell, C.P. Identification of mushrooms subjected to freeze damage using hyperspectral imaging. J. Food Eng. 2009, 93, 7–12. [Google Scholar] [CrossRef]
- Ariana, D.P.; Lu, R.; Guyer, D.E. Hyperspectral reflectance imaging for detection of bruises on pickling cucumbers. Comput. Electron. Agric. 2006, 53, 60–70. [Google Scholar] [CrossRef]
- Chao, K.; Yang, C.; Kim, M.S.; Chan, D. High throughput spectral imaging system for wholesomeness inspection of chicken. Appl. Eng. Agric. 2008, 24, 475–485. [Google Scholar] [CrossRef]
- Qin, J.; Lu, R. Measurement of the optical properties of fruits and vegetables using spatially resolved hyperspectral diffuse reflectance imaging technique. Postharvest Biol. Technol. 2008, 49, 355–365. [Google Scholar] [CrossRef]
- Lammertyn, J.; Peirs, A.; de Baerdemaeker, J.; Nicolaï, B. Light penetration properties of NIR radiation in fruit with respect to non-destructive quality assessment. Postharvest Biol. Technol. 2000, 18, 121–132. [Google Scholar] [CrossRef]
- Fernández Pierna, J.A.; Vincke, D.; Dardenne, P.; Yang, Z.; Han, L.; Baeten, V. Line scan hyperspectral imaging spectroscopy for the early detection of melamine and cyanuric acid in feed. J. Near Infrared Spectrosc. 2014, 22, 103–112. [Google Scholar] [CrossRef]
- Fu, X.; Kim, M.S.; Chao, K.; Qin, J.; Lim, J.; Lee, H.; Garrido-Varo, A.; Pérez-Marín, D.; Ying, Y. Detection of melamine in milk powders based on NIR hyperspectral imaging and spectral similarity analysis. J. Food Eng. 2014, 124, 97–104. [Google Scholar] [CrossRef]
- Kim, M.S.; Chen, Y.R.; Mehl, P.M. Hyperspectral reflectance and fluorescence imaging system for food quality and safety. Trans. ASAE 2001, 44, 721–729. [Google Scholar]
- Rinnan, A.; van den Berg, F.; Engelsen, S.B. Review of the most common pre-processing techniques for near-infrared spectra. Trends Anal. Chem. 2009, 28, 1201–1222. [Google Scholar] [CrossRef]
- Barker, M.; Rayens, W. Partial least squares for discrimination. J. Chemom. 2003, 17, 166–173. [Google Scholar] [CrossRef]
- Westerhuis, J.A.; Hoefsloot, H.C.J.; Smit, S.; Vis, D.J.; Smilde, A.K.; van Velzen, E.J.J.; van Duijnhoven, J.P.M.; van Dorsten, F.A. Assessment of PLSDA cross validation. Metabolomics 2008, 4, 81–89. [Google Scholar] [CrossRef]
- Huang, M.; Ma, Y.; Li, Y.; Zhu, Q.; Huang, G.; Bu, P. Hyperspectral image-based feature integration for insect-damaged hawthorn detection. Anal. Methods 2014, 6, 7793–7800. [Google Scholar] [CrossRef]
- Lu, R.; Huang, M.; Qin, J. Analysis of hyperspectral scattering characteristics for predicting apple fruit firmness and soluble solids content. In Proceedings of the International Conference on SPIE 7315, Sensing for Agriculture and Food Quality and Safety, Orlando, FL, USA, 13 April 2009.
- Nagarajan, R.; Singh, P.; Mehrotra, R. Direct determination of moisture in powder milk using near infrared spectroscopy. J. Autom. Methods Manag. Chem. 2006, 2006, 51342. [Google Scholar] [CrossRef] [PubMed]

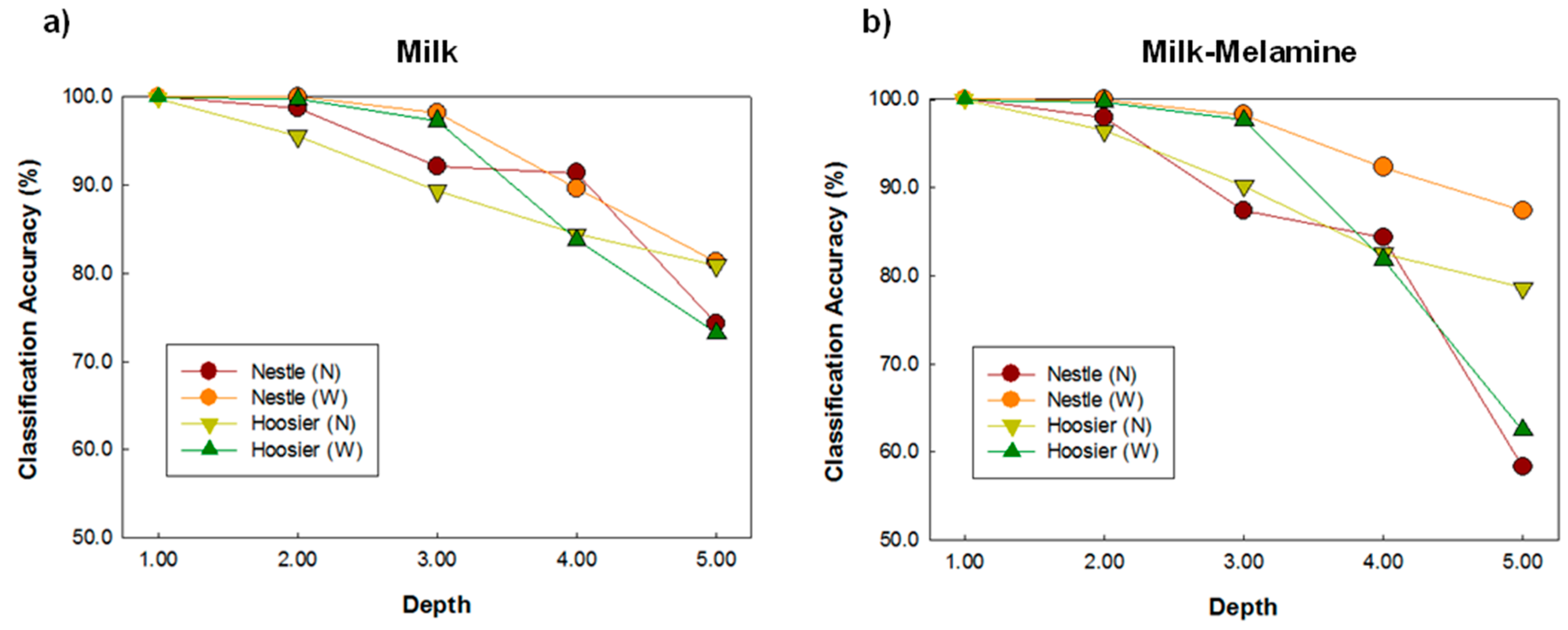
| Classification (%) | ||||||
|---|---|---|---|---|---|---|
| Depth | 1 mm | 2 mm | 3 mm | 4 mm | 5 mm | |
| valley (N) | 99.98 | 99.66 | 98.20 | 94.13 | 87.41 | |
| hoosier (N) | 99.86 | 95.53 | 89.37 | 84.43 | 80.91 | |
| nestle (N) | 100.00 | 98.72 | 92.08 | 91.39 | 74.26 | |
| bob (N) | 99.65 | 96.51 | 94.49 | 92.70 | 90.52 | |
| Milk | now (N) | 99.97 | 98.80 | 95.86 | 89.56 | 78.50 |
| nestle (W) | 100.00 | 99.98 | 98.19 | 89.57 | 81.29 | |
| hoosier (W) | 99.98 | 99.73 | 97.29 | 83.77 | 73.19 | |
| peak (W) | 99.99 | 99.98 | 98.86 | 87.81 | 83.85 | |
| Average | 99.93 | 98.61 | 95.54 | 89.17 | 81.24 | |
| valley (N) | 99.91 | 98.56 | 94.81 | 83.94 | 78.60 | |
| hoosier (N) | 99.96 | 96.43 | 90.21 | 82.47 | 78.62 | |
| nestle (N) | 100.00 | 97.97 | 87.34 | 84.35 | 58.26 | |
| Milk- | bob (N) | 99.06 | 95.82 | 95.29 | 93.42 | 90.30 |
| melamine | now (N) | 99.94 | 98.89 | 96.21 | 92.03 | 72.73 |
| nestle (W) | 100.00 | 100.00 | 98.27 | 92.32 | 87.42 | |
| hoosier (W) | 99.98 | 99.70 | 97.66 | 81.83 | 62.43 | |
| peak (W) | 99.99 | 99.99 | 99.70 | 95.76 | 92.92 | |
| Average | 99.86 | 98.42 | 94.93 | 88.26 | 77.66 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, M.; Kim, M.S.; Chao, K.; Qin, J.; Mo, C.; Esquerre, C.; Delwiche, S.; Zhu, Q. Penetration Depth Measurement of Near-Infrared Hyperspectral Imaging Light for Milk Powder. Sensors 2016, 16, 441. https://doi.org/10.3390/s16040441
Huang M, Kim MS, Chao K, Qin J, Mo C, Esquerre C, Delwiche S, Zhu Q. Penetration Depth Measurement of Near-Infrared Hyperspectral Imaging Light for Milk Powder. Sensors. 2016; 16(4):441. https://doi.org/10.3390/s16040441
Chicago/Turabian StyleHuang, Min, Moon S. Kim, Kuanglin Chao, Jianwei Qin, Changyeun Mo, Carlos Esquerre, Stephen Delwiche, and Qibing Zhu. 2016. "Penetration Depth Measurement of Near-Infrared Hyperspectral Imaging Light for Milk Powder" Sensors 16, no. 4: 441. https://doi.org/10.3390/s16040441






