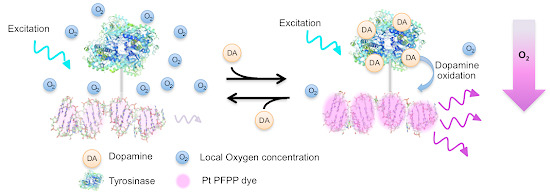
Quadruplex Integrated DNA (QuID) Nanosensors for Monitoring Dopamine
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Quadruplex Integrated DNA (QuID) Design
2.3. Azido-PEG4-Tyrosinase Attachment
2.4. Copper-Free Click Chemistry
2.5. QuID Nanosensor Assembly
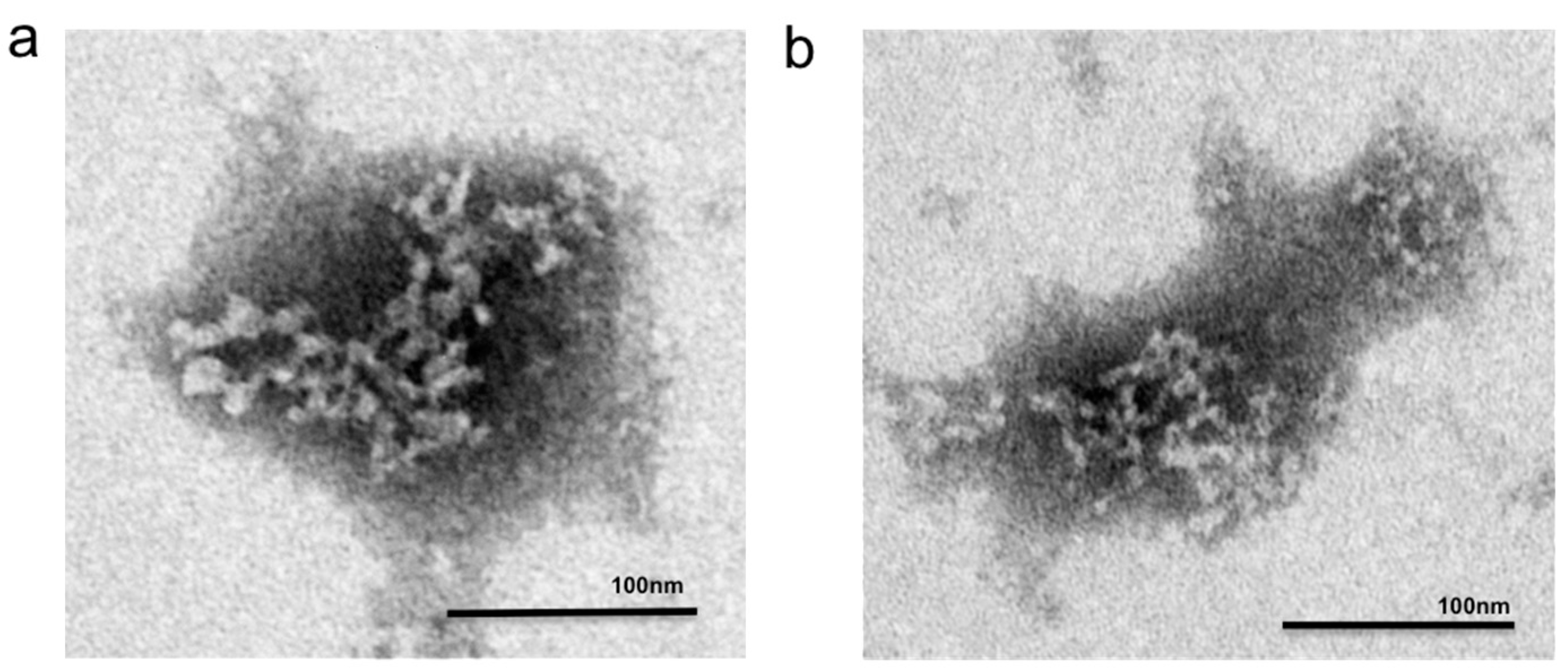
2.6. Transmission Electron Microscopy (TEM) Structural Determination
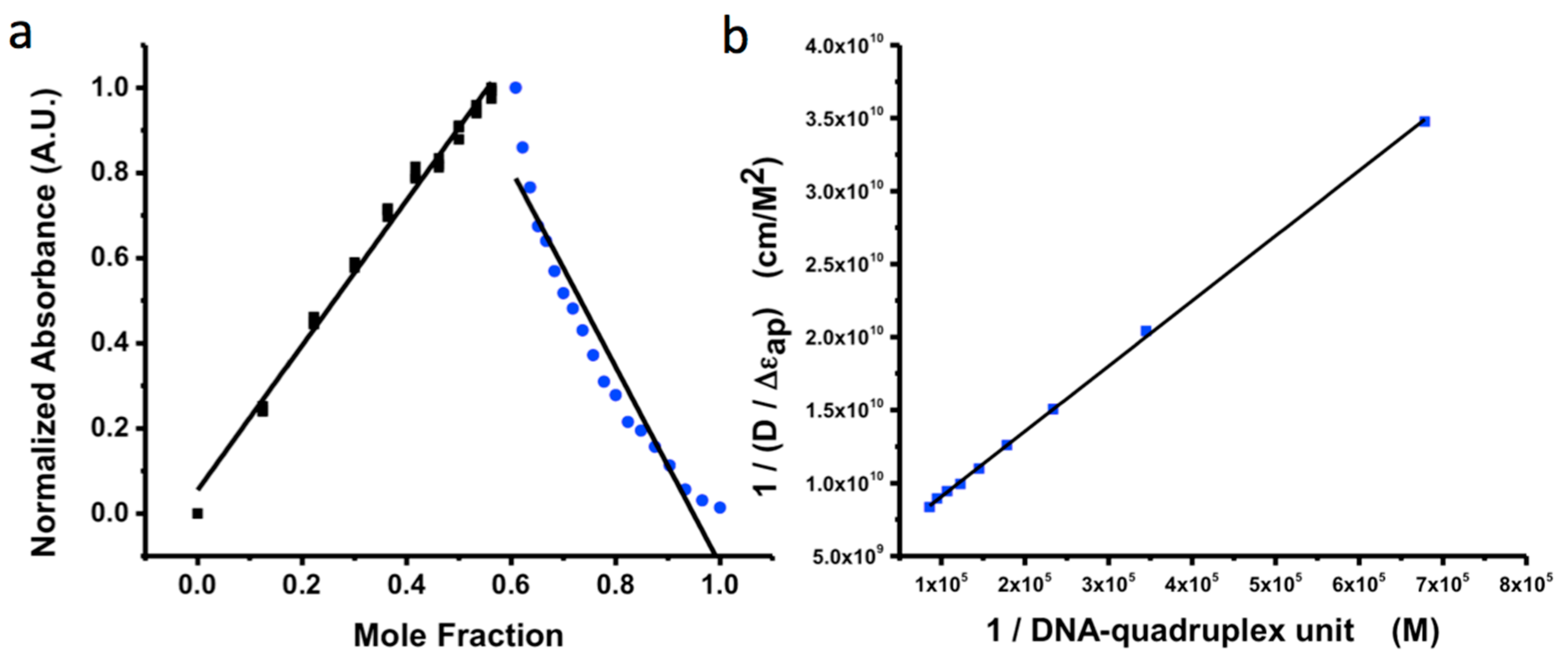
2.7. Continuous Variation Method (Job Plot)
2.8. QuID Size Determination
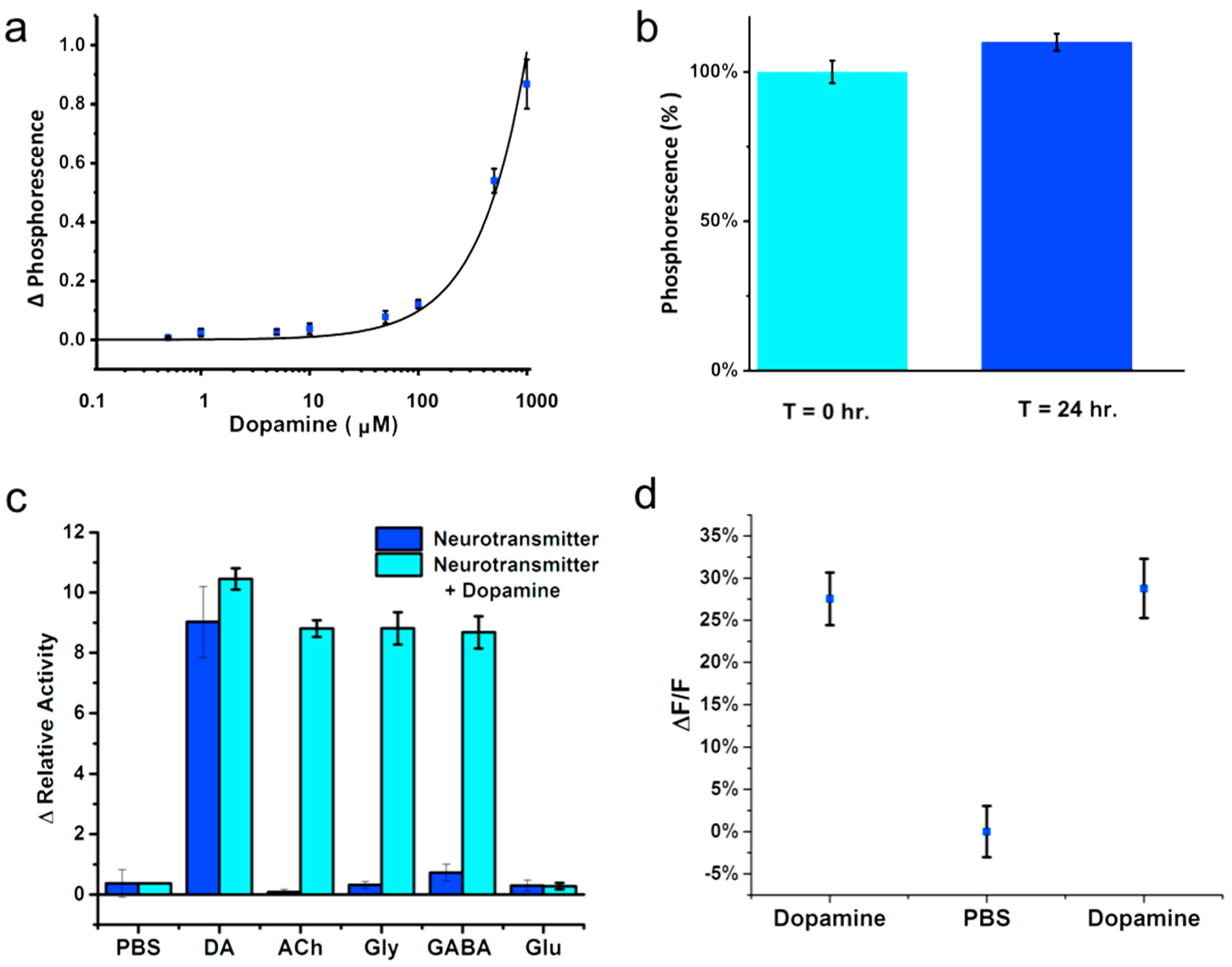
2.9. Tyrosinase Activity
2.10. Reversibility
2.11. Data Analysis
3. Results and Discussion

4. Conclusions/Outlook
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tritsch, N.X.; Sabatini, B.L. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron 2012, 76, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Vandecasteele, M.; Glowinski, J.; Deniau, J.-M.; Venance, L. Chemical transmission between dopaminergic neuron pairs. Proc. Natl. Acad. Sci. 2008, 105, 4904–4909. [Google Scholar] [CrossRef] [PubMed]
- Baskerville, T.A.; Douglas, A.J. Dopamine and oxytocin interactions underlying behaviors: Potential contributions to behavioral disorders. CNS Neurosci. Ther. 2010, 16, 92–123. [Google Scholar] [CrossRef] [PubMed]
- Uhlhaas, P.J.; Singer, W. Neuronal dynamics and neuropsychiatric disorders: Toward a translational paradigm for dysfunctional large-scale networks. Neuron 2012, 75, 963–980. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.L.; Sweedler, J.V.; Shen, M. Nanopipet-based Liquid-Liquid Interface Probes for the Electrochemical Detection of Acetylcholine, Tryptamine, and Serotonin via Ionic Transfer. Anal. Chem. 2015, 87, 5095–5100. [Google Scholar] [CrossRef] [PubMed]
- Haskins, W.E.; Watson, C.J.; Cellar, N.A.; Powell, D.H.; Kennedy, R.T. Discovery and neurochemical screening of peptides in brain extracellular fluid by chemical analysis of in vivo microdialysis samples. Anal. Chem. 2004, 76, 5523–5533. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.K.; Chaudhury, A. Structure activity relationship of synaptic and junctional neurotransmission. Auton. Neurosci. 2013, 176, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Kunori, N.; Takashima, I. A transparent epidural electrode array for use in conjunction with optical imaging. J. Neurosci. Methods 2015, 251, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Padamsey, Z.; Emptage, N.J. Imaging synaptic plasticity. Mol. Brain 2011, 4, 1756–6606. [Google Scholar] [CrossRef] [PubMed]
- Drummond, T.G.; Hill, M.G.; Barton, J.K. Electrochemical DNA sensors. Nat. Biotechnol. 2003, 21, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.S.; Dong, M.; Nielsen, M.M.; Jahn, K.; Subramani, R.; Mamdouh, W.; Golas, M.M.; Sander, B.; Stark, H.; Oliveira, C.L. Self-assembly of a nanoscale DNA box with a controllable lid. Nature 2009, 459, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Hu, C.; Wang, P.; Zhao, B.; Ouyang, X.; Zhou, J.; Liu, R.; He, D.; Fan, C.; Song, S. Growth and Origami Folding of DNA on Nanoparticles for High-Efficiency Molecular Transport in Cellular Imaging and Drug Delivery. Angew. Chem. 2015, 127, 2461–2465. [Google Scholar] [CrossRef]
- Kuzuya, A.; Sakai, Y.; Yamazaki, T.; Xu, Y.; Komiyama, M. Nanomechanical DNA origami “single-molecule beacons” directly imaged by atomic force microscopy. Nat. Commun. 2011, 2. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, P.W. Folding DNA to create nanoscale shapes and patterns. Nature 2006, 440, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, P.W.; Ekani-Nkodo, A.; Papadakis, N.; Kumar, A.; Fygenson, D.K.; Winfree, E. Design and characterization of programmable DNA nanotubes. J. Am. Chem. Soc. 2004, 126, 16344–16352. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.S.; Dong, M.; Nielsen, M.M.; Jahn, K.; Lind-Thomsen, A.; Mamdouh, W.; Gothelf, K.V.; Besenbacher, F.; Kjems, J. DNA origami design of dolphin-shaped structures with flexible tails. ACS Nano 2008, 2, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Maune, H.T.; Han, S.-P.; Barish, R.D.; Bockrath, M.; Goddard III, W.A.; Rothemund, P.W.; Winfree, E. Self-assembly of carbon nanotubes into two-dimensional geometries using DNA origami templates. Nat. Nanotechnol. 2010, 5, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.; Onodera, C.; Kidwell, C.; Tan, Y.; Graugnard, E.; Kuang, W.; Lee, J.; Knowlton, W.B.; Yurke, B.; Hughes, W.L. Programmable periodicity of quantum dot arrays with DNA origami nanotubes. Nano Lett. 2010, 10, 3367–3372. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Geng, Y.; Pound, E.; Gyawali, S.; Ashton, J.R.; Hickey, J.; Woolley, A.T.; Harb, J.N. Metallization of branched DNA origami for nanoelectronic circuit fabrication. ACS Nano 2011, 5, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Han, M.S.; Mirkin, C.A. Colorimetric Detection of Mercuric Ion (Hg2+) in Aqueous Media using DNA-Functionalized Gold Nanoparticles. Angew. Chem. 2007, 119, 4171–4174. [Google Scholar] [CrossRef]
- Liu, J.; Cao, Z.; Lu, Y. Functional nucleic acid sensors. Chem. Rev. 2009, 109, 1948–1998. [Google Scholar] [CrossRef] [PubMed]
- Tørring, T.; Voigt, N.V.; Nangreave, J.; Yan, H.; Gothelf, K.V. DNA origami: A quantum leap for self-assembly of complex structures. Chem. Soc. Rev. 2011, 40, 5636–5646. [Google Scholar] [CrossRef] [PubMed]
- Koirala, D.; Shrestha, P.; Emura, T.; Hidaka, K.; Mandal, S.; Endo, M.; Sugiyama, H.; Mao, H. Single-Molecule Mechanochemical Sensing Using DNA Origami Nanostructures. Angew. Chem. Int. Ed. 2014, 53, 8137–8141. [Google Scholar] [CrossRef] [PubMed]
- Georges, J.F.; Liu, X.; Eschbacher, J.; Nichols, J.; Mooney, M.A.; Joy, A.; Spetzler, R.F.; Feuerstein, B.G.; Preul, M.C.; Anderson, T. Use of a Conformational Switching Aptamer for Rapid and Specific Ex Vivo Identification of Central Nervous System Lymphoma in a Xenograft Model. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.; Buhlmann, P.; Pretsch, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 1. General Characteristics. Chem. Rev. 1997, 97, 3083–3132. [Google Scholar] [CrossRef] [PubMed]
- Buhlmann, P.; Pretsch, E.; Bakker, E. Carrier-Based Ion-Selective Electrodes and Bulk Optodes. 2. Ionophores for Potentiometric and Optical Sensors. Chem. Rev. 1998, 98, 1593–1688. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chen, P.; Niu, L.; Jin, J.; Liang, D.H.; Li, Z.B.; Yang, Z.Q.; Liu, D.S. pH-Responsive Size-Tunable Self-Assembled DNA Dendrimers. Angew. Chem. Int. Ed. 2012, 51, 11271–11274. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.-L.; Che, C.-M.; Yan, S.-C. Platinum (II) complexes with dipyridophenazine ligands as human telomerase inhibitors and luminescent probes for G-quadruplex DNA. J. Am. Chem. Soc. 2008, 131, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.-T.; Franz, S.; Zentgraf, H.; Sommerville, J. Electron Microscopy of Biomolecules. In Encyclopedia of Molecular Cell Biology and Molecular Medicine; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006. [Google Scholar]
- Kieltyka, R.; Englebienne, P.; Moitessier, N.; Sleiman, H. Quantifying interactions between G-quadruplex DNA and transition-metal complexes. In G-Quadruplex DNA; Springer: New York City, NY, USA, 2010; pp. 223–255. [Google Scholar]
- Bomble, Y.J.; Case, D.A. Multiscale modeling of nucleic acids: Insights into DNA flexibility. Biopolymers 2008, 89, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wu, Z.-S.; Shen, G.-L.; Yu, R.-Q. Intermolecular G-quadruplex structure-based fluorescent DNA detection system. Biosens. Bioelectron. 2013, 41, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Romera, C.; Bombarde, O.; Bonnet, R.; Gomez, D.; Dumy, P.; Calsou, P.; Gwan, J.-F.; Lin, J.-H.; Defrancq, E.; Pratviel, G. Improvement of porphyrins for G-quadruplex DNA targeting. Biochimie 2011, 93, 1310–1317. [Google Scholar] [CrossRef] [PubMed]
- Aidoo, A.Y.; Ward, K. Spatio-temporal concentration of acetylcholine in vertebrate synaptic cleft. Math. Comput. Model. 2006, 44, 952–962. [Google Scholar] [CrossRef]
- Martin, M.M.; Lindqvist, L. The pH dependence of fluorescein fluorescence. J. Lumin. 1975, 10, 381–390. [Google Scholar] [CrossRef]
- Erickson, H.P. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol. Proced. Online 2009, 11, 32–51. [Google Scholar] [CrossRef] [PubMed]
- Savtchenko, L.P.; Rusakov, D.A. The optimal height of the synaptic cleft. Proc. Natl. Acad. Sci. USA 2007, 104, 1823–1828. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Jia, G.; Yuan, J.; Feng, Z.; Li, C. A spectroscopic study on the interactions of porphyrin with G-quadruplex DNAs. Biochemistry 2006, 45, 6681–6691. [Google Scholar] [CrossRef] [PubMed]
- Cragg, S.J.; Rice, M.E. Dancing past the DAT at a DA synapse. Trends Neurosci. 2004, 27, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Cragg, S.J.; Nicholson, C.; Kume-Kick, J.; Tao, L.; Rice, M.E. Dopamine-mediated volume transmission in midbrain is regulated by distinct extracellular geometry and uptake. J. Neurophys. 2001, 85, 1761–1771. [Google Scholar]
- Garris, P.A.; Ciolkowski, E.L.; Pastore, P.; Wightman, R. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J. Neurosci. 1994, 14, 6084–6093. [Google Scholar] [PubMed]
- Marcott, P.F.; Mamaligas, A.A.; Ford, C.P. Phasic Dopamine Release Drives Rapid Activation of Striatal D2-Receptors. Neuron 2014, 84, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz Vieira, I.; Fatibello-Filho, O. Spectrophotometric determination of methyldopa and dopamine in pharmaceutical formulations using a crude extract of sweet potato root (Ipomoea batatas (L.) Lam.) as enzymatic source. Talanta 1998, 46, 559–564. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Characterization of the monophenolase activity of tyrosinase on betaxanthins: The tyramine-betaxanthin/dopamine-betaxanthin pair. Planta 2005, 222, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Kreft, O.; Javier, A.M.; Sukhorukov, G.B.; Parak, W.J. Polymer microcapsules as mobile local pH-sensors. J. Mater. Chem. 2007, 17, 4471–4476. [Google Scholar] [CrossRef]
- Li, H.; Yang, M.; Liu, J.; Zhang, Y.; Yang, Y.; Huang, H.; Liu, Y.; Kang, Z. A practical and highly sensitive C3N4-TYR fluorescent probe for convenient detection of dopamine. Nanoscale 2015, 7, 12068–12075. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, J.M.; Skipwith, C.G.; Clark, H.A. Quadruplex Integrated DNA (QuID) Nanosensors for Monitoring Dopamine. Sensors 2015, 15, 19912-19924. https://doi.org/10.3390/s150819912
Morales JM, Skipwith CG, Clark HA. Quadruplex Integrated DNA (QuID) Nanosensors for Monitoring Dopamine. Sensors. 2015; 15(8):19912-19924. https://doi.org/10.3390/s150819912
Chicago/Turabian StyleMorales, Jennifer M., Christopher G. Skipwith, and Heather A. Clark. 2015. "Quadruplex Integrated DNA (QuID) Nanosensors for Monitoring Dopamine" Sensors 15, no. 8: 19912-19924. https://doi.org/10.3390/s150819912
APA StyleMorales, J. M., Skipwith, C. G., & Clark, H. A. (2015). Quadruplex Integrated DNA (QuID) Nanosensors for Monitoring Dopamine. Sensors, 15(8), 19912-19924. https://doi.org/10.3390/s150819912