Study of Interdigitated Electrode Arrays Using Experiments and Finite Element Models for the Evaluation of Sterilization Processes
Abstract
:1. Introduction
2. Experimental Section
2.1. Sensor Design and Fabrication
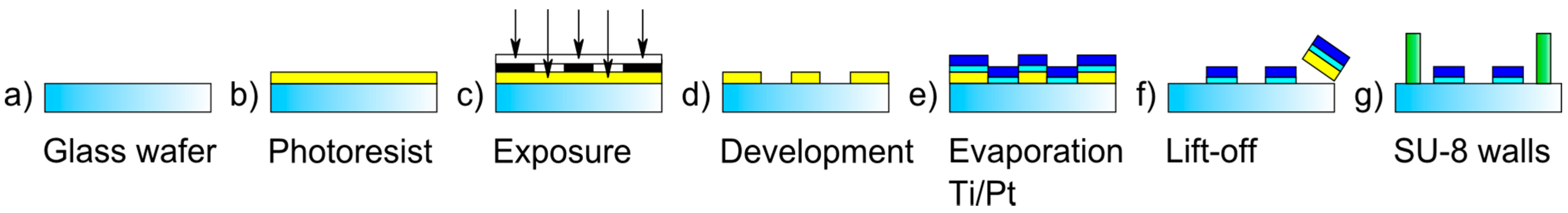
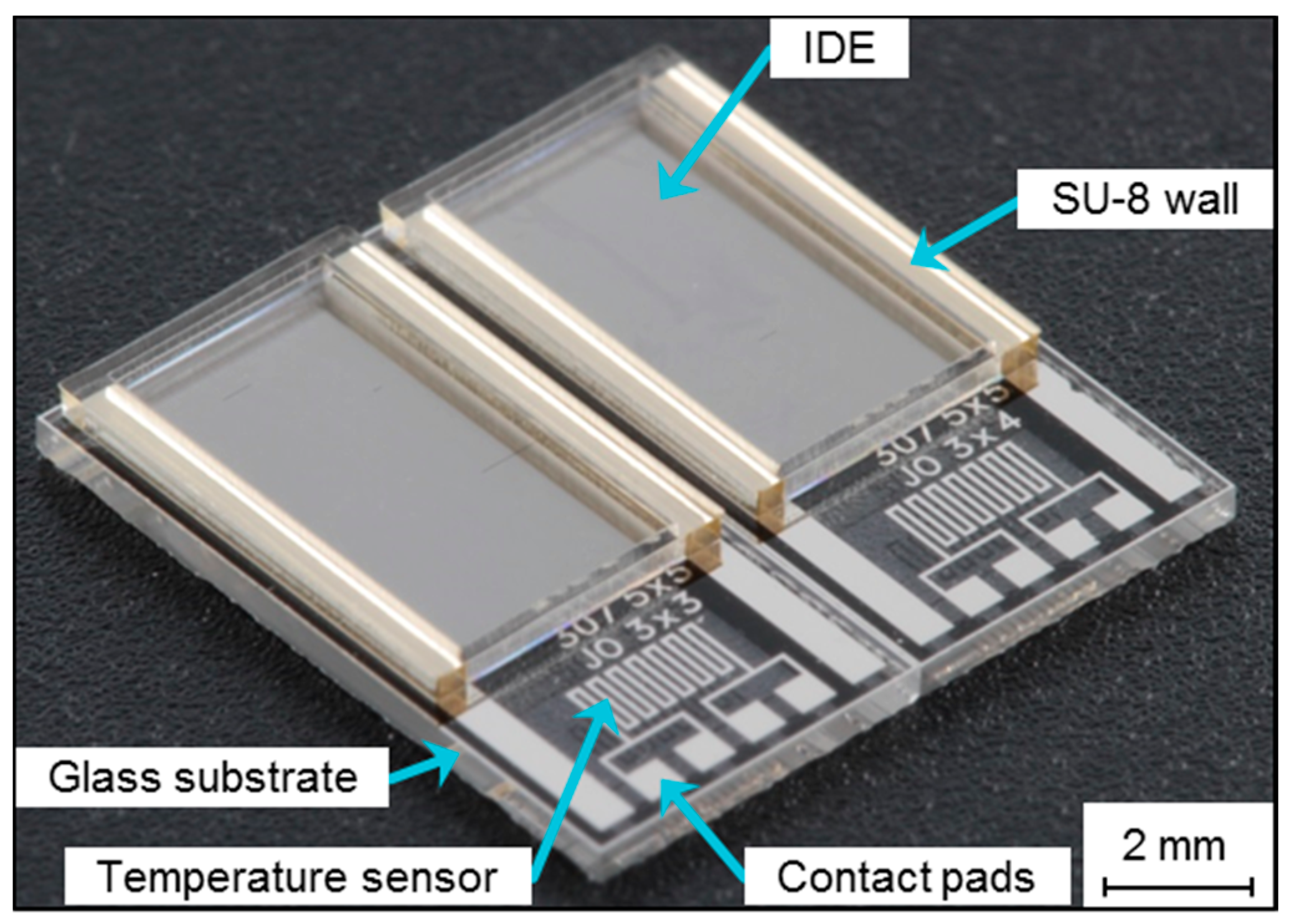
2.2. Impedimetric Analysis
2.3. Sterilization Process
3. Sensor Verification and Validation
3.1. Sensor Verification
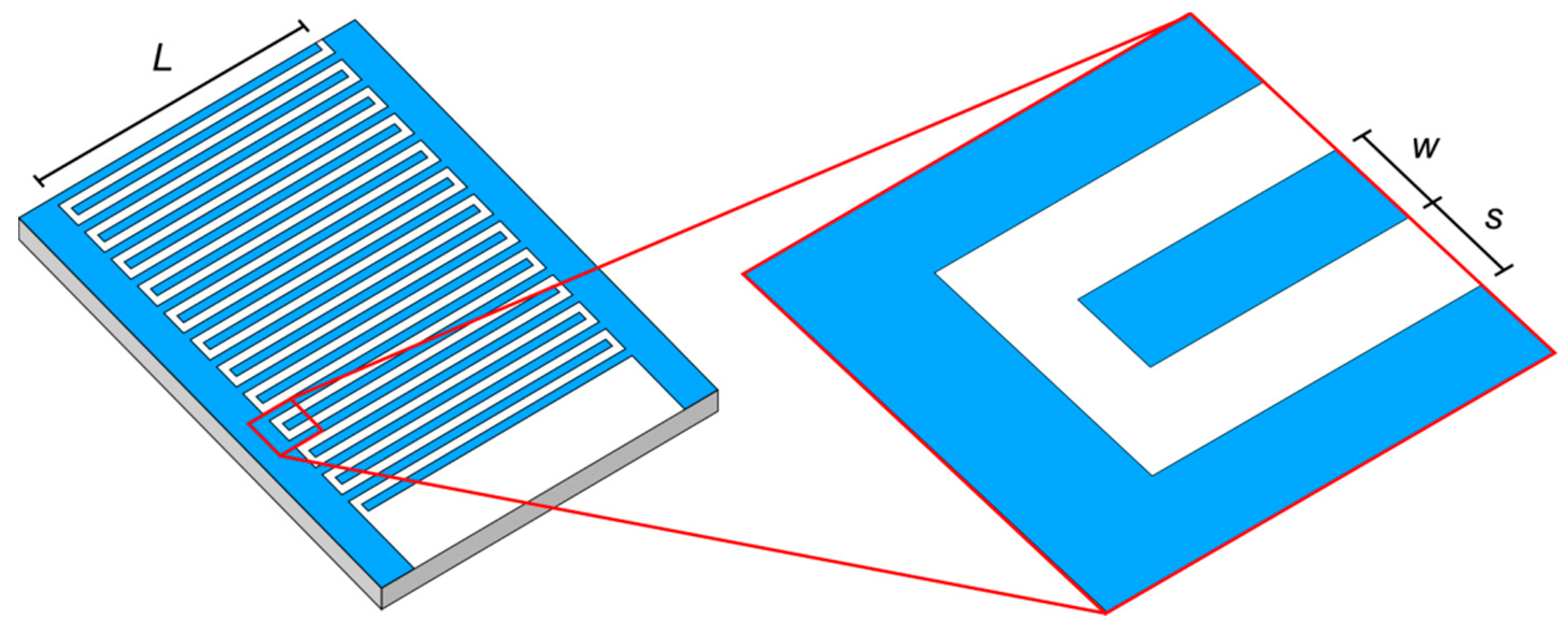
3.2. Sensor Validation
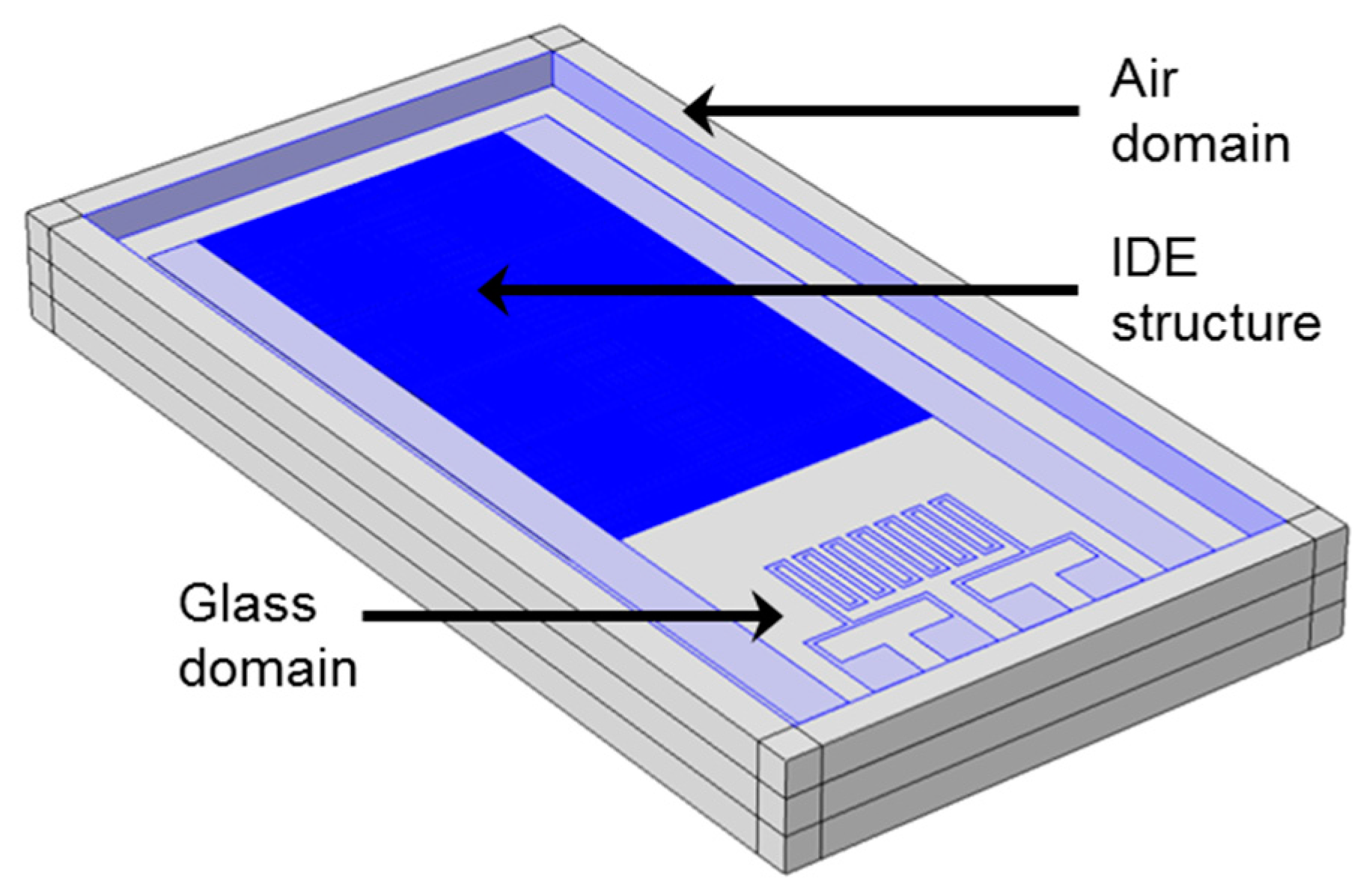
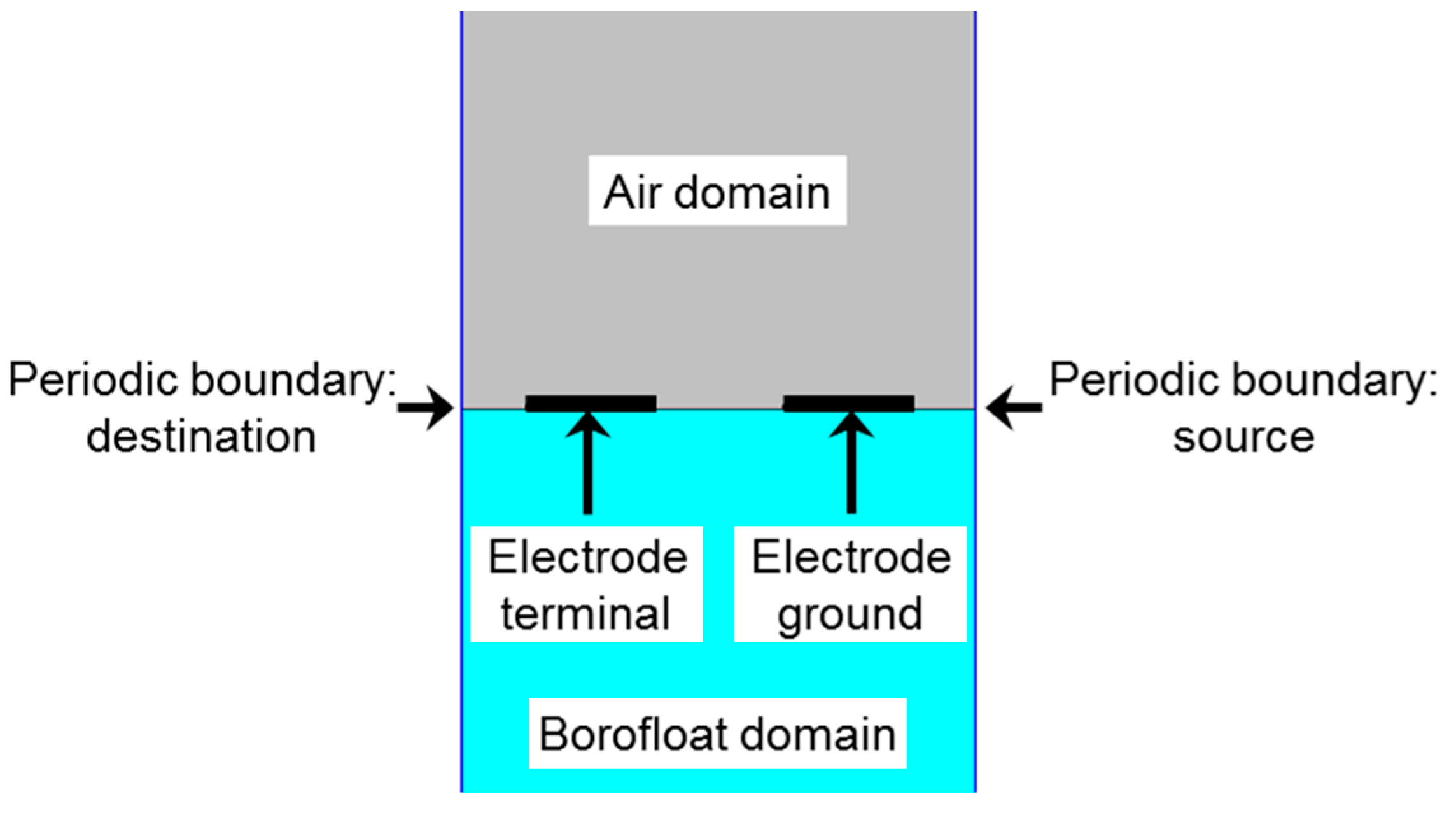
4. Results and Discussion
4.1. Impedimetric Characterization
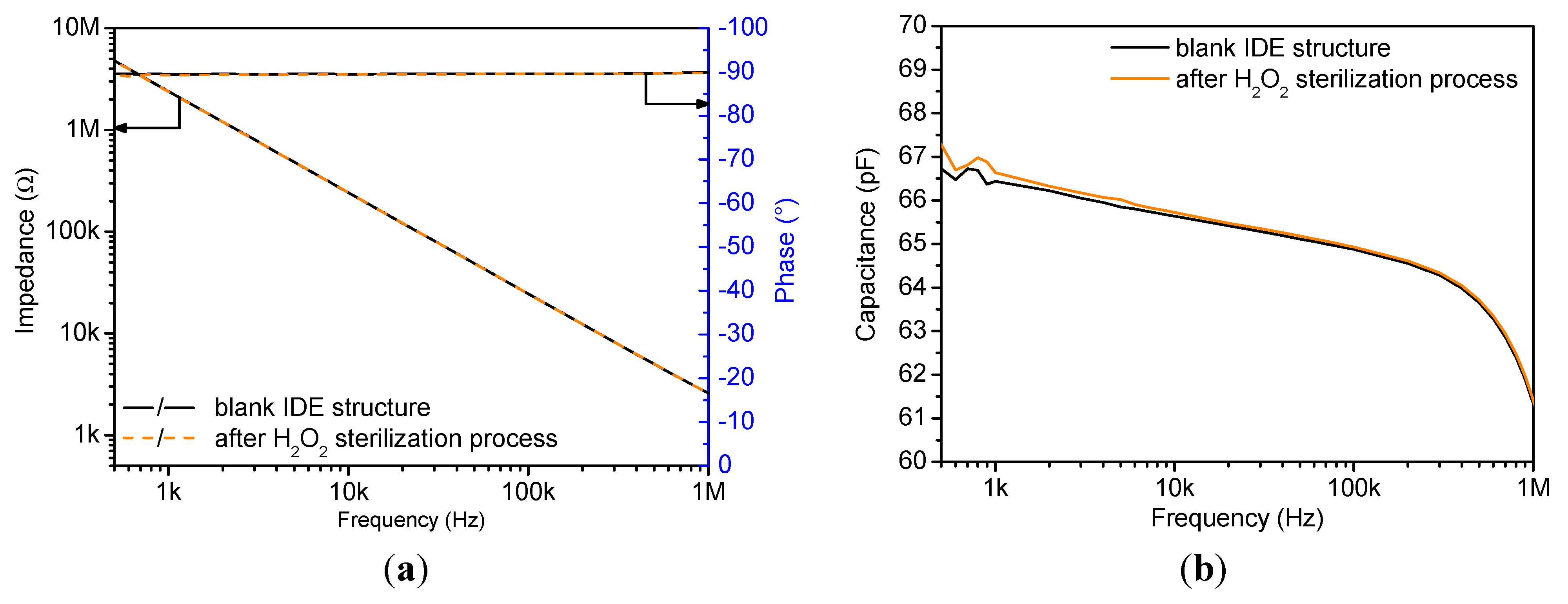
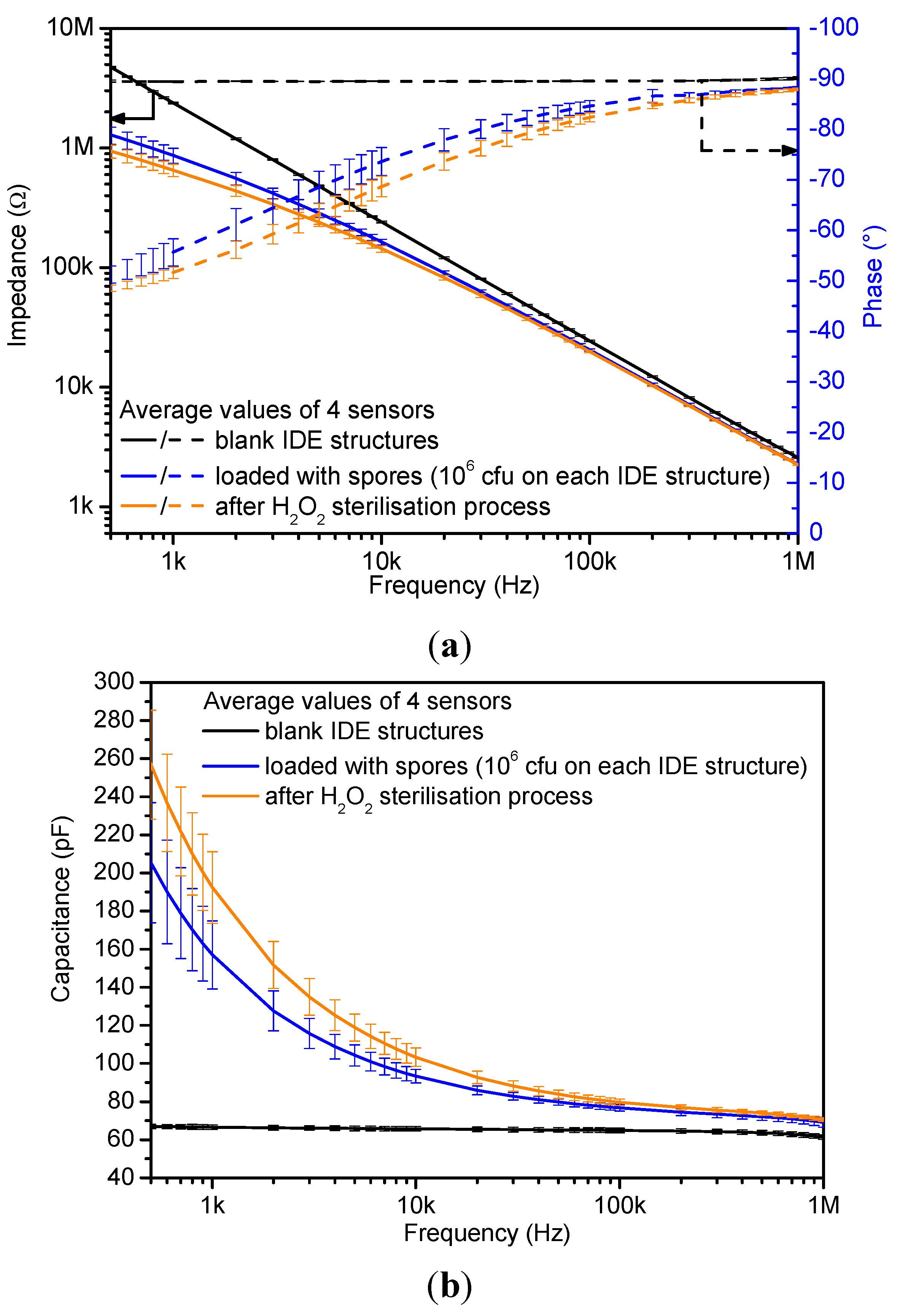
| Sensor State | C (f = 3 kHz) (pF) | Relative Error (%) |
|---|---|---|
| Blank sensors | 66.1 | 2.6 |
| Loaded with spores | 115.7 | 7.2 |
| After sterilization | 135.0 | 4.3 |
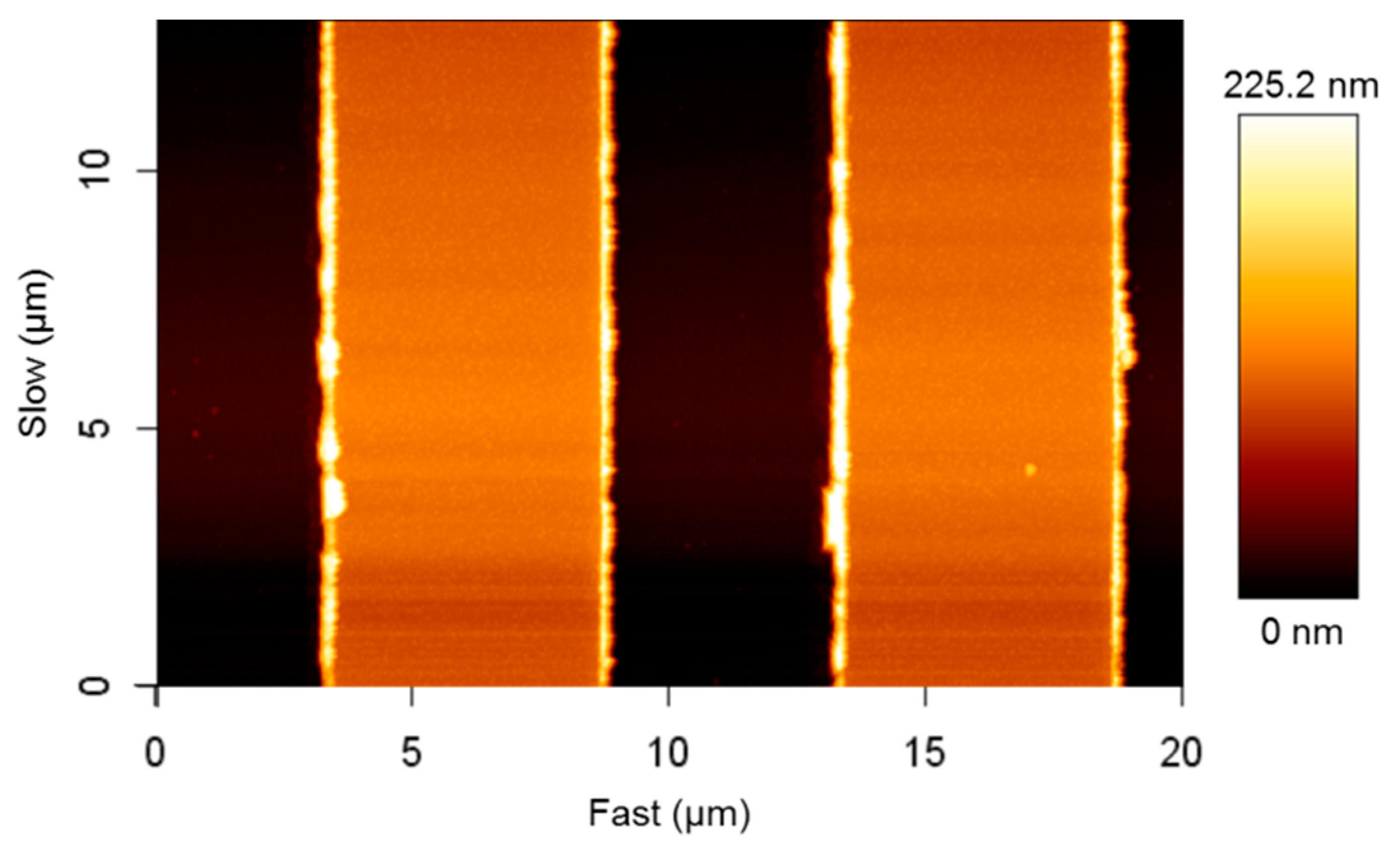
4.2. Verification and Validation of the IDE Sensor
| IDE Geometry | Results | |||||
|---|---|---|---|---|---|---|
| Experimental | Analytical (Equation (1)) | FEM Model | ||||
| w (μm) | s (μm) | t (nm) | Cexp (pF) | Canal (pF) | Cideal (pF) | CAFM (pF) |
| 5 | 5 | 110 | - | 50.09 | 50.07 | - |
| 6 | 4 | 110 | 66.1 | 57.98 | 57.91 | 60.05 |
| 6 | 4 | 220 | - | 58.95 | 58.57 | - |
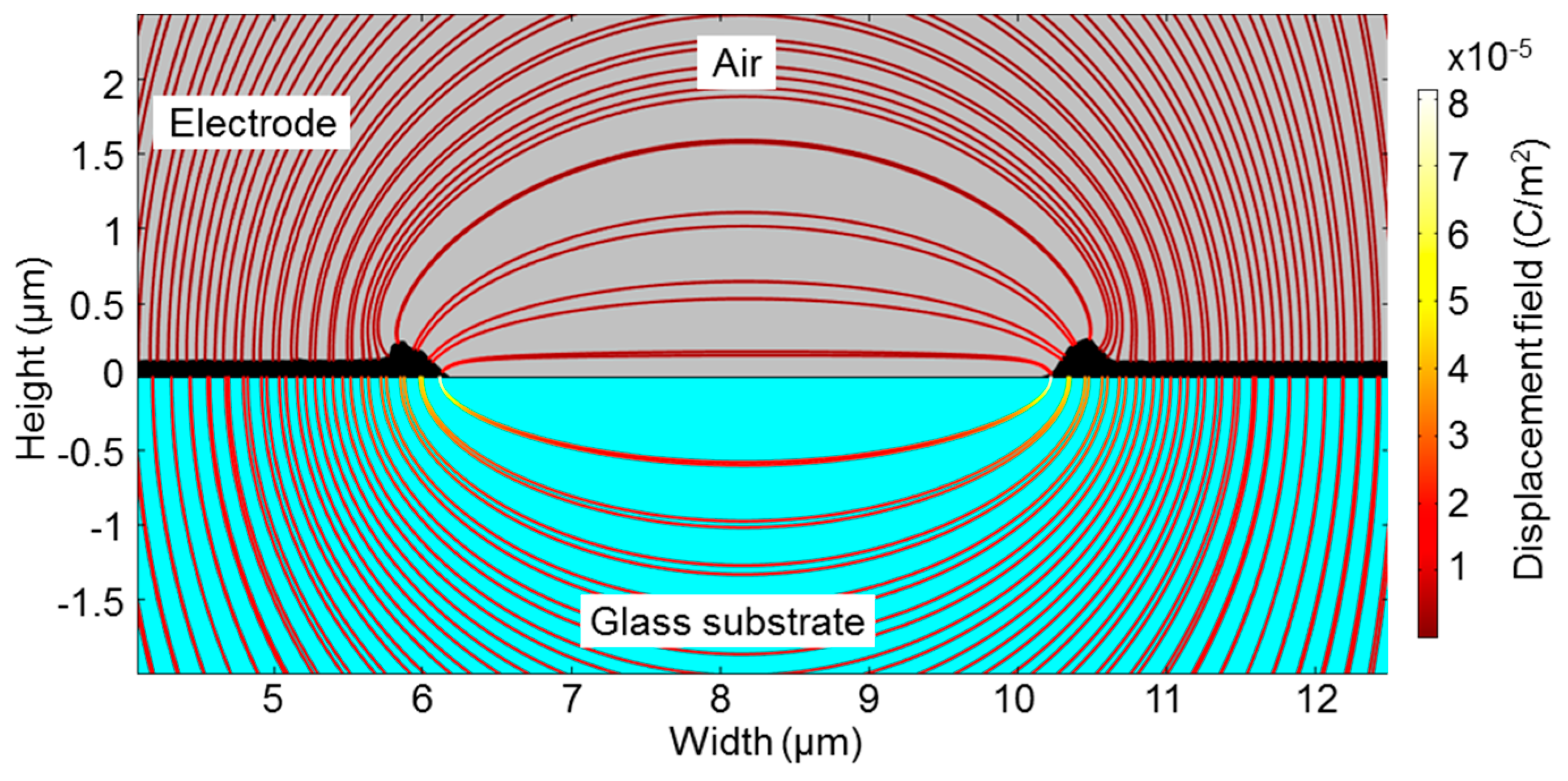
5. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.-Y. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Giguère, P.A.; Liu, I.D. Kinetics of the thermal decomposition of hydrogen peroxide vapor. Can. J. Chem. 1957, 35, 283–293. [Google Scholar] [CrossRef]
- Kirchner, P.; Oberländer, J.; Friedrich, P.; Berger, J.; Suso, H.-P.; Kupyna, A.; Keusgen, M.; Schöning, M.J. Optimization and fabrication of a calorimetric gas sensor built up on a polyimide substrate for H2O2 monitoring. Phys. Status Solidi A 2011, 208, 1235–1240. [Google Scholar] [CrossRef]
- Oberländer, J.; Kirchner, P.; Boyen, H.-G.; Schöning, M.J. Detection of hydrogen peroxide vapor by use of manganese(IV) oxide as catalyst for calorimetric gas sensors. Phys. Status Solidi A 2014, 211, 1372–1376. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Code of Federal Registrations: Title 21; Food and Drug Administration: Silver Spring, MD, USA, 2011.
- Verband Deutscher Maschinen- und Anlagenbau e.V. (VDMA). Code of Practice—Filling Machines of VDMA Hygiene Class V: Testing the Effectiveness of Packaging Sterilization Devices; Verband Deutscher Maschinen- und Anlagenbau e.V.: Frankfurt am Main, Germany, 2008; Volume 6. (In English) [Google Scholar]
- Oberländer, J.; Bromm, A.; Wendeler, L.; Iken, H.; Palomar Durán, M.; Greeff, A.; Kirchner, P.; Keusgen, M.; Schöning, M.J. Towards a biosensor to monitor the sterilization efficiency of aseptic filling machines. Phys. Status Solidi A 2015, 212, 1299–1305. [Google Scholar] [CrossRef]
- Lvovich, V.F.; Liu, C.; Smiechowski, M.F. Optimization and fabrication of planar interdigitated impedance sensors for highly resistive non-aqueous industrial fluids. Sens. Actuators B Chem. 2006, 119, 490–496. [Google Scholar] [CrossRef]
- Timmer, B.; Sparreboom, W.; Olthuis, W.; Bergveld, P.; van den Berg, A. Optimization of an electrolyte conductivity detector for measuring low ion concentrations. Lab Chip 2002, 2, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, M.C.; Mouayad, L.; Melcher, J.R. Continuum properties from interdigital electrode dielectrometry. IEEE Trans. Electr. Insul. 1988, 23, 897–917. [Google Scholar] [CrossRef]
- Olthuis, W.; Sprenkels, A.J.; Bomer, J.G.; Bergveld, P. Planar interdigitated electrolyte-conductivity sensors on an insulating substrate covered with Ta2O5. Sens. Actuators B Chem. 1997, 43, 211–216. [Google Scholar] [CrossRef]
- Kirchner, P.; Ng, Y.A.; Spelthahn, H.; Schneider, A.; Henkel, H.; Friedrich, P.; Kolstad, J.; Berger, J.; Keusgen, M.; Schöning, M.J. Gas sensor investigation based on a catalytically activated thin-film thermopile for H2O2 detection. Phys. Status Solidi A 2010, 207, 787–792. [Google Scholar] [CrossRef]
- Kirchner, P.; Oberländer, J.; Suso, H.-P.; Rysstad, G.; Keusgen, M.; Schöning, M.J. Monitoring the microbicidal effectiveness of gaseous hydrogen peroxide in sterilization processes by means of a calorimetric gas sensor. Food Control 2013, 31, 530–538. [Google Scholar] [CrossRef]
- Olthuis, W.; Streekstra, W.; Bergveld, P. Theoretical and experimental determination of cell constants of planar-interdigitated electrolyte conductivity sensors. Sens. Actuators B Chem. 1995, 24, 252–256. [Google Scholar] [CrossRef]
- Abu-Abed, A.S.; Lindquist, R.G. Capacitive interdigital sensor with inhomogeneous nematic liquid crystal film. Prog. Electromagn. Res. B 2008, 7, 75–87. [Google Scholar] [CrossRef]
- Carstensen, E.L.; Marquis, R.E.; Child, S.Z.; Bender, G.R. Dielectric properties of native and decoated spores Bacillus megaterium. J. Bacteriol. 1979, 140, 917–928. [Google Scholar] [PubMed]
- Setlow, B.; Loshon, C.; Genest, P.; Cowan, A.; Setlow, C.; Setlow, P. Mechanisms of killing spores of Bacillus subtilis by acid, alkali and ethanol. J. Appl. Microbiol. 2002, 92, 362–375. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oberländer, J.; Jildeh, Z.B.; Kirchner, P.; Wendeler, L.; Bromm, A.; Iken, H.; Wagner, P.; Keusgen, M.; Schöning, M.J. Study of Interdigitated Electrode Arrays Using Experiments and Finite Element Models for the Evaluation of Sterilization Processes. Sensors 2015, 15, 26115-26127. https://doi.org/10.3390/s151026115
Oberländer J, Jildeh ZB, Kirchner P, Wendeler L, Bromm A, Iken H, Wagner P, Keusgen M, Schöning MJ. Study of Interdigitated Electrode Arrays Using Experiments and Finite Element Models for the Evaluation of Sterilization Processes. Sensors. 2015; 15(10):26115-26127. https://doi.org/10.3390/s151026115
Chicago/Turabian StyleOberländer, Jan, Zaid B. Jildeh, Patrick Kirchner, Luisa Wendeler, Alexander Bromm, Heiko Iken, Patrick Wagner, Michael Keusgen, and Michael J. Schöning. 2015. "Study of Interdigitated Electrode Arrays Using Experiments and Finite Element Models for the Evaluation of Sterilization Processes" Sensors 15, no. 10: 26115-26127. https://doi.org/10.3390/s151026115







