The Effect of the Thickness of the Sensitive Layer on the Performance of the Accumulating NOx Sensor
Abstract
: A novel and promising method to measure low levels of NOx utilizes the accumulating sensor principle. During an integration cycle, incoming NOx molecules are stored in a sensitive layer based on an automotive lean NOx trap (LNT) material that changes its electrical resistivity proportional to the amount of stored NOx, making the sensor suitable for long-term detection of low levels of NOx. In this study, the influence of the thickness of the sensitive layer, prepared by multiple screen-printing, is investigated. All samples show good accumulating sensing properties for both NO and NO2. In accordance to a simplified model, the base resistance of the sensitive layer and the sensitivity to NOx decrease with increasing thickness. Contrarily, the sensor response time increases. The linear measurement range of all samples ends at a sensor response of about 30% resulting in an increase of the linearly detectable amount with the thickness. Hence, the variation of the thickness of the sensitive layer is a powerful tool to adapt the linear measurement range (proportional to the thickness) as well as the sensitivity (proportional to the inverse thickness) to the application requirements. Calculations combining the sensor model with the measurement results indicate that for operation in the linear range, about 3% of the LNT material is converted to nitrate.1. Introduction
Tightened emission and safety regulations have increased the demand for sensitive devices to detect reliably even low levels of NO and NO2 (NOx) over a long measurement period [1–3] (e.g., summarized as <100 ppm NOx in the automotive exhaust and 0.5–5 ppm NO2 in the interior by [1]). In the field of automotive or industrial exhausts or of air quality management, the interest is on the accurate determination of mean values (e.g., 1-h value for air quality monitoring [4]) or total amounts (e.g., cumulated vehicle emissions in g/km for on-board diagnostics [5,6]) rather than on the curve of the actual instantaneous concentration over time. However, most today's gas sensors measure time-continuously the actual analyte concentration [1]. Cumulated amount mean values are obtained by mathematical averaging (integration). Due to inaccuracies at low analyte levels, long sensor response times and recovery times, as well as due to drifts in the zero point level (baseline), these sensors are subject to errors in the determination of the accumulated analyte levels [2,3,7].
Alternatively the accumulating-type (or integrating-type or dosimeter-type) sensor measures directly the total amounts of analyte gases over a time interval. This novel principle is related to passive samplers being used to determine the cumulated analyte level in two steps. There, over a longer period (e.g., a month) analyte molecules from the ambience are collected in a diffusion controlled process on a sorption material, followed by a quantitative analysis with laboratory gas analysis methods [8,9]. Accumulating-type gas sensors presented here are also based on a sensitive layer that collects analyte molecules over a longer period, but in contrast to passive samplers, the analyte level is evaluated instantaneously and time-continuously by electrical means. While chemically sorbing the analyte molecules, the electrical properties of the sensitive layer, e.g., the resistivity, change with the amount of analyte stored. Like in passive samplers, the sorbent material needs to be regenerated periodically as saturation effects occur. The sensor signal of the accumulating NOx sensor correlates directly with the total amount of NOx (being the sum of NO and NO2), whereas the curve of the actual concentration can be obtained from the timely derivative of the sensor signal [10]. By collecting analyte molecules from the gas stream, even small levels contribute to the sensor signal, enabling accurate analyte detection over an extended time interval. Another important feature is the fact that errors in the sensor's zero level are minimized since the zero-level is redefined after each regeneration step. Both response times and recovery times of the signal derivative as the measurand for the actual concentration are quite low and in the range of the gas exchange time of the setup (<7 s [10]). The fast sensing characteristics of the accumulating sensor originate from the fact that the change (i.e., the time-based derivative) in the conductivity during NOx exposure correlates with the NOx concentration. This is in strong contrast to common gas sensors, in which the equilibrium conductivity of the sensor signal is of interest. In the following, the effect of the thickness of the NOx storage layer on the NOx accumulating sensing properties is addressed.
2. The Accumulating Sensing Concept
The accumulating or integrating-type sensor is intended to detect directly the total amount, A, of low levels of analyte by accumulation. Generally, A can be calculated according to Equation (1) from the analyte concentration, c(t), and the flow rate, V̇(t). Both may vary with time.
In [11] two setups of the integrating NOx sensor are presented. Utilizing a special channel-type setup with a large area of sensitive material compared to the small gas volume inside the channel, all analyte molecules become sorbed and the resulting sensor signal reflects the total amount, A, even if the flow rate of the gas varies (amount detector). Contrarily, it was investigated in [11] that by exposing the sensitive layer of the planar device to a large gas volume, always a constant fraction of the analyte molecules in the gas stream is stored independently on the gas flow rate in a wide range. The sensor signal correlates then with the timely integral of the concentration, ∫c(t)dt. If the flow rate, V̇(t), remains constant, A is directly proportional to the integral of c(t), i.e., A ∝ ∫c(t)dt and the total amount can be determined properly with the planar setup while the signal derivative reflects the curve of c(t) [10].
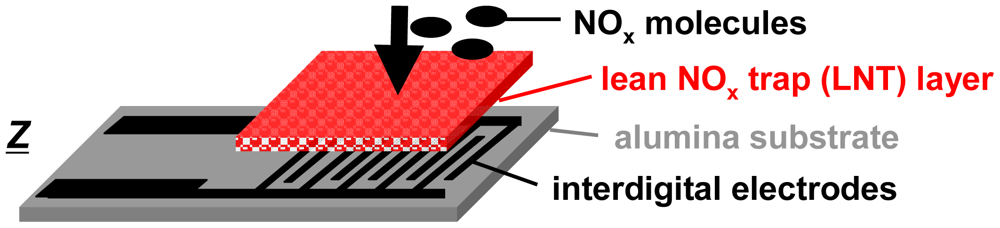
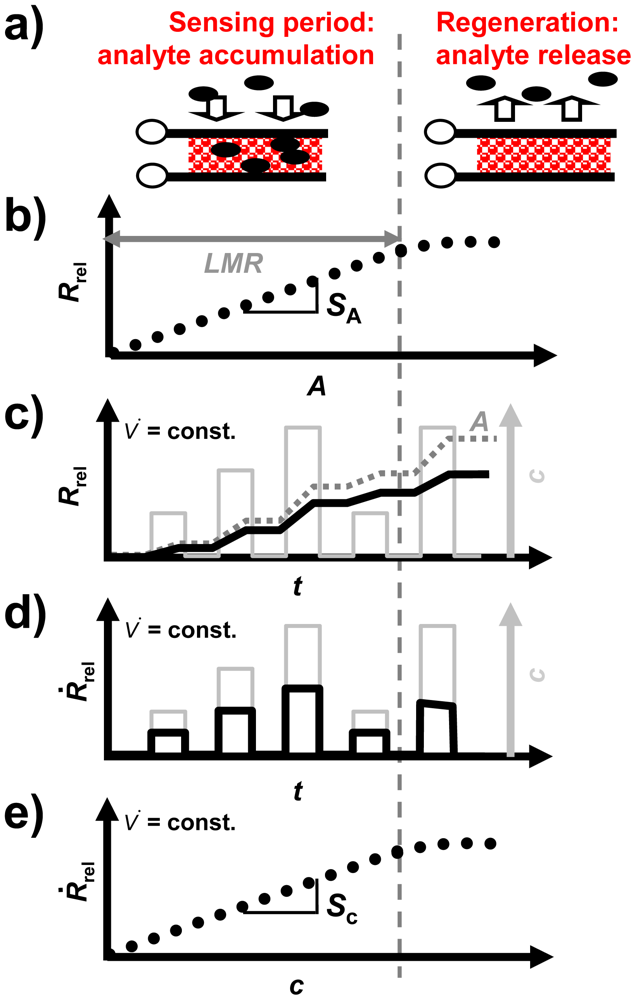
For the accumulating sensing principle sensitive layers of storage materials can be applied. They are able to sorb analyte molecules (e.g., by chemisorption or by a chemical reaction) and thereby they change their electrical properties. As illustrated in Figure 1, the accumulating NOx sensor in the planar setup (concentration integrator) consists of a lean NOx trap (LNT) layer deposited on an alumina substrate which is equipped with interdigital electrodes (IDEs). It is well known that LNT materials lower their resistivity when transformed from carbonates to nitrates upon NOx storage [12–15]. Since the accessible sorption sites in the storage material are becoming occupied with proceeding sorption, saturation effects limit the accumulating properties and a regeneration of the sorption sites is required to recover the original sorption capacity. Therefore, as illustrated in Figure 2(a), in the operation of accumulating sensors the sensing interval during which the sensitive layer (shaded area) accumulates the analyte molecules (black points) from the gas stream alternates with the regeneration interval. The absolute value of the relative resistance change, Rrel, calculated by Equation (2) with R0 being the base resistance in the unloaded state, is displayed as the accumulating NOx sensor signal:
Rrel depends on the loading level which, for low loading states, is proportional to the amount of NOx in the gas phase, A, as illustrated in Figure 2(b). The sensor signal on the time scale differs from those of conventional gas sensors due to the stepwise NOx accumulation. As shown in Figure 2(c), Rrel (black line) increases in the presence of NOx, whereas it remains constant in the NOx absence (holding ability)—Rrel is proportional to A (dark grey line, dotted). In the case of a constant flow rate, V̇(t), the timely increase of the sensor signal is proportional to the NOx concentration c (light grey line, right axis). This proportionality enables to determine the curve of the instantaneous concentration using the timely derivative, dRrel/dt. In the following, dRrel/dt will be denoted as Ṙrel:
Ṙrel is illustrated as a function of time in Figure 2(d). As depicted in Figure 2(e), there is a linear correlation between Ṙrel and c. Hence, to compare the performance of the accumulating sensor with conventional gas sensors, the characteristics of Ṙrel are appropriate. The sensitivity is commonly defined as the slope of the characteristic line which is the correlation between the sensor signal and the measurand [16]. Hence, for the accumulating type NOx sensor, which is intended to determine A, the amount sensitivity, SA, (Figure 2(b)) can be calculated from the correlation between Rrel and A according to Equation (4) resulting in the unit %/μL. Additionally, in the case of a constant flow rate, the proportionality between Ṙrel and c, as shown in Figure 2(e), allows to calculate the concentration sensitivity, Sc, according to Equation (5) which is analogous to the sensitivity known of conventional gas sensors:
If one transforms Equation (4) using Equation (1), one obtains:
If Sc = const, i.e., Ṙrel ∝ c, then dṘrel/dc can be replaced by Ṙrel/c. Then the relation between Sc and SA yields:
In other words, the amount-related sensitivity, SA, and the “classical” sensitivity with respect to the concentration, Sc, are proportional to each other, as long as the gas flow remains constant. As shown in Figure 2(b,e), as soon as saturation effects occur and the linear measurement range, LMR, is exceeded, SA and Sc decrease, and the slope of the signal no longer reflects the concentration (Figure 2(b–e))—the accumulating sensor demands regeneration.
LNT materials are known from automotive NOx storage and reduction catalysts to reduce the NOx emissions in the exhaust [17–19]. Since NOx molecules can be stored in a lean gas atmosphere, whereas they are released and reduced in rich gas compositions, the engine operation cycles between long lean and short rich intervals to ensure low emissions [5,17–19]. LNTs usually consist of alkaline (earth-) oxides or carbonates (e.g., BaCO3 or K2CO3) as storage components, finely dispersed precious metal particles to catalyze oxidation and reduction reactions, and support oxides like Al2O3 to provide high surface areas for the catalytic processes [19,20]. The storage mechanism is based on the conversion of alkaline (earth-) carbonates MCO3 or oxides to nitrates M(NO3)2 upon NO2 exposure according to Equation (8). NO needs to be oxidized to NO2 on the catalytic active particles prior to the nitrate formation according to Equation (9) [17,18,20]:
We recently demonstrated the integrating or accumulating NOx sensing principle under various gas conditions (base gas composition, temperature) [10,21,22]. Additionally, it was found that the sensitivity to NO is the same as that to NO2, thus allowing for total NOx detection, and that the sensor is suitable for long-term detection of low levels of NOx [10]. O2 and CO2 concentration variations were found to be negligible in a wide range in lean gas containing O2, CO2 and H2O [10,21].
In order to understand further how NOx storage occurs in the catalyst material, the influence of the thickness of the sensitive layer on the performance of the accumulating NOx sensor is the focus of this study. This is motivated by the idea that the number of accessible storage sites and hence the fraction of sites occupied by NOx upon NOx exposure should depend on the thickness of the LNT coating if the LNT coated area remains the same. The obtained results may even be of interest for LNT catalyst research and may help to elucidate more details about the storage reactions.
3. Expected Influence of the Thickness of the Sensitive Layer—Some Pre-Considerations
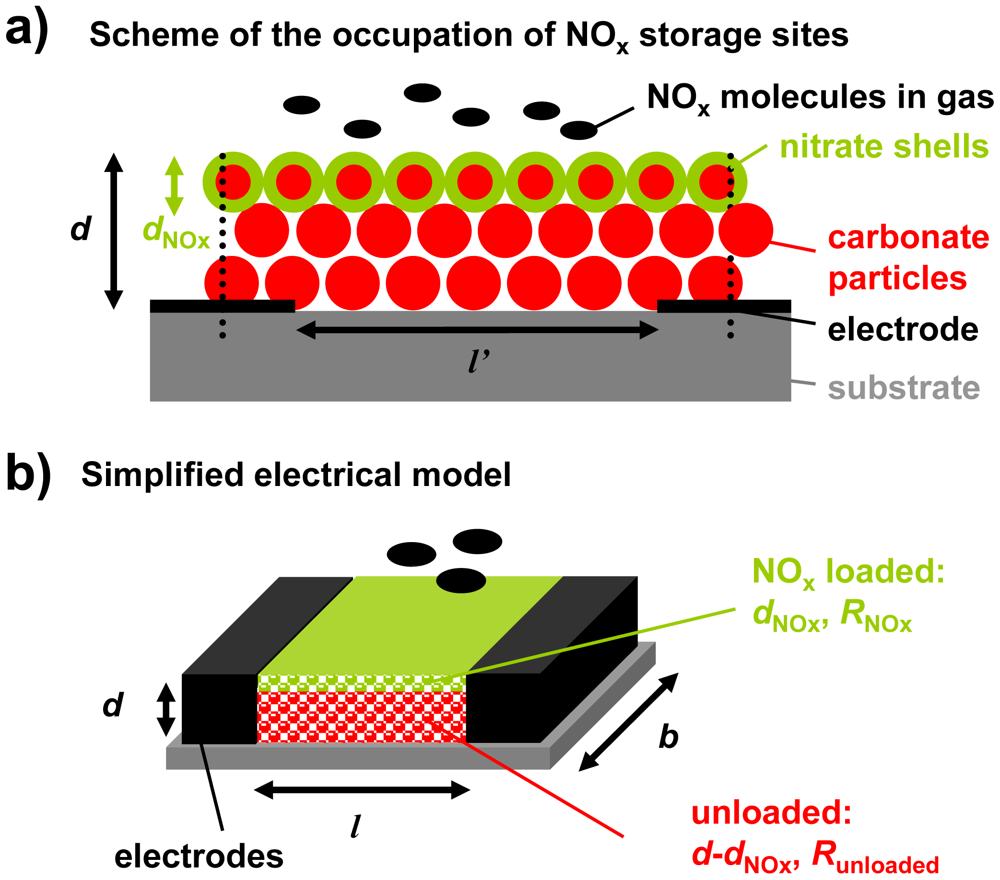
The storage capacity and hence the number of accessible storage sites control the analyte accumulation properties of LNT catalysts. Hence, it is expected that the thickness of the carbonate layer affects the accumulating sensing properties. As described in Figure 3, a simplified model of the configuration containing the storage material (red) and the interdigital electrodes (black) was developed for a rough estimation of this influence.
From catalyst research it has been known that NOx storage occurs mainly at the surface of the LNT material that is in contact with the gas phase, resulting in less than 40% utilization of the available storage sites upon saturation [23–25]. This means that even in the highly loaded state, only a fraction of the storage sites are involved in the storage process. Additionally, nitrate formation is accompanied by a shrinking of the pore structure since the nitrates have a higher molar volume than the corresponding carbonates [26]. In the case of K2CO3, the volume theoretically increases by almost 70% upon storing NOx. The decreased diameter of the pores lowers the diffusion of the NOx molecules into the carbonate particles and the NOx loaded zones can be modeled as dense nitrate shells at the surface of the LNT particles (shrinking core type model [26,27]). Since the accumulating NOx sensor is exposed to small NOx concentrations and is only operated in the low loading state, nitrate shells are expected to form mainly at the upper surface area of the LNT layer, which is in close contact to the analyte gas phase. Hence, the occupation of NOx storage sites in the sensitive layer by NOx molecules can be illustrated as shown in Figure 3(a). Thereby, the thickness of the NOx loaded area, dNOx, increases with progressive NOx exposure. If the distance between the electrodes, l′, is much larger than the film thickness, d, the electrical field lines between the electrode fingers are parallel and homogenously distributed. Almost the entire flux is inside the material [28]. Hence, in a simplified model of the sensor setup, the LNT layer can be described as a resistive material in between two parallel electrodes with the distance l (Figure 3(b)). The term l is related to the distance of the planar electrodes of the IDEs, l′. The relation between l and l′ can be calculated which has even been experimentally proven in [29].
Applying this simplified model of Figure 3(b) for the case of the regenerated state (dNOx = 0), the base resistance of the accumulating NOx sensor in the unloaded state, R0, can be calculated from the geometry and the resistivity of the carbonate material, ρ0, by Equation (10). Therefore, it is expected that R0 correlates with the inverse thickness, 1/d:
Upon exposure to NOx, it is assumed that surface nitrate is formed. Hence, the corresponding simplified model in the partly loaded state contains a thin nitrate film with the resistance RNOx and the thickness dNOx on top of the remaining unloaded material with the resistance Runloaded, as shown in Figure 3(b). The resulting resistance of the sensitive layer, R, can be calculated as a parallel circuit of both fractions (RNOx‖Runloaded). The sensor signal Rrel can then be calculated from the sensor geometry, the resistivity of the sensitive material in the unloaded state, ρ0, and the resistivity of the NOx loaded material, ρNOx, according to Equation (11):
Since A is independent of d and the same amount of NOx is expected to result in the same thickness of the formed NOx loaded layer, Equations (4) and (12) lead to Equation (13) and the amount-sensitivity, SA, should correlate with 1/d as well:
As the resistivity decreases by at least one order upon saturation in NOx, (ρ0/ρNOx−1) ≥ 10 and Equation (13) can be simplified to Equation (14):
Since SA ∝ Sc for constant gas flows, even the classical concentration-related sensitivity, Sc, is expected to depend on 1/d and to increase the thinner the sensing layers are.
This simplified model points out that the thickness of the sensitive layer might be an effective tool to vary the sensing properties (especially the sensitivity) of the accumulating NOx sensor. In the following study, the model was validated by exposing samples with various thicknesses to NOx containing gas flows and monitoring the sensing performance.
4. Experimental
Samples with coatings of different thicknesses were prepared and exposed to various gas compositions containing NO, NO2 or total NOx. As illustrated in Figure 1, the accumulating NOx sensor consists of an LNT layer (potassium-based LNT material provided by Johnson Matthey, composition details given in [30]) deposited on platinum interdigital electrodes (IDEs) with an area of 5 × 6 mm2 and an electrode width and spacing of 100 μm on an alumina substrate with a purity of 96%. After drying and milling the catalyst powder, a screen-printable paste was made by adding organic additives (KD 2721, Zschimmer & Schwarz). To obtain samples with sensitive layers in various thicknesses, the IDE area was screen-printed multiple times with the LNT-paste with intermediate drying periods. After firing at 650 °C to remove organic additives, the sensing properties of the samples were analyzed at 380 °C in a sensor test bench. Thereby, lean measurements periods and rich desorption periods were periodically applied. The gas flow was kept constant at V̇(t) = 2 L/min. The base lean gas consisted of 10% O2, 5% CO2, and 50% N2 humidified (by a water bubbler at room temperature) in N2, whereas the rich gas for regeneration contained 1.5% H2, 5% CO2, and 50% N2 humidified in N2. Different NOx gas compositions were admixed. The NO and NO2 concentrations were monitored by a chemiluminescence detector downstream of the sensor sample. The complex impedances of the sensitive devices were measured in the frequency range from 0.1 Hz to 20 MHz. The electrical characteristics of the bulk material can be described by an R‖C parallel equivalent circuit. In time-continuous measurements, R was calculated from the impedance taken at 1 kHz applying the R‖C model. The thicknesses of the sensitive layers were estimated using SEM micrographs.
5. Results and Discussion
5.1. Thickness Determination from SEM Analysis
SEM images from the cross sections of the sensor samples printed multiple times with the LNT paste and an illustration for clarification reasons are shown in Figure 4. The microstructure of the sensitive layers is dominated by loose grains of different diameters. The thickness increased with each printing step from about 30 μm (printed only once) to 150 μm (printed five times). The samples printed four and five times had almost the same thickness. This might be due to a densification of the LNT material with successive printing as it is well known when printing porous films or due to a erroneous thickness determination as a result of the increased roughness. In general, the surface is very rough, with an increasing roughness in the case of thicker coatings lowering the accuracy of the thickness evaluation.
5.2. Base Resistance as a Function of the Thickness
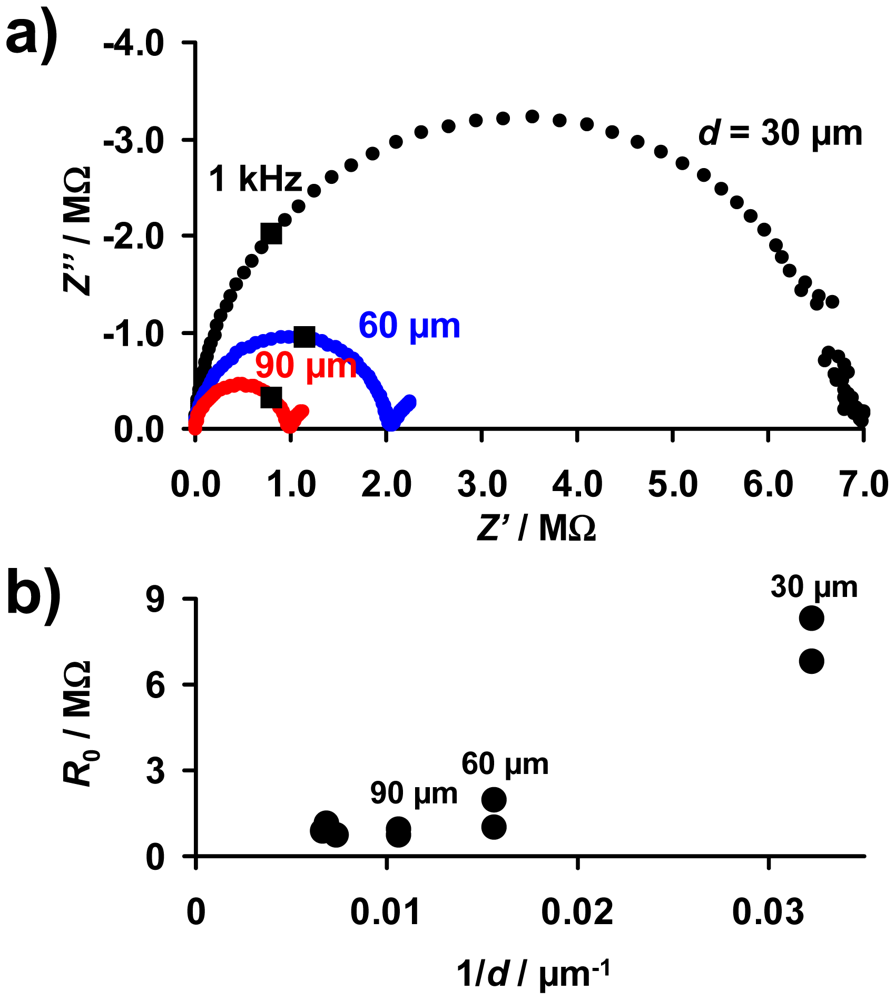
As described above, the base resistance of the sensitive layer in the unloaded state, R0, is expected to be proportional to 1/d (Equation (10)). For an electrical characterization of the sensitive layer, complex impedance plots of the samples with sensitive LNT layers of 30 to 150 μm were taken. The electrical properties of all investigated sensor samples between 10 Hz and 20 MHz can be described by an R‖C equivalent circuit. Figure 5(a) shows exemplarily Nyquist plots of samples with layers of 30, 60, and 90 μm. The data points measured at 1 kHz are marked. Fitting the spectra by an R‖C model, the values of R0 were obtained and are plotted as a function of 1/d in Figure 5(b). R0 increases with decreasing thickness, although no exact 1/d-dependency occurs. The resistances of the samples with the thinnest layers (30 μm) are especially high, resulting in deviations from the expected 1/d-behavior (Equation (10)). This may be caused by the loose packing of the catalyst particles in the coating and by inaccuracies in the thickness estimation due to the film unevenness. Furthermore, the simple model depicted in Figure 3(a) that leads to Equation (10) is only valid if the layers are by far thinner than the distance between the IDE fingers (d ≪ l′). This was confirmed in [28] by modeling the electrical flux lines in a system of a highly resistive substrate, metallic IDEs, and a resistive sensitive layer covering the IDEs. Since the distance between the electrodes of the applied IDE samples is 100 μm, it is assumed that in samples with coating of 100 μm and above there are less flux lines in the outer LNT material. Therefore, the outer parts of the LNT layer do not or only slightly contribute to the overall resistance. As a result, these thicker films may no longer exhibit the R0 ∝ 1/d behavior.
5.3. Direct Accumulative Amount Detection
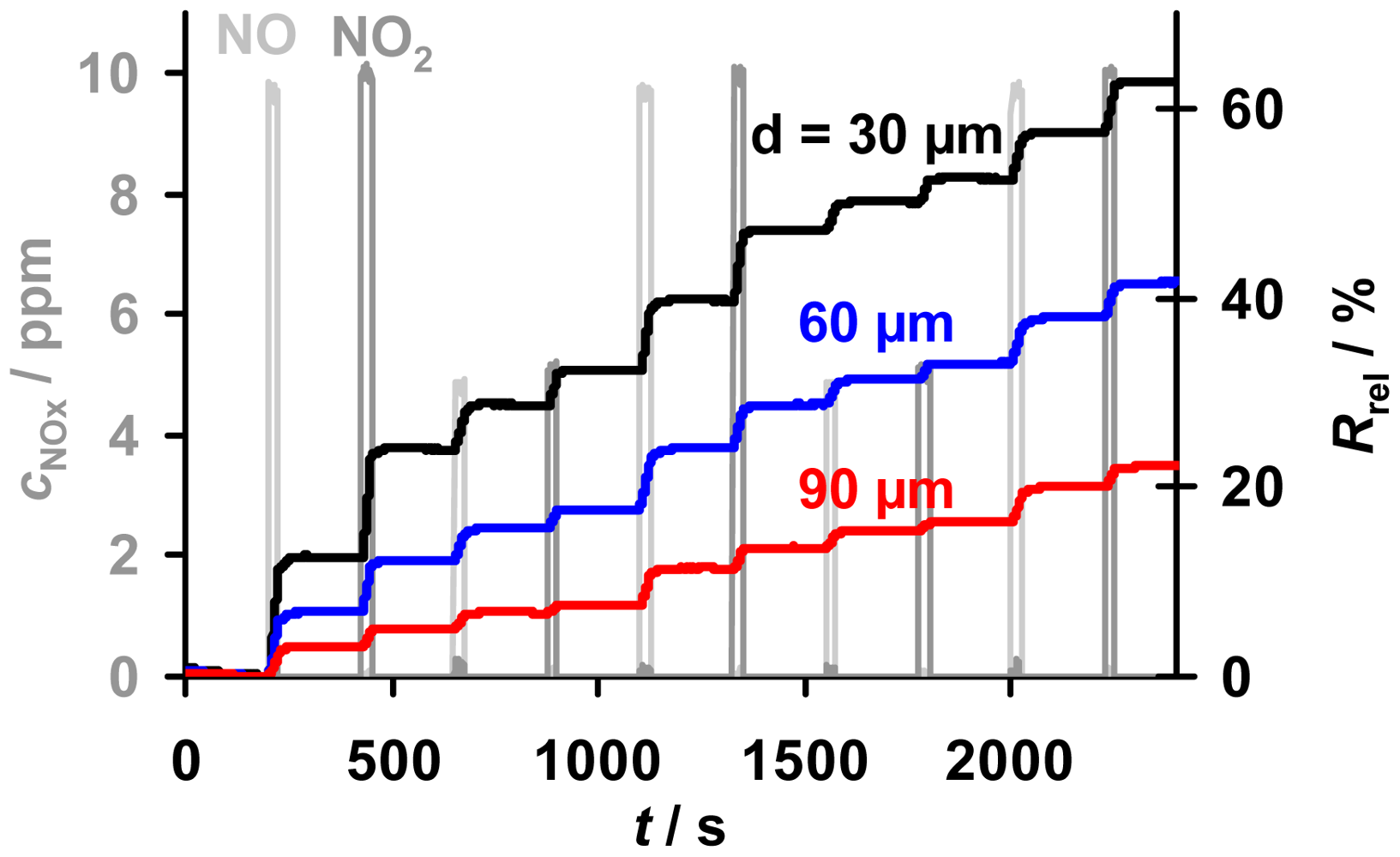
A cyclic test program to investigate the accumulating sensing properties of the samples with various thicknesses in low levels of NOx (here 10 ppm and less), especially the holding capability in the absence of NOx, is given in Figure 6. Also shown is a comparison of the sensor responses, Rrel, towards NO and NO2. The samples with layers from 30 to 90 μm were exposed to alternating NO and NO2 steps of 25 s with concentrations of 5 and 10 ppm interrupted by NOx pauses of 200 s. Due to the lower resistivity of the nitrate compared to the carbonate form of the storage material, the resistance decreased during NOx loading, yielding an increase in Rrel [10,13]. For all samples in Figure 6, Rrel increases linearly at constant NOx concentration, with an almost equal response to NO and NO2, while Rrel remains constant in 0 ppm NOx. It is evident that the sensors are less sensitive to NOx the thicker the storage layers are, albeit the samples are exposed to the same NOx profile. The constancy of Rrel during the 200 s intervals without NOx reveals that all sensitive layers are able to keep the stepwise accumulated NOx molecules - even in the absence of NOx. This indicates that the storage capacities of the samples with various thicknesses are not nearing saturation and that the formed nitrates are highly stable in the applied conditions enabling accumulating NOx sensing. Hence, all applied samples with various thicknesses provide accumulating NOx sensing properties.
A more detailed analysis of the dependency of the sensitivity, the linear measurement range and the sensor response time on the thickness of the sensitive layer is given in the next sections.
5.4. Concentration Detection by the Signal Derivative
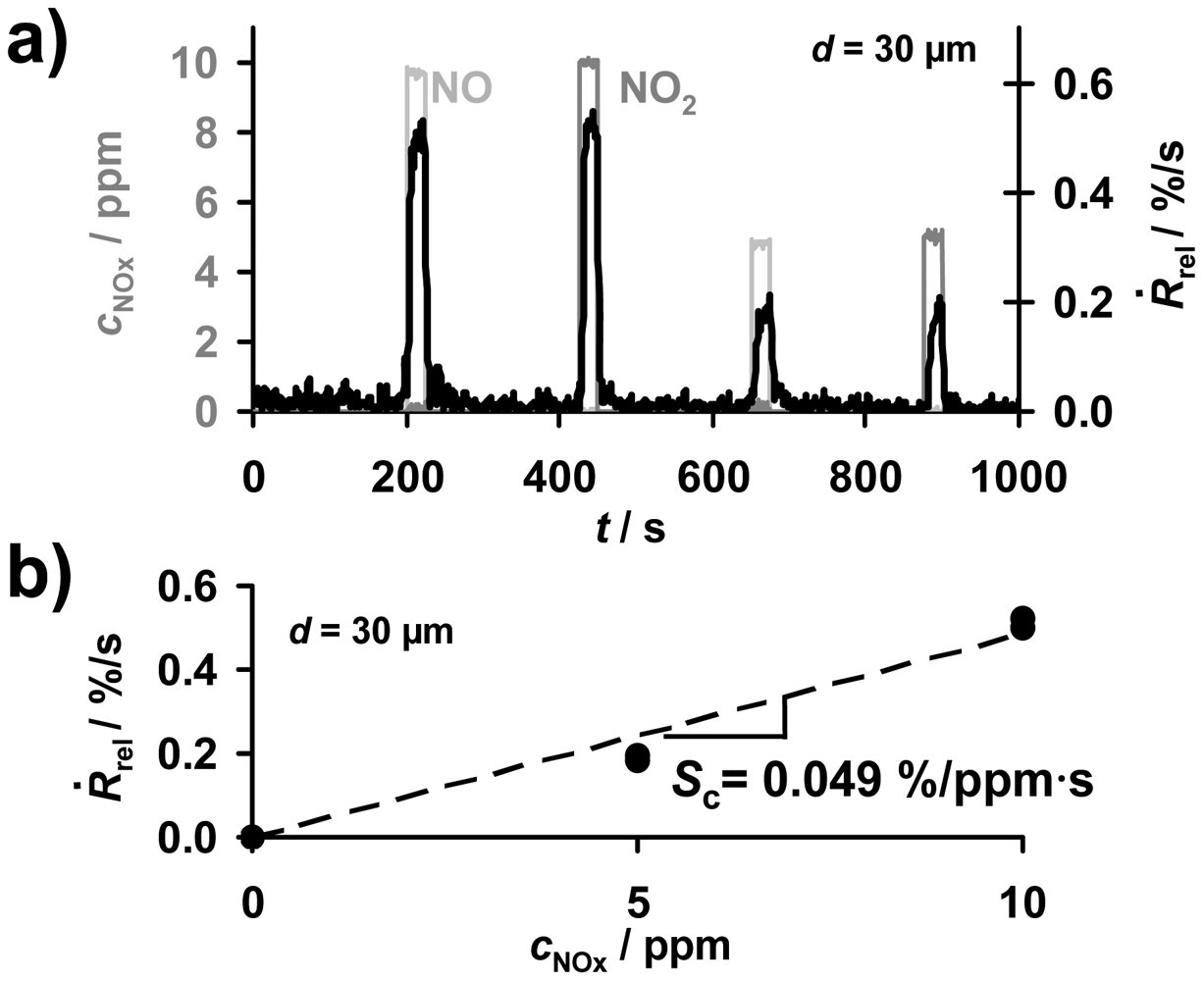
The progressive accumulation of NOx molecules in the sensitive layer in the presence of NOx enables the direct detection of the total NOx amount. As sketched in Figure 2, in the case of a constant flow rate, the time-based signal derivative, Ṙrel according to Equation (3), of the ideal accumulating sensor in the low loaded state can be applied to obtain information about the actual NOx concentration, cNOx. To compare accumulating-type sensors with conventional gas sensors, one has to analyze the concentration sensitivity Sc, i.e., the derivative Sc = dṘrel/dc according to Equation (5). Exemplarily, the corresponding data of the signal derivative Ṙrel of the sample coated with a 30 μm storage layer are plotted in Figure 7(a). During the first four NOx periods, Ṙrel reflects the curve of cNOx being the sum of cNO and cNO2 allowing for determining the actual NOx concentration. The corresponding characteristic line for the concentration detection correlating Ṙrel and cNOx is given in Figure 7(b). Ṙrel increases with an increased NOx concentration in the gas. Hence, the NOx sensitivity, Sc, of the 30 μm sample can be calculated from Equation (5) and was found to be 0.049%/ppm·s. This means that the resistance decreases by 0.049%/s upon exposure to 1 ppm NOx. The analysis of the sensor response times for the concentration detection by Ṙrel in dependency of the thickness of the sensitive layer will be discussed in detail in Sections 5 and 6. From Ṙrel in Figure 7, one obtains a sensor response and recovery time of about 5 to 8 s, which is in the range of the gas exchange time of the setup. Figure 7 demonstrates that besides detecting directly the total NOx amount, the signal derivative of the accumulating NOx sensor allows NOx concentration monitoring with a high sensitivity and a fast sensor response.
5.5. The Effect of the Thickness on the Sensitivity
According to the simplified model that is based on the assumption that a thin nitrate layer forms at the surface of the sensitive storage layer upon NOx exposure, as shown in Figure 3, and the resulting Equation (13), both sensitivities, SA and Sc, should increase with 1/d. The characteristic lines, correlating Rrel with the total NOx amount, A, extracted from the cyclic measurement data presented in Figure 6 are shown in Figure 8(a). For each sample, the data points of Rrel measured at the end of each NOx step form straight lines through the origin up to a sensor response of about 30%, independent of the NOx concentration or the type of NOx (NO or NO2). Comparing the characteristic lines for the amount detection of the samples with different thicknesses in Figure 8(a), it becomes clear that SA decreases when the sensitive layer gets thicker. To evaluate the thickness effect in detail, SA is plotted as a function of 1/d in Figure 8(b). SA increases linearly with 1/d with small deviations in the case of layers being 90 μm or thicker (1/d ≤ 0.011 μm−1).
From the concentration related sensitivity Sc ≈ 0.049%/ppm·s of the 30 μm sample determined from Figure 7(b) and the applied gas flow of V̇ = 2 L/min = 0.033 L/s, the sensitivity SA can be calculated. Applying the relationship between SA and Sc described by Equation (7) one obtains SA ≈ 1.48%/μL, which agrees with the value shown in Figure 8(b) for the 30 μm sample. This good agreement verifies that validity of the correlation between SA and Sc (Equation (7)).
These results verify the simplified model as illustrated in Figure 3 and demonstrate that the thickness of the accumulating NOx storage layer can be used as an effective tool to adapt the sensor sensitivity to the application requirements.
According to Equation (14) a linear correlation between SA and 1/d with a proportionality factor of ρ0/ρNOx·d(dNOx)/dA can be expected from the simplified model. Hence, applying the slope of the correlation in Figure 8(b), dS/d(1/d), the thickness of the formed nitrate layer, dNOx, dependent on the exposed amount of NOx can be estimated. Since the resistivity of the samples was found to decrease by at least one order upon saturation in NOx (result not shown here), ρ0/ρNOx ≈ 10 is assumed as a minimal value. With the slope dS/d(1/d) being 0.43 μm/μL (dotted line in Figure 8(b) for samples <100 μm), dNOx increases by less than about 43 nm per μL NOx in the gas phase.
5.6. Variations of the Linear Measurement Range
As sketched in Figure 2, the linear measurement range, LMR, is defined as the amount of NOx that can be detected when a linear correlation between Rrel and A exists. The LMR ends when saturation effects occur and the sensitivities SA and Sc decrease. Therefore, besides the sensitivity and the sensor response time, the LMR is an important feature of the accumulating-type sensor. The effect of the sensitive layer thickness on the LMR is addressed in Figure 9. In the presence of 10 ppm NOx (consisting of 5 ppm NO and 5 ppm NO2) in a lean gas mixture, Rrel of all samples increases continuously with time (Figure 9(a)). In accordance to Equation (14), the sensitivity is higher in the case of thinner sensitive storage layers. From Figure 9(a) it seems that, independent on the sensitive layer thickness, the LMR ends at about Rrel = 30%. This results in an increase of the LMR-amount with the layer thickness as shown in Figure 9(b). The slope in Figure 9(b) indicates that 0.80 μL NOx can be detected linearly per μm LNT layer deposited on the sensor.
In Section 5.5, it was calculated that the thickness of the nitrate fraction of the sensitive layer increases by about 43 nm/μL. This value can be combined with the dependency of the LMR on d of 0.80 μL/μm from Figure 9(b). One obtains that if reaching the end of the linear measurement range upon NOx exposure 43 nm/μL·0.80 μL/μm = 34 nm nitrate is formed per μm LNT material. In other words, independent of the LNT thickness only about 3% of the sensitive material is converted to nitrate in the case of NOx storage in the linear measurement range. This estimated nitrate fraction of the sensitive layer is much less than the values reported for the storage sites utilization of LNT catalysts of maximal 20 to 40% upon saturation [23–25]. This difference indicates that the end of the linear measurement range of the accumulating NOx sensor might be limited rather by the non-linear relation between resistivity change and NOx loading than by the storage capacity of the LNT material.
5.7. Evaluation of the Sensor Response Time
With the accumulating NOx sensor the amount of NOx is detected by looking on the changes in the electrical properties and not on the equilibrium values like with conventional gas sensors. Hence, no sensor response time of Rrel can be defined in the classical way. Instead one has to apply the timely derivative, Ṙrel, which is a function of the concentration and therefore corresponds to the sensor signal of conventional gas sensors. From the measured data of the samples in 10 ppm NOx, the sensor response time of the slope Ṙrel was analyzed. Therefore, the time to reach 90% of the maximum value of Ṙrel, t90, is compared for the samples with different coating thicknesses. Figure 10 shows that t90 increases with d. While t90 of the sample with a 30 μm storage layer is about 7 s, it is in the range of 30 s in the case of the 90 μm sample. In the case of very thin coatings the sensor response is limited by the gas exchange of the test bench, which is in the range of 7 s. For very thick coatings (d ≈ l), NOx storage occurs in a region far away from the electrodes. Due to the increasingly weaker electrical field lines, NOx uptake in this region of the LNT layer is most probably not reflected properly by Rrel and Ṙrel. Additionally, it is expected that the accessibility of the storage sites is dependent on the LNT thickness as with progressive NOx loading upcoming NOx molecules need to diffuse into the catalyst material to reach unoccupied storage sites [26,27,31]. The analysis of the sensitivity and the sensor response time in Figures 8 and 10 clarifies that for a highly sensitive and fast low level detection of NOx, accumulating NOx sensors with a thin sensitive layer are preferable.
6. Conclusions
The intent of this study was to investigate the influence of the sensitive layer thickness on the sensing properties of the accumulating NOx sensor. In several NOx loading experiments it was demonstrated that the general accumulating NOx amount sensing properties seem not to be affected by the thickness of the sensitive layer in the studied range (i.e., the increase of the sensor signal in the presence of NOx due to NOx accumulation, the correlation between the slope and the NOx concentration, and the constancy of the sensor signal in the NOx absence due to the strength of sorption). The linearity of the sensor signal, Rrel, with the total NOx amount enables the detection of the actual NOx concentration by Ṙrel with all applied samples.
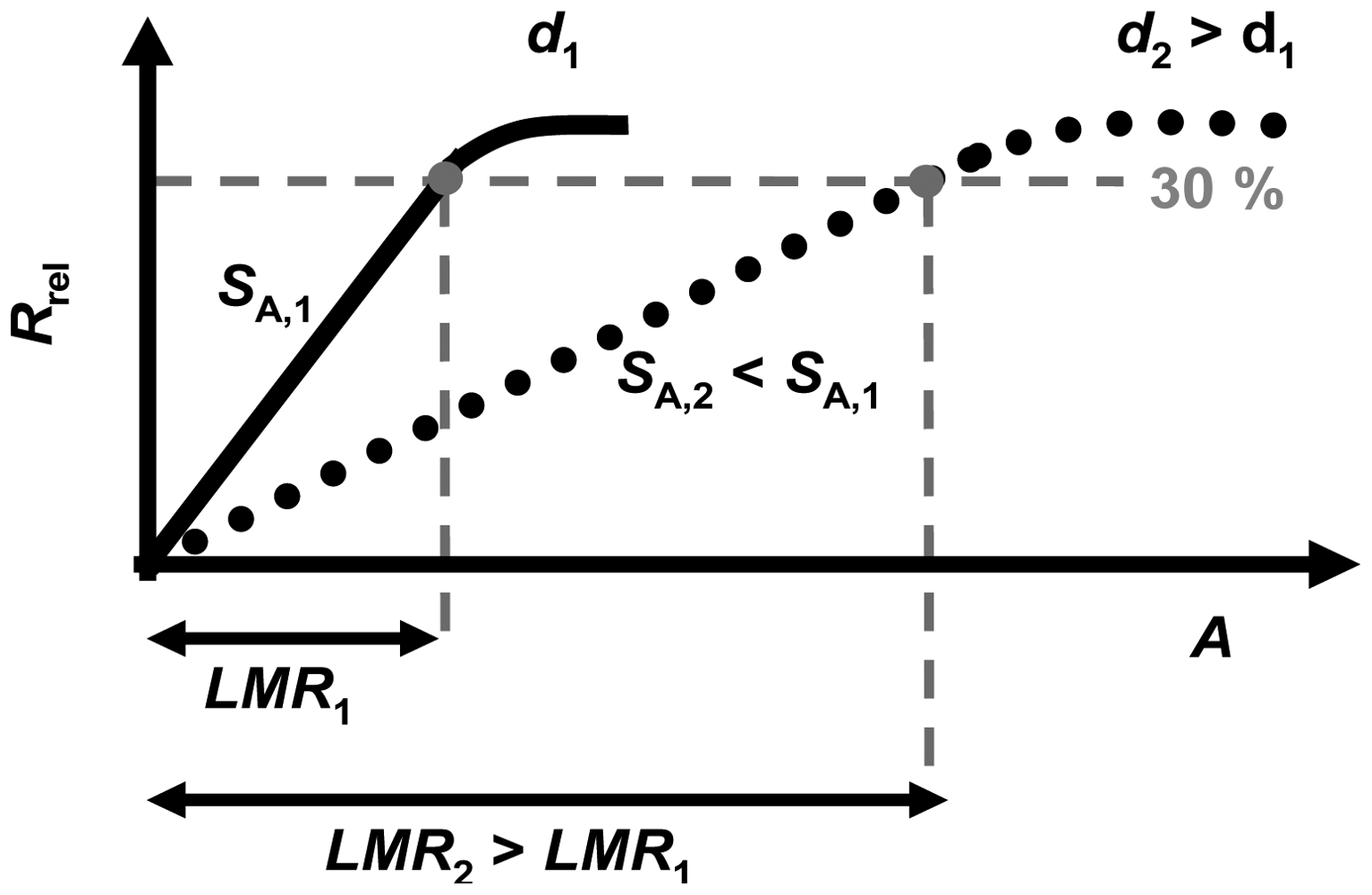
However, like the base resistance, the sensitivity to NOx is inversely proportional to the film thickness d. This agrees with a simple model concerning nitrate formation at the surface of the sensitive layer. It was demonstrated that NOx can be detected linearly until the sensor resistances reaches about 30%. This limit was found to be independent on the thickness of the sensitive layer. This controversial effect of the sensitive layer thickness on the sensitivity, SA (and also on Sc), and on the linear measurement range, LMR, is illustrated in Figure 11 for two samples with two different thicknesses, d2 (dark grey line) being higher than d1 (light grey line). While S1 is higher than S2, the resulting LMR2 is larger than LMR1. More particularly, LMR increases with d allowing for a measurement range adaption depending on the requirements of the application conditions. However, there is a trade-off between a large linear measurement range and a high sensitivity.
The presented measurement results also point out that the timely sensor response characteristic depends on the thickness of the storage material. In the case of very thin layers (30 μm) the sensor response time corresponds to the gas exchange time of the gas flow stand, whereas the sensor signal becomes slower as the thickness increases. An estimation based on the presented simplified model of the sensor setup reveals that, independently on the thickness of the LNT material only a small fraction of the sensitive layer—probably about 3%—is involved in the NOx storage process as the accumulating sensor is operated in the linear measurement range.
Acknowledgments
The authors gratefully acknowledge the LNT material preparation by Shadab Mulla, Todd H. Ballinger, Hai-Ying Chen (Johnson Matthey) and the SEM analysis by Angelika Mergner (Lehrstuhl Funktionsmaterialien). Ralf Moos thanks the German Research Foundation (DFG) for supporting this work under grant number MO 1060/15-1. This publication was funded by the German Research Foundation (DFG) and the University of Bayreuth in the funding program “Open Access Publishing”.
References
- Afzal, A.; Cioffi, N.; Sabbatini, L.; Torsi, L. NOx sensors based on semiconducting metal oxide nanostructures: Progress and perspectives. Sens. Actuators B Chem. 2012, 171–172, 25–42. [Google Scholar]
- Kim, Y.-W.; van Nieuwstadt, M. Threshold Monitoring of Urea SCR Systems; SAE Technical Paper 2006-01-3548; SAE 2006 Commercial Vehicle Engineering Congress & Exhibition: Rosemont, IL, USA; October; 2006. [Google Scholar]
- Sasaki, H.; Scholl, D.; Parsons, M.; Inagaki, H.; Shiotani, K.; Visser, J.; Zawacki, G.; Kawai, T.; Teramoto, S.; Kubinski, D. Development of an Al2O3/ZrO2-Composite High-Accuracy NOx Sensor; SAE Technical Paper 2010-01-0041; SAE 2010 World Congress & Exhibition: Detroit, MI, USA; April; 2010. [Google Scholar]
- Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on Ambient Air Quality and Cleaner Air for Europe; Official Journal of the European Union, 2008; p. L152/1.
- Alkemade, U.G.; Schumann, B. Engines and exhaust after treatment systems for future automotive applications. Solid State Ionics. 2006, 177, 2291–2296. [Google Scholar]
- Twigg, M.V. Progress and future challenges in controlling automotive exhaust gas emissions. Appl. Catal. B Environ. 2007, 70, 2–15. [Google Scholar]
- Padilla, M.; Perera, A.; Montoliu, I.; Chaudry, A.; Persaud, K.; Marco, S. Drift compensation of gas sensor array data by orthogonal signal correction. Chemometr. Intell. Lab. Syst. 2010, 100, 28–35. [Google Scholar]
- Sickles, J.E.; Grohse, P.M.; Hodson, L.L.; Salmons, C.A.; Cox, K.W.; Turner, A.R.; Estes, E.D. Development of a method for the sampling and analysis of sulfur dioxide and nitrogen dioxide from ambient air. Anal. Chem. 1990, 62, 338–346. [Google Scholar]
- Varshney, C.K.; Singh, A.P. Passive samplers for NOx monitoring: A critical review. Environmentalist 2003, 23, 127–136. [Google Scholar]
- Groβ, A.; Beulertz, G.; Marr, I.; Kubinski, D.J.; Visser, J.H.; Moos, R. Dual mode NOx sensor: Measuring both the accumulated amount and instantaneous level at low concentrations. Sensors 2012, 12, 2831–2850. [Google Scholar]
- Beulertz, G.; Groβ, A.; Moos, R.; Kubinski, D.J.; Visser, J.H. Determining the total amount of NOx in a gas stream—Advances in the accumulating gas sensor principle. Sens. Actuators B Chem. 2012. [Google Scholar] [CrossRef]
- Moos, R.; Wedemann, M.; Spörl, M.; Reiβ, S.; Fischerauer, G. Direct catalyst monitoring by electrical means: An overview on promising novel principles. Top. Catal. 2009, 52, 2035–2040. [Google Scholar]
- Fremerey, P.; Reiβ, S.; Geupel, A.; Fischerauer, G.; Moos, R. Determination of the NOx loading of an automotive lean NOx trap by directly monitoring the electrical properties of the catalyst material itself. Sensors 2011, 11, 8261–8280. [Google Scholar]
- Moos, R.; Zimmermann, C.; Birkhofer, T.; Knezevic, A.; Plog, C.; Busch, M.R.; Ried, T. Sensor for Directly Determining the State of a Nox Storage Catalyst; SAE Technical Paper 2008-01-0447; SAE World Congress & Exhibition: Detroit, MI, USA; April; 2008. [Google Scholar] [CrossRef]
- Groβ, A.; Bishop, S.R.; Yang, D.J.; Tuller, H.L.; Moos, R. The electrical properties of NOx-storing carbonates during NOx exposure. Solid State Ionics. 2012. [Google Scholar] [CrossRef]
- Morrison, S.R.; Madou, M.J. Chemical Sensing with Solid State Devices; Academic Press Inc.: London, UK; May; 1989. [Google Scholar]
- Epling, W.S.; Campbell, L.E.; Yezerets, A.; Currier, N.W.; Parks, J.E., II. Overview of the fundamental reactions and degradation mechanism of NOx storage/reduction catalysts. Catal. Rev. 2004, 46, 163–245. [Google Scholar]
- Roy, S.; Baiker, A. NOx storage-reduction catalysis: From mechanism and materials properties to storage-reduction performance. Chem. Rev. 2009, 109, 4054–4091. [Google Scholar]
- Liu, G.; Gao, P.-X. A review of NOx storage/reduction catalysts: Mechanism, materials and degradation studies. Catal. Sci. Technol. 2011, 1, 552–568. [Google Scholar]
- Lesage, T.; Saussey, J.; Malo, S.; Hervieu, M.; Hedouin, C.; Blanchard, G.; Daturi, M. Operando FTIR study of NOx storage over a Pt/K/Mn/Al2O3-CeO2 catalyst. Appl. Catal. B Environ. 2007, 72, 166–177. [Google Scholar]
- Geupel, A.; Schönauer, D.; Röder-Roith, U.; Kubinski, D.J.; Mulla, S.; Ballinger, T.H.; Chen, H.-Y.; Visser, J.H.; Moos, R. Integrating nitrogen oxide sensor: A novel concept for measuring low concentrations in the exhaust gas. Sens. Actuators B Chem. 2010, 145, 756–761. [Google Scholar]
- Geupel, A.; Kubinski, D.J.; Mulla, S.; Ballinger, T.H.; Chen, H-Y.; Visser, J.H.; Moos, R. Integrating NOx sensor for automotive exhausts—A novel concept. Sens. Lett. 2011, 9, 311–315. [Google Scholar]
- Castoldi, L.; Lietti, L.; Forzatti, P.; Morandi, S.; Ghiotti, G.; Vindigni, F. The NOx storage-reduction on Pt-K/Al2O3 lean NOx trap catalyst. J. Catal. 2010, 276, 335–350. [Google Scholar]
- Muncrief, R.L.; Khanna, P.; Kabin, K.S.; Harold, M.P. Mechanistic and kinetic studies of NOx storage and reduction on Pt/BaO/Al2O3. Catal. Today 2004, 98, 393–402. [Google Scholar]
- Scholz, C.M.L.; Gangwal, V.R.; de Croon, M.H.J.M.; Schouten, J.C. Influence of CO2 and H2O on NOx storage and reduction on a Pt–Ba/γ-Al2O3 catalyst. Appl. Catal. B Environ. 2007, 71, 143–150. [Google Scholar]
- Tuttlies, U.; Schmeiβer, V.; Eigenberger, G. A mechanistic simulation model for NOx storage catalyst dynamics. Chem. Eng. Sci. 2004, 59, 4731–4738. [Google Scholar]
- Olsson, L.; Blint, R.J.; Fridell, E. Global kinetic model for lean NOx traps. Ind. Eng. Chem. Res. 2005, 44, 3021–3032. [Google Scholar]
- Hagen, G. Impedimetric Zeolite-Based Gas Sensors. Ph.D. Dissertation, Universität Bayreuth, Bayreuth, Germany, 2009. [Google Scholar]
- Schönauer, D.; Moos, R. Detection of water droplets on exhaust gas sensors. Sens. Actuators B Chem. 2010, 148, 624–629. [Google Scholar]
- Chen, H.-Y.; Mulla, S.; Ballinger, T.H. Nox Storage Materials for Sensor Applications. U.S. Patent 20,100,146,935, 17 June 2010. [Google Scholar]
- Rettig, F.; Moos, R.; Plog, C. Sulfur adsorber for thick-film exhaust gas sensors. Sens. Actuators B Chem. 2003, 93, 36–42. [Google Scholar]




© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Groß, A.; Richter, M.; Kubinski, D.J.; Visser, J.H.; Moos, R. The Effect of the Thickness of the Sensitive Layer on the Performance of the Accumulating NOx Sensor. Sensors 2012, 12, 12329-12346. https://doi.org/10.3390/s120912329
Groß A, Richter M, Kubinski DJ, Visser JH, Moos R. The Effect of the Thickness of the Sensitive Layer on the Performance of the Accumulating NOx Sensor. Sensors. 2012; 12(9):12329-12346. https://doi.org/10.3390/s120912329
Chicago/Turabian StyleGroß, Andrea, Miriam Richter, David J. Kubinski, Jacobus H. Visser, and Ralf Moos. 2012. "The Effect of the Thickness of the Sensitive Layer on the Performance of the Accumulating NOx Sensor" Sensors 12, no. 9: 12329-12346. https://doi.org/10.3390/s120912329
APA StyleGroß, A., Richter, M., Kubinski, D. J., Visser, J. H., & Moos, R. (2012). The Effect of the Thickness of the Sensitive Layer on the Performance of the Accumulating NOx Sensor. Sensors, 12(9), 12329-12346. https://doi.org/10.3390/s120912329




