Recent Advances in Understanding the Effects of Climate Change on Coral Reefs
Abstract
:1. Introduction
2. Reef-Building Corals
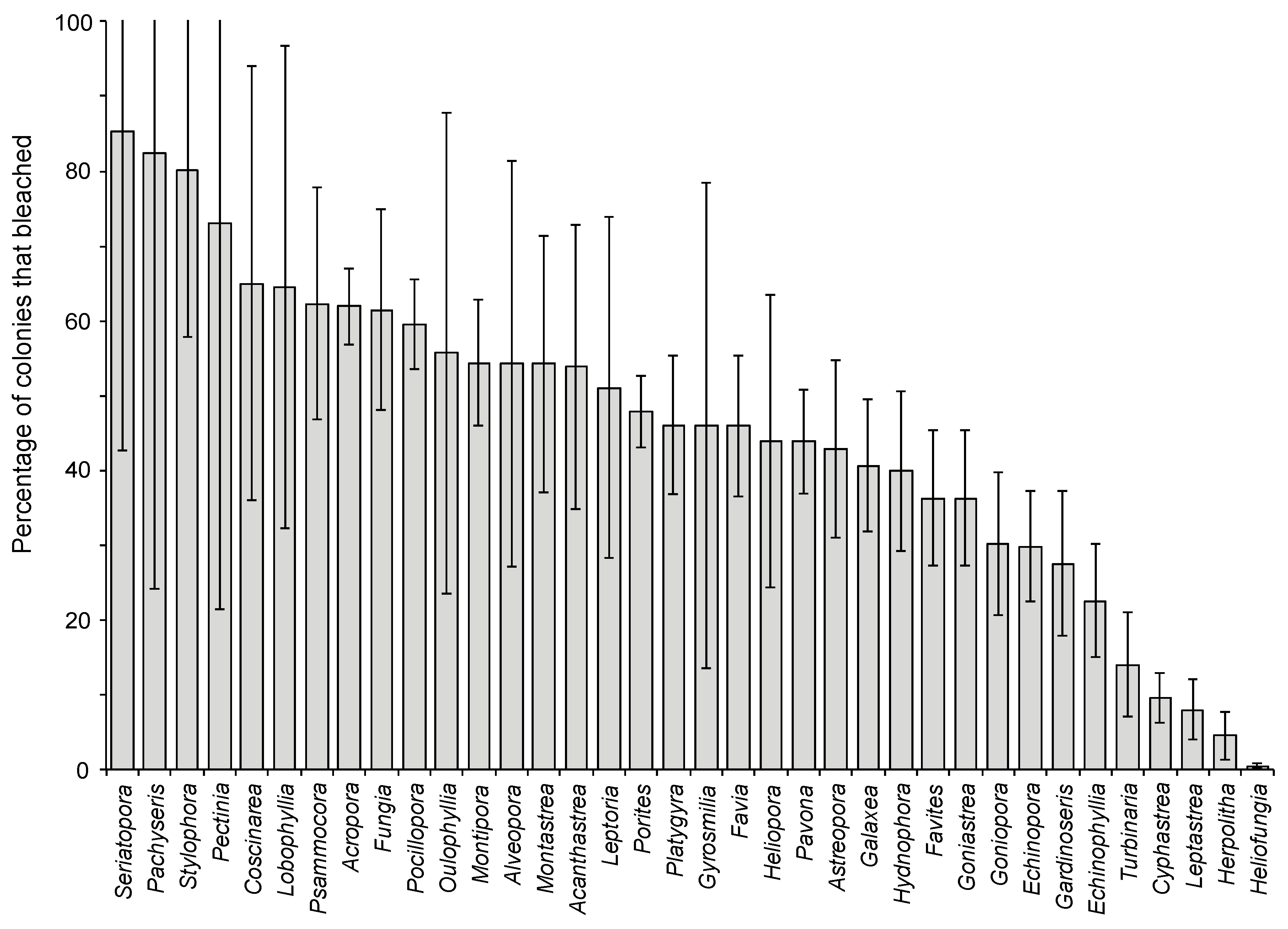
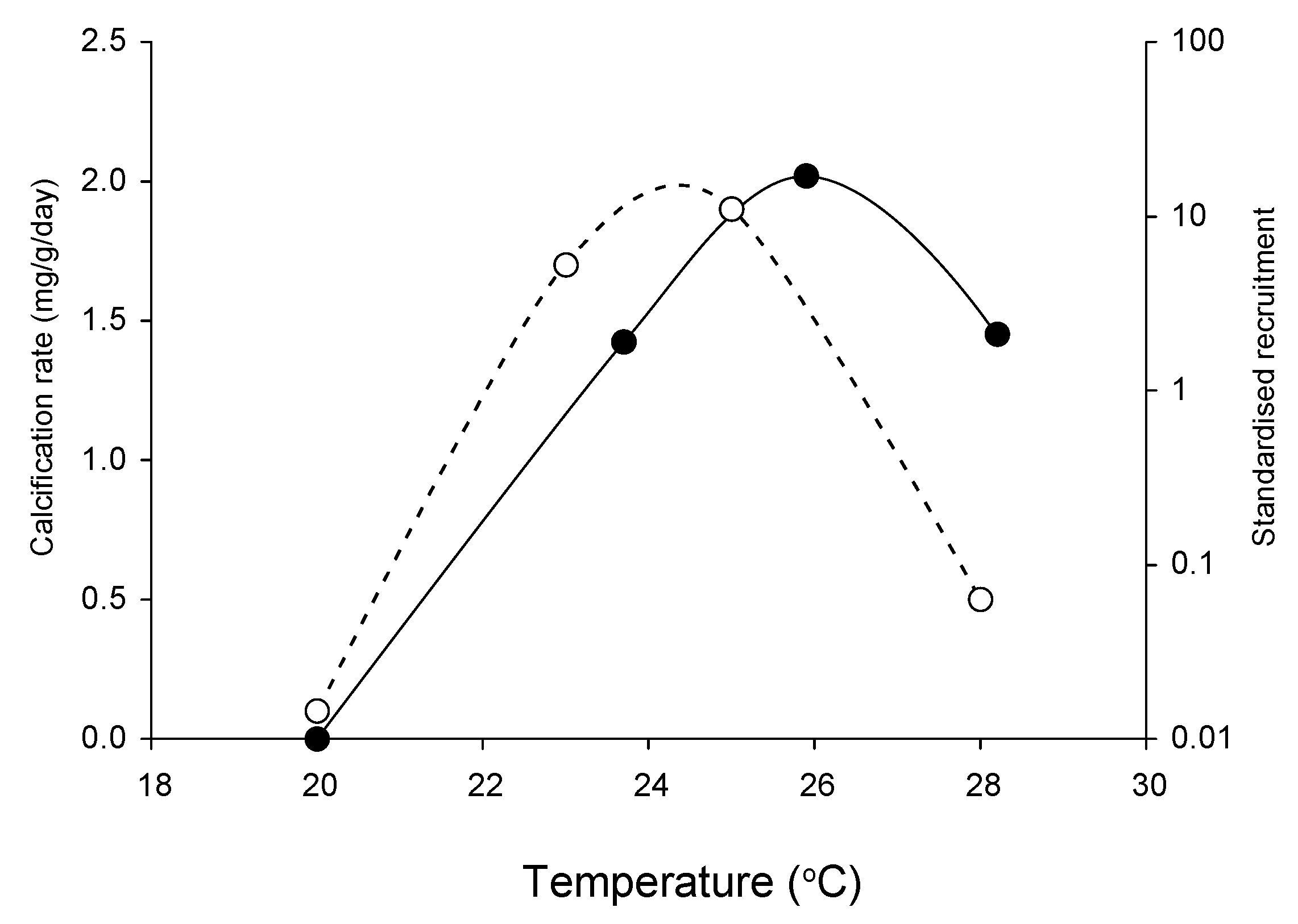
2.1. Effects of Increasing Temperature
2.2. Effects of Ocean Acidification
2.3. Acclimatisation and Adaptation of Reef-Building Corals
3. Reef Fishes
3.1. Direct Effects
3.2. Acclimation and Adaptation of Reef Fish
3.3. Indirect Effects
3.4. Ecological Feedbacks
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Intergovermental Panel on Climate Change. Climate Change 2013: The Physical Science Basis. In Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013; p. 1535. [Google Scholar]
- Hoegh-Guldberg, O. Climate change, coral bleaching and the future of the world’s coral reefs. Mar. Freshw. Res. 1999, 50, 839–866. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O.; Bruno, J.F. The impact of climate change on the world’s marine ecosystems. Science 2010, 328, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.O.; Farrell, A.P. Physiology and climate change. Science 2008, 322, 690–692. [Google Scholar] [CrossRef] [PubMed]
- Jokiel, P.L.; Coles, S.L. Response of Hawaiian and other Indo-Pacific reef corals to elevated temperature. Coral Reefs 1990, 8, 155–162. [Google Scholar] [CrossRef]
- Pandolfi, J.M.; Connolly, S.R.; Marshall, D.J.; Cohen, A.L. Projecting coral reef futures under global warming and ocean acidification. Science 2011, 333, 418–422. [Google Scholar] [CrossRef] [PubMed]
- DiBattista, J.D.; Choat, J.H.; Gaither, M.R.; Hobbs, J-P.A.; Lozano-Cortes, D.F.; Myers, R.F.; Paulay, G.; Rocha, L.A.; Toonen, R.J.; Westneat, M.W.; et al. On the origin of endemics species in the Red Sea. J. Biogeogr. 2016, 43, 13–30. [Google Scholar] [CrossRef]
- Pandolfi, J.M.; Kiessling, W. Gaining insights from past reefs to inform understanding of coral reef response to global climate change. Curr. Opin. Environ. Sustain. 2014, 7, 52–58. [Google Scholar] [CrossRef]
- Baird, A.H.; Marshall, P.A. Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2002, 237, 133–141. [Google Scholar] [CrossRef]
- Wilson, S.K.; Graham, N.A.J.; Pratchett, M.S.; Jones, G.P.; Polunin, N.V.C. Multiple disturbances and the global degradation of coral reefs: Are reef fishes at risk or resilient? Glob. Chang. Biol. 2006, 12, 2220–2234. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Munday, P.L.; Wilson, S.K.; Graham, N.A.J.; Cinner, J.E.; Bellwood, D.R.; Jones, G.P.; Polunin, N.V.C.; McClanahan, T.R. Effects of climate-induced coral bleaching on coral-reef fishes—Ecological and economic consequences. Oceanogr. Mar. Biol. Annu. Rev. 2008, 46, 251–296. [Google Scholar]
- Doney, S.C.; Fabry, V.J.; Feely, R.A.; Kleypas, J.A. Ocean Acidification: The Other CO2 Problem. Annu. Rev. Mar. Sci. 2009, 1, 169–192. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P.; Baird, A.H.; Bellwood, D.R.; Card, M.; Connolly, S.R.; Folke, C.; Grosberg, R.; Hoegh-Guldberg, O.; Jackson, J.B.; Kleypas, J.; et al. Climate change, human impacts, and the resilience of coral reefs. Science 2003, 301, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, C.R.C. Predicted recurrences of mass coral mortality in the Indian Ocean. Nature 2003, 425, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Cantin, N.E.; Cohen, A.L.; Karnauskas, K.B.; Tarrant, A.M.; McCorkle, D.C. Ocean warming slows coral growth in the central Red Sea. Science 2010, 329, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Donner, S.D.; Skirving, W.J.; Little, C.M.; Oppenheimer, M.; Hoegh-Guldberg, O. Global assessment of coral bleaching and required rates of adaptation under climate change. Glob. Chang. Biol. 2005, 11, 2251–2265. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O. Coral reef sustainability through adaptation: Glimmer of hope or persistent mirage? Curr. Opin. Environ. Sustain. 2014, 7, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Hoegh-Guldberg, O.; Mumby, P.J.; Hooten, A.J.; Steneck, R.S.; Greenfield, P.; Gomez, E.; Harvell, C.D.; Sale, P.F.; Edwards, A.J.; Caldeira, K.; et al. Coral reefs under rapid climate change and ocean acidification. Science 2007, 318, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Loya, Y.; Sakai, K.; Yamazato, K.; Nakano, Y.; Sambali, H.; van Woesik, R. Coral bleaching: The winners and the losers. Ecol. Lett. 2001, 4, 122–131. [Google Scholar] [CrossRef]
- Grottoli, A.G.; Warner, M.E.; Levas, S.J.; Aschaffenberg, M.D.; Schoepf, V.; McGinley, M.; Baumann, J.; Matsui, Y. The cumulative impact of annual coral bleaching can turn some coral species winners into losers. Glob. Change Biol. 2014, 20, 3823–3833. [Google Scholar] [CrossRef] [PubMed]
- Riegl, B.M.; Purkis, S.J. Model of coral population response to accelerated bleaching and mass mortality in a changing climate. Ecol. Model. 2009, 220, 192–208. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Weil, E.; Cortés, J.; Baird, A.H.; Ateweberhan, M. Consequences of coral bleaching for sessile reef organisms. In Coral Bleaching: Patterns, Processes, Causes and Consequenes; VanOppen, M.J.H., Lough, J.M., Eds.; Springer: Berlin, Germany, 2009; Volume 205, pp. 121–138. [Google Scholar]
- Hughes, T.P.; Bellwood, D.R.; Connolly, S.R.; Cornell, H.V.; Karlson, R.H. Double jeopardy and global extinction risk in corals and reef fishes. Curr. Biol. 2014, 24, 2946–2951. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.C.; Glynn, P.W.; Riegl, B. Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coastal Shelf Sci. 2008, 80, 435–471. [Google Scholar] [CrossRef]
- Linares, C.; Pratchett, M.S.; Coker, D.J. Recolonisation of Acropora hyacinthus following climate-induced coral bleaching on the Great Barrier Reef. Mar. Ecol. Prog. Ser. 2011, 438, 97–104. [Google Scholar] [CrossRef]
- Darling, E.S.; McClanahan, T.R.; Côté, I.M. Life histories predict coral community disassembly under multiple stressors. Glob. Chang. Biol. 2013, 19, 1930–1940. [Google Scholar] [CrossRef] [PubMed]
- Fabina, N.S.; Baskett, M.L.; Gross, K. The differential effects of increasing frequency and magnitude of extreme events on coral populations. Ecol. Appl. 2015, 25, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, D.M. Inter- and Intra-Specific Variation in Bleaching Susceptibility among Scleractinian Corals. Ph.D. Thesis, James Cook University, Townsville, Australia, 2014. [Google Scholar]
- Jokiel, P.L.; Coles, S.L. Effects of temperature on the mortality and growth of Hawaiian reef corals. Mar. Biol. 1977, 43, 201–208. [Google Scholar] [CrossRef]
- Jokiel, P.L.; Guinther, E.B. Effects of temperature on reproduction in the hermatypic coral Pocillopora damicornis. Bull. Mar. Sci. 1978, 28, 786–789. [Google Scholar]
- De’ath, G.; Lough, J.M.; Fabricius, K.E. Declining coral calcification on the Great Barrier Reef. Science 2009, 323, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Cantin, N.E.; Lough, J.M. Surviving Coral bleaching events: Porites growth anomalies on the Great Barrier Reef. PLoS ONE 2014, 9, e88720. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, A.; Burns, K.; Lough, J.; Brinkman, D.; Delean, S. Trace analysis of hydrocarbons in coral cores from Saudi Arabia. Org. Geochem. 2006, 37, 1913–1930. [Google Scholar] [CrossRef]
- Tanzil, J.T.; Brown, B.E.; Dunne, R.P.; Lee, J.N.; Kaandorp, J.A.; Todd, P.A. Regional decline in growth rates of massive Porites corals in Southeast Asia. Glob. Chang. Biol. 2013, 19, 3011–3023. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.F.; O’Leary, R.A.; Lough, J.M. Growth of Western Australian corals in the Anthropocene. Science 2012, 335, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Pratchett, M.S.; Anderson, K.D.; Hoogenboom, M.O.; Widman, E.; Baird, A.H.; Pandolfi, J.M.; Edmunds, P.J. Spatial, temporal and taxonomic variation in coral growth: Implications for the structure and function of coral reef ecosystems. Oceanogr. Mar. Biol. Annu. Rev. 2015, 53, 215–295. [Google Scholar]
- Pratchett, M.S.; Wilson, S.K.; Munday, P.L. Effects of climate change on coral reef fishes. In Ecology of Fishes on Coral Reefs; Mora, C., Ed.; Cambridge University Press: Cambridge, UK, 2015; pp. 127–134. [Google Scholar]
- Walther, G.R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.; Fromentin, J.M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Kleypas, J.A.; Buddemeier, R.W.; Archer, D.; Gattuso, J.P.; Langdon, C.; Opdyke, B.N. Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 1999, 284, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.; Connolly, S.R. Sensitivity of coral calcification to ocean acidification: A meta-analysis. Glob. Chang. Biol. 2013, 19, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Comeau, S.; Edmunds, P.J.; Spindel, N.B.; Carpenter, R.C. The responses of eight coral reef calcifiers to increasing partial pressure of CO2 do not exhibit a tipping point. Limnol. Oceanogr. 2013, 58, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Comeau, S.; Edmunds, P.J.; Spindel, N.B.; Carpenter, R.C. Fast coral reef calcifiers are more sensitive to ocean acidification in short-term laboratory incubations. Limnol. Oceanogr. 2014, 59, 1081–1091. [Google Scholar] [CrossRef]
- Anthony, K.; Maynard, J.A.; Diaz-Pulido, G.; Mumby, P.J.; Marshall, P.A.; Cao, L.; Hoegh-Guldberg, O. Ocean acidification and warming will lower coral reef resilience. Glob. Change Biol. 2011, 17, 1798–1808. [Google Scholar] [CrossRef]
- Dove, S.G.; Kline, D.I.; Pantos, O.; Angly, F.E.; Tyson, G.W.; Hoegh-Guldberg, O. Future reef decalcification under a business-as-usual CO2 emission scenario. Proc. Natl. Acad. Sci. USA 2013, 110, 15342–15347. [Google Scholar] [CrossRef] [PubMed]
- Van Woesik, R.; Sakai, K.; Ganase, A.; Loya, Y. Revisiting the winners and the losers a decade after coral bleaching. Mar. Ecol. Prog. Ser. 2011, 434, 67–76. [Google Scholar] [CrossRef]
- McCulloch, M.; Falter, J.; Trotter, J.; Montagna, P. Coral resilience to ocean acidification and global warming through pH up-regulation. Nat. Clim. Chang. 2012, 2, 623–627. [Google Scholar] [CrossRef]
- Venn, A.A.; Tambutté, E.; Holcomb, M.; Laurent, J.; Allemand, D.; Tambutté, S. Impact of seawater acidification on pH at the tissue-skeleton interface and calcification in reef corals. Proc. Natl. Acad. Sci. USA 2013, 110, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Riegl, B.M.; Purkis, S.J.; Al-Cibahy, A.S.; Abdel-Moati, M.A.; Hoegh-Guldberg, O. Present limits to heat-adaptability in corals and population-level responses to climate extremes. PLoS ONE 2011, 6, e24802. [Google Scholar] [CrossRef] [PubMed]
- Crook, E.D.; Cohen, A.L.; Rebolledo-Vieyra, M.; Hernandez, L.; Paytan, A. Reduced calcification and lack of acclimatization by coral colonies growing in areas of persistent natural acidification. Proc. Natl. Acad. Sci. USA 2013, 110, 11044–11049. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, K.E.; Langdon, C.; Uthicke, S.; Humphrey, C.; Noonan, S.; De’ath, G.; Okazaki, R.; Muehllehner, N.; Glas, M.S.; Lough, J.M. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Chang. 2011, 1, 165–169. [Google Scholar] [CrossRef]
- Shamberger, K.E.; Cohen, A.L.; Golbuu, Y.; McCorkle, D.C.; Lentz, S.J.; Barkley, H.C. Diverse coral communities in naturally acidified waters of a Western Pacific Reef. Geophys. Res. Lett. 2014, 41, 499–504. [Google Scholar] [CrossRef]
- Bauman, A.G.; Feary, D.A.; Heron, S.F.; Pratchett, M.S.; Burt, J.A. Multiple environmental factors influence the spatial distribution and structure of reef communities in the northeastern Arabian peninsula. Mar. Poll. Bull. 2013, 72, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Barkley, H.C.; Cohen, A.L.; Golbuu, Y.; Starczak, V.R.; DeCarlo, T.M.; Shamberger, K.E. Changes in coral reef communities across a natural gradient in seawater pH. Sci. Adv. 2015, 1, e1500328. [Google Scholar] [CrossRef] [PubMed]
- Munday, P.L.; Warner, R.R.; Monro, K.; Pandolfi, J.M.; Marshall, D.J. Predicting evolutionary responses to climate change in the sea. Ecol. Lett. 2013, 16, 1488–1500. [Google Scholar] [CrossRef] [PubMed]
- Maynard, J.; Anthony, K.; Marshall, P.; Masiri, I. Major bleaching events can lead to increased thermal tolerance in corals. Mar. Biol. 2008, 155, 173–182. [Google Scholar] [CrossRef]
- Berkelmans, R. Bleaching and mortality thresholds: How much is too much? In Coral Bleaching: Patterns, Processes, Causes and Consequenes; VanOppen, M.J.H., Lough, J.M., Eds.; Springer: Berlin, Germany, 2009; Volume 205, pp. 103–119. [Google Scholar]
- Guest, J.R.; Baird, A.H.; Maynard, J.A.; Muttaqin, E.; Edwards, A.J.; Campbell, S.J.; Yewdall, K.; Affendi, Y.A.; Chou, L.M. Contrasting patterns of coral bleaching susceptibility in 2010 suggest an adaptive response to thermal stress. PLoS ONE 2012, 7, e33353. [Google Scholar] [CrossRef] [PubMed]
- Pratchett, M.S.; McCowan, D.; Maynard, J.A.; Heron, S.F. Changes in bleaching susceptibility among corals subject to ocean warming and recurrent bleaching in Moorea, French Polynesia. PLoS ONE 2013, 8, e70443. [Google Scholar] [CrossRef] [PubMed]
- McClanahan, T.; Muthiga, N. Community change and evidence for variable warm-water temperature adaptation of corals in northern Male Atoll, Maldives. Mar. Poll. Bull. 2014, 80, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.E.; Dunne, R.P.; Goodson, M.S.; Douglas, A.E. Experience shapes the susceptibility of a reef coral to bleaching. Coral Reefs 2002, 21, 119–126. [Google Scholar]
- Brown, B.E.; Cossins, A.R. The potential for temperature acclimatisation of reef corals in the face of climate change. In Coral Reefs: An Ecosystem in Transition; Springer: Berlin, Germany, 2011; pp. 421–433. [Google Scholar]
- Rosenberg, E.; Koren, O.; Reshef, L.; Efrony, R.; Zilber-Rosenberg, I. The role of microorganisms in coral health, disease and evolution. Nature Rev. Microbiol. 2007, 5, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Weis, V.M. The susceptibility and resilience of corals to thermal stress: Adaptation, acclimatization or both? Mol. Ecol. 2010, 19, 1515–1517. [Google Scholar] [CrossRef] [PubMed]
- Baird, A.H.; Bhagooli, R.; Ralph, P.J.; Takahashi, S. Coral bleaching: The role of the host. Trends Ecol. Evol. 2009, 24, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Linan-Cabello, M.A.; Flores-Ramirez, L.A.; Zenteno-Savin, T.; Olguin-Monroy, N.O.; Sosa-Avalos, R.; Patino-Barragan, M.; Olivos-Ortiz, A. Seasonal changes of antioxidant and oxidative parameters in the coral Pocillopora capitata on the Pacific coast of Mexico. Mar. Ecol. 2010, 31, 407–417. [Google Scholar]
- Grottoli, A.G.; Rodrigues, L.J.; Palardy, J.E. Heterotrophic plasticity and resilience in bleached corals. Nature 2006, 440, 1186–1189. [Google Scholar] [CrossRef] [PubMed]
- Cunning, R.; Vaughan, N.; Gillette, P.; Capo, T.R.; Maté, J.L.; Baker, A.C. Dynamic regulation of partner abundance mediates response of reef coral symbioses to environmental change. Ecology 2015, 96, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Barshis, D.J.; Ladner, J.T.; Oliver, T.A.; Seneca, F.O.; Traylor-Knowles, N.; Palumbi, S.R. Genomic basis for coral resilience to climate change. Proc. Natl. Acad. Sci. USA 2013, 110, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Dixon, G.B.; Davies, S.W.; Aglyamova, G.A.; Meyer, E.; Bay, L.K.; Matz, M.V. Genomic determinants of coral heat tolerance across latitudes. Science 2015, 348, 1460–1462. [Google Scholar] [CrossRef] [PubMed]
- Kenkel, C.; Meyer, E.; Matz, M. Gene expression under chronic heat stress in populations of the mustard hill coral (Porites astreoides) from different thermal environments. Mol. Ecol. 2013, 22, 4322–4334. [Google Scholar] [CrossRef] [PubMed]
- Palumbi, S.R.; Barshis, D.J.; Traylor-Knowles, N.; Bay, R.A. Mechanisms of reef coral resistance to future climate change. Science 2014, 344, 895–898. [Google Scholar] [CrossRef] [PubMed]
- Barshis, D.; Stillman, J.; Gates, R.; Toonen, R.; Smith, L.; Birkeland, C. Protein expression and genetic structure of the coral Porites lobata in an environmentally extreme Samoan back reef: Does host genotype limit phenotypic plasticity? Mol. Ecol. 2010, 19, 1705–1720. [Google Scholar] [CrossRef] [PubMed]
- Bellantuono, A.J.; Hoegh-Guldberg, O.; Rodriguez-Lanetty, M. Resistance to thermal stress in corals without changes in symbiont composition. Proc. R. Soc. B 2012, 279, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, T.D.; Heron, S.F.; Ortiz, J.C.; Mumby, P.J.; Grech, A.; Ogawa, D.; Eakin, C.M.; Leggat, W. Climate change disables coral bleaching protection on the Great Barrier Reef. Science 2016, 352, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Howells, E.J.; Berkelmans, R.; van Oppen, M.J.; Willis, B.L.; Bay, L.K. Historical thermal regimes define limits to coral acclimatization. Ecology 2013, 94, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Bay, R.A.; Palumbi, S.R. Multilocus adaptation associated with heat resistance in reef-building corals. Curr. Biol. 2014, 24, 2952–2956. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.; Davies, S.; Wang, S.; Willis, B.L.; Abrego, D.; Juenger, T.E.; Matz, M.V. Genetic variation in responses to a settlement cue and elevated temperature in the reef-building coral Acropora millepora. Mar. Ecol. Prog. Ser. 2009, 392, 81–92. [Google Scholar] [CrossRef]
- Polato, N.R.; Voolstra, C.R.; Schnetzer, J.; DeSalvo, M.K.; Randall, C.J.; Szmant, A.M.; Medina, M.; Baums, I.B. Location-specific responses to thermal stress in larvae of the reef-building coral Montastraea faveolata. PLoS ONE 2010, 5, e11221. [Google Scholar] [CrossRef] [PubMed]
- Woolsey, E.; Keith, S.; Byrne, M.; Schmidt-Roach, S.; Baird, A. Latitudinal variation in thermal tolerance thresholds of early life stages of corals. Coral Reefs 2015, 34, 471–478. [Google Scholar] [CrossRef]
- Howells, E.J.; Abrego, D.; Meyer, E.; Kirk, N.L.; Burt, J.A. Host adaptation and unexpected symbiont partners enable reef-building corals to tolerate extreme temperatures. Glob. Chang. Biol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Kenkel, C.; Setta, S.; Matz, M. Heritable differences in fitness-related traits among populations of the mustard hill coral, Porites astreoides. Heredity 2015, 115, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Putnam, H.M.; Gates, R.D. Preconditioning in the reef-building coral Pocillopora damicornis and the potential for trans-generational acclimatization in coral larvae under future climate change conditions. J. Exp. Biol. 2015, 218, 2365–2372. [Google Scholar] [CrossRef] [PubMed]
- Omori, M. Degradation and restoration of coral reefs: Experience in Okinawa, Japan. Mar. Biol. Res. 2011, 7, 3–12. [Google Scholar] [CrossRef]
- Babcock, R.C. Comparative demography of three species of scleractinian corals using age-and size-dependent classifications. Ecol. Monogr. 1991, 61, 225–244. [Google Scholar] [CrossRef]
- Csaszar, N.B.M.; Ralph, P.J.; Frankham, R.; Berkelmans, R.; van Oppen, M.J.H. Estimating the potential for adaptation of corals to climate warming. PLoS ONE 2010, 5, e9751. [Google Scholar] [CrossRef] [PubMed]
- Fogarty, N.D. Caribbean acroporid coral hybrids are viable across life history stages. Mar. Ecol. Prog. Ser. 2011, 446, 145–159. [Google Scholar] [CrossRef]
- Van Oppen, M.J.; Oliver, J.K.; Putnam, H.M.; Gates, R.D. Building coral reef resilience through assisted evolution. Proc. Natl. Acad. Sci. USA 2015, 112, 2307–2313. [Google Scholar] [CrossRef] [PubMed]
- Van Oppen, M.J.H.; Souter, P.; Howells, E.J.; Heyward, A.; Berkelmans, R. Novel genetic diversity through somatic mutations: Fuel for adaptation of reef corals? Diversity 2011, 3, 405–423. [Google Scholar] [CrossRef]
- Iglesias-Prieto, R.; Trench, R.K. Acclimation and adaptation to irradiance in symbiotic dinoflagellates. I. Responses of the photosynthetic unit to changes in photon flux density. Mar. Ecol. Prog. Ser. 1994, 113, 163–175. [Google Scholar] [CrossRef]
- Tchernov, D.; Gorbunov, M.Y.; de Vargas, C.; Yadav, S.N.; Milligan, A.J.; Haggblom, M.; Falkowski, P.G. Membrane lipids of symbiotic algae are diagnostic of sensitivity to thermal bleaching in corals. Proc. Natl. Acad. Sci. USA 2004, 101, 13531–13535. [Google Scholar] [CrossRef] [PubMed]
- McGinty, E.S.; Pieczonka, J.; Mydlarz, L.D. Variations in reactive oxygen release and antioxidant activity in multiple symbiodinium types in response to elevated temperature. Microb. Ecol. 2012, 64, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Krueger, T.; Becker, S.; Pontasch, S.; Dove, S.; Hoegh-Guldberg, O.; Leggat, W.; Fisher, P.L.; Davy, S.K. Antioxidant plasticity and thermal sensitivity in four types of Symbiodinium sp. J. Phycol. 2014, 50, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Whitney, S.M.; Badger, M.R. Different thermal sensitivity of the repair of photodamaged photosynthetic machinery in cultured symbiodinium species. Proc. Natl. Acad. Sci. USA 2009, 106, 3237–3242. [Google Scholar] [CrossRef] [PubMed]
- Coffroth, M.A.; Santos, S.R.; Goulet, T.L. Early ontogenetic expression of specificity in a cnidarian-algal symbiosis. Mar. Ecol. Prog. Ser. 2001, 222, 85–96. [Google Scholar] [CrossRef]
- Padilla-Gamiño, J.L.; Pochon, X.; Bird, C.; Concepcion, G.T.; Gates, R.D. From parent to gamete: Vertical transmission of Symbiodinium (Dinophyceae) ITS2 sequence assemblages in the reef building coral Montipora capitata. PLoS ONE 2012, 7, e38440. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.A.; Wang, J.-T.; Fang, L.S.; Yang, Y.-W. Fluctuating algal symbiont communities in Acropora palifera (Scleractinia: Acroporidae) from Taiwan. Mar. Ecol. Prog. Ser. 2005, 295, 113–121. [Google Scholar] [CrossRef]
- Berkelmans, R.; van Oppen, M.J.H. The role of zooxanthellae in the thermal tolerance of corals: A ‘nugget of hope’ for coral reefs in an era of climate change. Proc. R. Soc. B 2006, 273, 2305–2312. [Google Scholar] [CrossRef] [PubMed]
- Abrego, D.; Willis, B.L.; van Oppen, M.J. Impact of light and temperature on the uptake of algal symbionts by coral juveniles. PLoS ONE 2012, 7, e50311. [Google Scholar] [CrossRef] [PubMed]
- Thornhill, D.J.; LaJeunesse, T.C.; Kemp, D.W.; Fitt, W.K.; Schmidt, G.W. Multi-year, seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or post-bleaching reversion. Mar. Biol. 2006, 148, 711–722. [Google Scholar] [CrossRef]
- Boulotte, N.M.; Dalton, S.J.; Carroll, A.G.; Harrison, P.L.; Putnam, H.M.; Peplow, L.M.; van Oppen, M.J.H. Exploring the Symbiodinium rare biosphere provides evidence for symbiont switching in reef-building corals. ISME J. 2016. [Google Scholar] [CrossRef] [PubMed]
- LaJeunesse, T.C.; Wham, D.C.; Pettay, D.T.; Parkinson, J.E.; Keshavmurthy, S.; Chen, C.A. Ecologically differentiated stress-tolerant endosymbionts in the dinoflagellate genus Symbiodinium (Dinophyceae) clade d are different species. Phycologia 2014, 53, 305–319. [Google Scholar] [CrossRef]
- Baker, A.C. Reef corals bleach to survive change. Nature 2001, 411, 765–766. [Google Scholar] [CrossRef] [PubMed]
- Fabricius, K.E.; Mieog, J.C.; Colin, P.L.; Idip, D.; van Oppen, M.J.H. Identity and diversity of coral endosymbionts (zooxanthellae) from three palauan reefs with contrasting bleaching, temperature and shading histories. Mol. Ecol. 2004, 13, 2445–2458. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Berkelmans, R.; van Oppen, M.J.H.; Mieog, J.C.; Sinclair, W. A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: Field evidence of acclimatization. Proc. R. Soc. B 2008, 275, 1359–1365. [Google Scholar] [CrossRef] [PubMed]
- Keshavmurthy, S.; Hsu, C.M.; Kuo, C.Y.; Meng, P.J.; Wang, J.T.; Chen, C.A. Symbiont communities and host genetic structure of the brain coral Platygyra verweyi, at the outlet of a nuclear power plant and adjacent areas. Mol. Ecol. 2012, 21, 4393–4407. [Google Scholar] [CrossRef] [PubMed]
- Abrego, D.; Ulstrup, K.E.; Willis, B.L.; van Oppen, M.J.H. Species-specific interactions between algal endosymbionts and coral hosts define their bleaching response to heat and light stress. Proc. R. Soc. B 2008, 275, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Howells, E.J.; Beltran, V.H.; Larsen, N.W.; Bay, L.K.; Willis, B.L.; van Oppen, M.J.H. Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat. Clim. Chang. 2012, 2, 116–120. [Google Scholar] [CrossRef]
- Hume, B.; D’Angelo, C.; Smith, E.; Stevens, J.; Burt, J.; Wiedenmann, J. Symbiodinium thermophilum sp. Nov., a thermotolerant symbiotic alga prevalent in corals of the world’s hottest sea, the Persian/Arabian Gulf. Sci. Rep. 2015, 5, Article 8562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correa, A.M.S.; Baker, A.C. Disaster taxa in microbially mediated metazoans: How endosymbionts and environmental catastrophes influence the adaptive capacity of reef corals. Glob. Chang. Biol. 2011, 17, 68–75. [Google Scholar] [CrossRef]
- Wilkinson, S.P.; Fisher, P.L.; van Oppen, M.J.; Davy, S.K. Intra-genomic variation in symbiotic dinoflagellates: Recent divergence or recombination between lineages? BMC Evol. Biol. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.; Parrow, M.W.; Dunthorn, M. Cryptic sex in Symbiodinium (alveolata, dinoflagellata) is supported by an inventory of meiotic genes. J. Eukaryotic Microbiol. 2014, 61, 322–327. [Google Scholar] [CrossRef]
- Anthony, K.R.; Kline, D.I.; Diaz-Pulido, G.; Dove, S.; Hoegh-Guldberg, O. Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. USA 2008, 105, 17442–17446. [Google Scholar] [CrossRef] [PubMed]
- Schoepf, V.; Grottoli, A.G.; Warner, M.E.; Cai, W.-J.; Melman, T.F.; Hoadley, K.D.; Pettay, D.T.; Hu, X.; Li, Q.; Xu, H. Coral energy reserves and calcification in a high-CO2 world at two temperatures. PLoS ONE 2013, 8, e75049. [Google Scholar] [CrossRef] [PubMed]
- Strahl, J.; Stolz, I.; Uthicke, S.; Vogel, N.; Noonan, S.; Fabricius, K. Physiological and ecological performance differs in four coral taxa at a volcanic carbon dioxide seep. Comp. Biochem. Physiol. A 2015, 184, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.; Huisman, L.; Forêt, S.; Gattuso, J.P.; Hayward, D.; Ball, E.; Miller, D. Rapid acclimation of juvenile corals to CO2-mediated acidification by upregulation of heat shock protein and bcl-2 genes. Mol. Ecol. 2015, 24, 438–452. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, P.J. Zooplanktivory ameliorates the effects of ocean acidification on the reef coral Porites spp. Limnol. Oceanogr. 2011, 56, 2402–2410. [Google Scholar] [CrossRef]
- Brading, P.; Warner, M.E.; Davey, P.; Smith, D.J.; Achterberg, E.P.; Suggett, D.J. Differential effects of ocean acidification on growth and photosynthesis among phylotypes of Symbiodinium (Dinophyceae). Limnol. Oceanogr. 2011, 56, 927–938. [Google Scholar] [CrossRef]
- Noonan, S.H.; Fabricius, K.E.; Humphrey, C. Symbiodinium community composition in scleractinian corals is not affected by life-long exposure to elevated carbon dioxide. PLoS ONE 2013, 8, e63985. [Google Scholar] [CrossRef] [PubMed]
- Sunday, J.M.; Calosi, P.; Dupont, S.; Munday, P.L.; Stillman, J.H.; Reusch, T.B. Evolution in an acidifying ocean. Trends Ecol. Evol. 2014, 29, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Pörtner, H.O.; Schulte, P.M.; Wood, C.M.; Schiemer, F. Niche dimensions in fishes: An integrative view. Physiol. Biochem. Zool. 2010, 83, 808–826. [Google Scholar] [CrossRef] [PubMed]
- Esbaugh, A.J.; Heuer, R.; Grosell, M. Impacts of ocean acidification on respiratory gas exchange and acid-base balance in a marine teleost, Opsanus beta. J. Comp. Physiol. B 2012, 182, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Munday, P.L.; Crawley, N.E.; Nilsson, G.E. Interacting effects of elevated temperature and ocean acidification on the aerobic performance of coral reef fishes. Mar. Ecol. Prog. Ser. 2009, 388, 235–242. [Google Scholar] [CrossRef]
- Donelson, J.M.; Munday, P.L.; McCormick, M.I.; Nilsson, G.E. Acclimation to predicted ocean warming through developmental plasticity in a tropical reef fish. Glob. Chang. Biol. 2011, 17, 1712–1719. [Google Scholar] [CrossRef]
- Donelson, J.M.; Munday, P.L.; McCormick, M.I. Climate change may affect fish through an interaction of parental and juvenile environments. Coral Reefs 2012, 31, 753–762. [Google Scholar] [CrossRef]
- Johansen, J.L.; Jones, G.P. Increasing ocean temperature reduces the metabolic performance and swimming ability of coral reef damselfishes. Glob. Chang. Biol. 2011, 17, 2971–2979. [Google Scholar] [CrossRef]
- Rummer, J.L.; Couturier, C.S.; Stecyk, J.A.; Gardiner, N.M.; Kinch, J.P.; Nilsson, G.E.; Munday, P.L. Life on the edge: Thermal optima for aerobic scope of equatorial reef fishes are close to current day temperatures. Glob. Chang. Biol. 2014, 20, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Hein, A.M.; Keirsted, K.J. The rising cost of warming waters: Effects of temperature on the cost of swimming in fishes. Biol. Lett. 2012, 8, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Johansen, J.L.; Pratchett, M.S.; Messmer, V.; Coker, D.J.; Tobin, A.J.; Hoey, A.S. Large predatory coral trout species unlikely to meet increasing energetic demands in a warming ocean. Sci. Rep. 2015, 5, Article 13830. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.D.; Sandblom, E.; Jutfelt, F. Aerobic scope measurements in an era of climate change: Respiromery, relevance and recommendations. J. Exp. Biol. 2013, 216, 2771–2782. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.E.; Nilsson, S.O.; Munday, P.L. Effects of elevated temperature on coral reef fishes: Loss of hypoxia tolerance and inability to acclimate. Comp. Biochem. Phys. A 2010, 156, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Killen, S.S.; Mitchell, M.D.; Rummer, J.L.; Chivers, D.P.; Ferrari, M.C.O.; Meekan, M.G.; McCormick, M.I. Aerobic scope predicts dominance during early life in a tropical damselfish. Func. Ecol. 2014, 28, 1367–1376. [Google Scholar] [CrossRef]
- Deutsch, C.; Ferrel, A.; Seibel, B.; Pörtner, H.O.; Huey, R.B. Climate change tightens a metabolic constraint on marine habitats. Science 2015, 348, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Nay, T.J.; Johansen, J.L.; Habary, A.; Steffensen, J.F.; Rummer, J.L. Behavioural thermoregulation in a temperature-sensitive coral reef fish, the five-lined cardinalfish (Cheilodipterus quinquelineatus). Coral Reefs 2015, 34, 1261–1265. [Google Scholar] [CrossRef]
- Green, B.S.; Fisher, R. Temperature influences swimming speed, growth and larval duration in coral reef fish larvae. J. Exp. Mar. Biol. Ecol. 2004, 299, 115–132. [Google Scholar] [CrossRef]
- Rankin, T.L.; Sponaugle, S. Temperature influences selective mortality during the early life stages of a coral reef fish. PLoS ONE 2011, 6, e16814. [Google Scholar] [CrossRef] [PubMed]
- Donelson, J.M.; Munday, P.L.; McCormick, M.I.; Pankhurst, N.W.; Pankhurst, P.M. Effects of elevated water temperature and food availability on the reproductive performance of a coral reef fish. Mar. Ecol. Prog. Ser. 2010, 401, 233–245. [Google Scholar] [CrossRef]
- McLeod, I.M.; Rummer, J.L.; Clark, T.D.; Jones, G.P.; McCormick, M.I.; Wenger, A.S.; Munday, P.L. Climate change and the performance of larval coral reef fishes: The interaction between temperature and food availability. Conserv. Physiol. 2013, 1. [Google Scholar] [CrossRef]
- Johansen, J.L.; Messmer, V.; Coker, D.J.; Hoey, A.S.; Pratchett, M.S. Increasing ocean temperatures reduce activity patterns of a large commercially important coral reef fish. Glob. Chang. Biol. 2014, 20, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, J.A.; Bickford, D. Shrinking body size as an ecological response to climate change. Nat. Clim. Chang. 2011, 1, 401–406. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Sarmiento, J.L.; Dunne, J.; Frolicher, T.L.; Lam, V.W.Y.; Palomares, M.L.D.; Watson, R.; Pauly, D. Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat. Clim. Chang. 2013, 3, 254–258. [Google Scholar] [CrossRef]
- Lefort, S.; Aumont, O.; Bopp, L.; Arsouze, T.; Gehlen, M.; Maury, O. Spatial and body-size dependent response of marine pelagic communities to projected global climate change. Glob. Chang. Biol. 2015, 21, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Colin, P.L. Timing and location of aggregation and spawning in reef fishes. In Reef Fish Spawning Aggregations: Biology Research and Management; Sadovy de Mitcheson, Y., Colin, P.L., Eds.; Springer: New York, NY, USA, 2012; pp. 117–158. [Google Scholar]
- Miller, G.M.; Kroon, F.J.; Metcalfe, S.; Munday, P.L. Temperature is the evil twin: Effects of increased temperature and ocean acidification on reproduction in a reef fish. Ecol. Appl. 2015, 25, 603–620. [Google Scholar] [CrossRef] [PubMed]
- Potts, W.M.; Booth, A.J.; Richardson, T.J.; Sauer, W.H. Ocean warming affects the distribution and abundance of resident fishes by changing their reproductive scope. Rev. Fish Biol. Fish. 2014, 24, 493–504. [Google Scholar] [CrossRef]
- Donelson, J.M.; McCormick, M.I.; Booth, D.J.; Munday, P.L. Reproductive acclimation to increased water temperature in a tropical reef fish. PLoS ONE 2014, 9, e97223. [Google Scholar] [CrossRef] [PubMed]
- Ishimatsu, A.; Kikkawa, T.; Hayashi, M.; Lee, K.S.; Kita, J. Effects of CO2 on marine fish: Larvae and adults. J. Oceanogr. 2004, 60, 731–741. [Google Scholar] [CrossRef]
- Munday, P.L.; Dixson, D.L.; Donelson, J.M.; Jones, G.P.; Pratchett, M.S.; Devitsina, G.V.; Døving, K.B. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl. Acad. Sci. USA 2009, 106, 1848–1852. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.M.; Watson, S.A.; Donelson, J.M.; McCormick, M.I.; Munday, P.L. Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat. Clim. Chang. 2012, 2, 858–861. [Google Scholar] [CrossRef]
- Couturier, C.S.; Stecyk, J.A.W.; Rummer, J.L.; Munday, P.L.; Nilsson, G.E. Species-specific effects of near-future CO2 on the respiratory performance of two tropical prey fish and their predator. Com. Biochem. Physiol. 2013, 166, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.M.; Watson, S.A.; McCormick, M.I.; Munday, P.L. Increased CO2 stimulates reproduction in a coral reef fish. Glob. Chang. Biol. 2013, 19, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Heuer, R.M.; Grosell, M. Physiological impacts of elevated carbon dioxide and ocean acidification on fish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 307, R1061–R1084. [Google Scholar] [CrossRef] [PubMed]
- Dixson, D.L.; Munday, P.L.; Jones, G.P. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 2010, 13, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Munday, P.L.; Dixson, D.L.; McCormick, M.I.; Meekan, M.; Ferrari, M.C.O.; Chivers, D.P. Replenishment of fish populations is threatened by ocean acidification. Proc. Natl. Acad. Sci. USA 2010, 107, 12930–12934. [Google Scholar] [CrossRef] [PubMed]
- Munday, P.L.; Pratchett, M.S.; Dixson, D.L.; Donelson, J.M.; Endo, G.G.; Reynolds, A.D.; Knuckey, R. Elevated CO2 affects the behavior of an ecologically and economically important coral reef fish. Mar. Biol. 2013, 160, 2137–2144. [Google Scholar] [CrossRef]
- Ferrari, M.C.O.; McCormick, M.I.; Munday, P.L.; Meekan, M.G.; Dixson, D.L.; Lönnstedt, O.; Chivers, D.P. Putting prey and predator into the CO2 equation—Qualitative and quantitative effects of ocean acidification on predator-prey interactions. Ecol. Lett. 2011, 14, 1143–1148. [Google Scholar] [CrossRef] [PubMed]
- Devine, B.M.; Munday, P.L.; Jones, G.P. Homing ability of adult cardinalfish is affected by elevated carbon dioxide. Oecologia 2012, 168, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.D.; Munday, P.L.; Wittenrich, M.L.; Manassa, R.; Dixson, D.L.; Gagliano, M.; Yan, H.Y. Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol. Lett. 2011, 7, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.C.O.; McCormick, M.I.; Munday, P.L.; Meekan, M.G.; Dixson, D.L.; Lönnstedt, O.; Chivers, D.P. Effects of ocean acidification on visual risk assessment in coral reef fishes. Func. Ecol. 2012, 26, 553–558. [Google Scholar] [CrossRef]
- Domenici, P.; Allan, B.; McCormick, M.I.; Munday, P.L. Elevated carbon dioxide affects behavioural lateralization in coral reef fish. Biol. Lett. 2012, 8, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Allan, B.J.; Miller, G.M.; McCormick, M.I.; Domenici, P.; Munday, P.L. Parental effects improve escape performance of juvenile reef fish in a high-CO2 world. Proc. R. Soc. B 2012, 281, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Almany, G.R.; Webster, M.S. The predation gauntlet: Early post-settlement mortality in reef fishes. Coral Reefs 2006, 25, 19–22. [Google Scholar] [CrossRef]
- Hoey, A.S.; McCormick, M.I. Selective predation for low body condition at the larval-juvenile transition of a coral reef fish. Oecologia 2004, 139, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.E.; Dixson, D.L.; Domenici, P.; McCormick, M.I.; Sorensen, C.; Watson, S.-A.; Munday, P.L. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat. Clim. Chang. 2012, 2, 201–204. [Google Scholar] [CrossRef]
- Sørensen, C.; Munday, P.L.; Nilsson, G.E. Aerobic vs. anaerobic scope: Sibling species of fish indicate that temperature dependence of hypoxia tolerance can predict future survival. Glob. Chang. Biol. 2014, 20, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Munday, P.L.; Cheal, A.J.; Dixson, D.L.; Rummer, J.L.; Fabricius, K.E. Behavioural impairment in reef fishes caused by ocean acidification at CO2 seeps. Nat. Clim. Chang. 2012, 4, 487–492. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Russell, B.D.; Gillanders, B.M.; Connell, S.D. Ocean acidification alters fish populations indirectly through habitat modification. Nat. Clim. Chang. 2016, 6, 89–93. [Google Scholar] [CrossRef]
- Lawton, R.J.; Cole, A.J.; Berumen, M.L.; Pratchett, M.S. Geographic variation in resource use by specialist versus generalist butterflyfishes. Ecography 2012, 35, 566–576. [Google Scholar] [CrossRef]
- Caley, M.J.; Munday, P.L. Growth trades off with habitat specialization. Proc. R. Soc. B 2003, 270, S175–S177. [Google Scholar] [CrossRef] [PubMed]
- Graham, N.A.; Cinner, J.E.; Norström, A.V.; Nyström, M. Coral reefs as novel ecosystems: Embracing new futures. Curr. Opin. Environ. Sustain. 2014, 7, 9–14. [Google Scholar] [CrossRef]
- Albins, M.A. Effects of invasive Pacific red lionfish Pterois volitans versus a native predator on Bahamian coral-reef fish communities. Biol. Invasions 2013, 15, 29–43. [Google Scholar] [CrossRef]
- Feary, D.A.; Pratchett, M.S.; Emslie, M.J.; Fowler, A.M.; Figueira, W.F.; Luiz, O.J.; Nakamura, Y.; Booth, D.J. Latitudinal shifts in coral reef fishes: Why some species do and others do not shift. Fish Fish. 2014, 15, 593–615. [Google Scholar] [CrossRef]
- Vergés, A.; Steinberg, P.D.; Hay, M.E.; Poore, A.G.; Campbell, A.H.; Ballesteros, E.; Heck, K.L.; Booth, D.J.; Coleman, M.A.; Feary, D.A.; et al. The tropicalization of temperate marine ecosystems: Climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B 2014, 281, rspb20140846. [Google Scholar]
- Pratchett, M.S.; Hoey, A.S.; Wilson, S.K. Reef degradation and the loss of critical ecosystem goods and services provided by coral reef fishes. Curr. Opin. Environ. Sustain. 2014, 7, 37–43. [Google Scholar] [CrossRef]
- Rogers, A.; Blanchard, J.L.; Mumby, P.J. Vulnerability of oral reef fisheries to a loss of structural complexity. Curr. Biol. 2014, 24, 1000–1005. [Google Scholar] [CrossRef] [PubMed]
- Nyström, M.; Folke, C.; Moberg, F. Coral reef disturbance and resilience in a human-dominated environment. Trends Ecol. Evol. 2000, 15, 413–417. [Google Scholar] [CrossRef]
- Bonin, M.C.; Almany, G.R.; Jones, G.P. Contrasting effects of habitat loss and fragmentation on coral-associated reef fishes. Ecology 2011, 92, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Munday, P.L.; Jones, G.P.; Pratchett, M.S.; Williams, A.J. Climate change and the future for coral reef fishes. Fish Fish. 2008, 9, 261–285. [Google Scholar] [CrossRef]
- Coker, D.J.; Wilson, S.K.; Pratchett, M.S. Importance of live coral habitat for reef fishes. Rev. Fish Biol. Fish. 2014, 24, 89–126. [Google Scholar] [CrossRef]
- Jones, G.P.; McCormick, M.I.; Srinivasan, M.; Eagle, J.V. Coral decline threatens fish biodiversity in marine reserves. Proc. Natl. Acad. Sci. USA 2004, 101, 8251–8253. [Google Scholar] [CrossRef] [PubMed]
- Pratchett, M.S.; Hoey, A.S.; Wilson, S.K.; Messmer, V.; Graham, N.A.J. Changes in biodiversity and functioning of reef fish assemblages following coral bleaching and coral loss. Diversity 2011, 3, 424–452. [Google Scholar] [CrossRef]
- Alevizon, W.S.; Porter, J.W. Coral loss and fish guild stability on a Caribbean coral reef: 1974–2000. Environ. Biol. Fish. 2015, 98, 1035–1045. [Google Scholar] [CrossRef]
- Chong-Seng, K.M.; Nash, K.L.; Bellwood, D.R.; Graham, N.A. Macroalgal herbivory on recovering versus degrading coral reefs. Coral Reefs 2014, 33, 409–419. [Google Scholar] [CrossRef]
- Mangubhai, S.; Strauch, A.M.; Obura, D.O.; Stone, G.; Rotjan, R.D. Short-term changes of fish assemblages observed in the near-pristine reefs of the Phoenix Islands. Rev. Fish Biol. Fish. 2014, 24, 505–518. [Google Scholar] [CrossRef]
- Williamson, D.H.; Ceccarelli, D.M.; Evans, R.D.; Jones, G.P.; Russ, G.R. Habitat dynamics, marine reserve status, and the decline and recovery of coral reef fish communities. Ecol. Evol. 2014, 4, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Pratchett, M.S.; Blowes, S.A.; Coker, D.; Kubacki, E.; Nowicki, J.; Hoey, A.S. Indirect benefits of high coral cover for non-corallivorous butterflyfishes. Coral Reefs 2014, 34, 665–672. [Google Scholar] [CrossRef]
- Emslie, M.J.; Pratchett, M.S.; Cheal, A.J.; Osborne, K. Great Barrier Reef butterflyfish community structure: The role of shelf position and benthic community type. Coral Reefs 2010, 29, 705–715. [Google Scholar] [CrossRef]
- Munday, P.L. Habitat loss, resource specialization, and extinction on coral reefs. Glob. Chang. Biol. 2004, 10, 1642–1647. [Google Scholar] [CrossRef]
- Pratchett, M.S. Dietary overlap among coral-feeding butterflyfishes (Chaetodontidae) at Lizard Island, northern Great Barrier Reef. Mar. Biol. 2005, 148, 373–382. [Google Scholar] [CrossRef]
- Hoey, J.; McCormick, M.I.; Hoey, A.S. Influence of depth on sex-specific energy allocation patterns in a tropical reef fish. Coral Reefs 2007, 26, 603–613. [Google Scholar] [CrossRef]
- Bellwood, D.R.; Hoey, A.S.; Ackerman, J.L.; Depczynski, M. Coral bleaching, reef fish community phase shifts and the resilience of coral reefs. Glob. Chang. Biol. 2006, 12, 1587–1594. [Google Scholar] [CrossRef]
- Brooker, R.M.; Munday, P.L.; Brandl, S.J.; Jones, G.P. Local extinction of a coral reef fish explained by inflexible prey choice. Coral Reefs 2014, 33, 891–896. [Google Scholar] [CrossRef]
- Hoey, A.S.; Bellwood, D.R. Cross-shelf variation in the role of parrotfishes on the Great Barrier Reef. Coral Reefs 2008, 27, 37–47. [Google Scholar] [CrossRef]
- Madin, J.S.; Connolly, S.R. Ecological consequences of major hydrodynamic disturbances on coral reefs. Nature 2006, 444, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Filip, L.; Dulvy, N.K.; Gill, J.A.; Côté, I.M.; Watkinson, A.R. Flattening of Caribbean coral reefs: Region-wide declines in architectural complexity. Proc. R. Soc. B 2009, 276, 3019–3025. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Filip, L.; Gill, J.A.; Dulvy, N.K.; Perry, A.L.; Watkinson, A.R.; Côté, I.M. Drivers of region-wide declines in architectural complexity on Caribbean reefs. Coral Reefs 2011, 30, 1051–1060. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Nash, K.L. The importance of structural complexity in coral reef ecosystems. Coral Reefs 2013, 32, 315–326. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; Gill, J.A.; Dulvy, N.K. Complex reef architecture supports more small-bodied fishes and longer food chains on Caribbean reefs. Ecosphere 2011, 2. [Google Scholar] [CrossRef]
- Emslie, M.J.; Cheal, A.J.; Johns, K.A. Retention of habitat complexity minimizes disassembly of reef fish communities following disturbance: A large-scale natural experiment. PLoS ONE 2014, 9, e105384. [Google Scholar] [CrossRef] [PubMed]
- Richardson, L.E.; Graham, N.A.J.; Pratchett, M.S.; Hoey, A.S. Structural complexity mediates functional structure of reef fish assemblages among coral habitats. Mar. Biol. 2016. under review. [Google Scholar]
- Nash, K.L.; Graham, N.A.J.; Wilson, S.K.; Bellwood, D.R. Cross-scale habitat structure drives fish body size distributions on coral reefs. Ecosystems 2013, 16, 478–490. [Google Scholar] [CrossRef]
- Graham, N.A.; Chabanet, P.; Evans, R.D.; Jennings, S.; Letourneur, Y.; MacNeil, M.A.; McClanahan, T.R.; Öhman, M.C.; Polunin, N.V.; Wilson, S.K. Extinction vulnerability of coral reef fishes. Ecol. Lett. 2011, 14, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Kerry, J.T.; Bellwood, D.R. The effect of coral morphology on shelter selection by coral reef fishes. Coral Reefs 2012, 31, 415–424. [Google Scholar] [CrossRef]
- Wen, C.K.; Pratchett, M.S.; Almany, G.R.; Jones, G.P. Patterns of recruitment and microhabitat associations for three predatory coral reef fishes on the southern Great Barrier Reef, Australia. Coral Reefs 2013, 32, 389–398. [Google Scholar] [CrossRef]
- Wilson, S.K.; Fisher, R.; Pratchett, M.S.; Graham, N.A.; Dulvy, N.K.; Turner, R.A.; Cakacaka, A.; Polunin, N.V. Habitat degradation and fishing effects on the size structure of coral reef fish communities. Ecol. Appl. 2010, 20, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.D.; Ganachaud, A.; Gehrke, P.C.; Griffiths, S.P.; Hobday, A.J.; Hoegh-Guldberg, O.; Johnson, J.E.; Le Borgne, R.; Lehodey, P.; Lough, J.M.; et al. Mixed responses of tropical Pacific fisheries and aquaculture to climate change. Nat. Clim. Chang. 2013, 3, 591–599. [Google Scholar] [CrossRef]
- McCormick, M.I.; Moore, J.A.Y.; Munday, P.L. Influence of habitat degradation on fish replenishment. Coral Reefs 2010, 29, 537–546. [Google Scholar] [CrossRef]
- Cole, A.J.; Lawton, R.J.; Pisapia, C.; Pratchett, M.S. The effects of coral bleaching on settlement preferences and growth of juvenile butterflyfishes. Mar. Environ. Res. 2014, 98, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Coker, D.J.; Pratchett, M.S.; Munday, P.L. Coral bleaching and habitat degradation increase susceptibility to predation for coral-dwelling fishes. Behav. Ecol. 2009, 20, 1204–1210. [Google Scholar] [CrossRef]
- McCormick, M.I. Lethal effects of habitat degradation on fishes through changing competitive advantage. Proc. R. Soc. B 2012, 279, 3899–3904. [Google Scholar] [CrossRef] [PubMed]
- Bonin, M.C. Specializing on vulnerable habitat: Acropora selectivity among damselfish recruits and the risk of bleaching-induced habitat loss. Coral Reefs 2012, 31, 287–297. [Google Scholar] [CrossRef]
- Brooker, R.M.; Munday, P.L.; McLeod, I.M.; Jones, G.P. Habitat preferences of a corallivorous reef fish: Predation risk versus food quality. Coral Reefs 2013, 32, 613–622. [Google Scholar] [CrossRef]
- Coker, D.J.; Hoey, A.S.; Wilson, S.K.; Depczynski, M.; Graham, N.A.J.; Hobbs, J.-P.A.; Holmes, T.H.; Pratchett, M.S. Habitat selectivity and reliance on live corals for Indo-Pacific hawkfishes (Family: Cirrhitidae). PLoS ONE 2015, 10, e0138136. [Google Scholar] [CrossRef] [PubMed]
- Pratchett, M.S.; Coker, D.J.; Jones, G.P.; Munday, P.L. Specialization in habitat use by coral reef damselfishes and their susceptibility to habitat loss. Ecol. Evol. 2012, 2, 2168–2180. [Google Scholar] [CrossRef] [PubMed]
- Messmer, V.; Jones, G.P.; Munday, P.L.; Holbrook, S.J.; Schmitt, R.J.; Brooks, A.J. Habitat biodiversity as a determinant of fish community structure on coral reefs. Ecology 2011, 92, 2285–2298. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Palacios, M.; Zapata, F.A. Fish community structure on coral habitats with contrasting architecture in the Tropical Eastern Pacific. Rev. Biol. Trop. 2014, 62, 343–357. [Google Scholar] [CrossRef]
- Komyakova, V.; Munday, P.L.; Jones, G.P. Relative importance of coral cover, habitat complexity and diversity in determining the structure of reef fish communities. PLoS ONE 2013, 8, e83178. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, S.J.; Schmitt, R.J.; Messmer, V.; Brooks, A.J.; Srinivasan, M.; Munday, P.L.; Jones, G.P. Reef fishes in biodiversity hotspots are at greatest risk from loss of coral species. PLoS ONE 2015, 10, e0124054. [Google Scholar] [CrossRef] [PubMed]
- Rasher, D.B.; Hoey, A.S.; Hay, M.E. Consumer diversity interacts with prey defenses to drive ecosystem function. Ecology 2013, 94, 1347–1358. [Google Scholar] [CrossRef] [PubMed]
- Messmer, V.; Blowes, S.A.; Jones, G.P.; Munday, P.L. Experimental evaluation of diversity-productivity relationships in a coral reef fish assemblage. Oecologia 2014, 176, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, J.P.; Smith, L.D.; Heyward, A.J.; Baird, A.H.; Pratchett, M.S. Recovery of an isolated coral reef system following severe disturbance. Science 2013, 340, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Adam, T.C.; Schmitt, R.J.; Holbrook, S.J.; Brooks, A.J.; Edmunds, P.J.; Carpenter, R.C.; Bernardi, G. Herbivory, connectivity, and ecosystem resilience: Response of a coral reef to a large-scale perturbation. PLoS ONE 2011, 6, e23717. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.K.; Bellwood, D.R.; Choat, J.H.; Furnas, M.J. Detritus in the epilithic algal matrix and its use by coral reef fishes. Oceanogr. Mar. Biol. Annu. Rev. 2003, 41, 279–310. [Google Scholar]
- Hoey, A.S.; Bellwood, D.R. Suppression of herbivory by macroalgal density: A critical feedback on coral reefs? Ecol. Lett. 2011, 14, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Dixson, D.L.; Abrego, D.; Hay, M.E. Chemically mediated behavior of recruiting corals and fishes: A tipping point that may limit reef recovery. Science 2014, 345, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Green, A.L. Spatio-temporal patterns of recruitment of labroid fishes (Pisces: Labridae and Scaridae) to damselfish territories. Environ. Biol. Fish. 1998, 51, 235–244. [Google Scholar] [CrossRef]
- Wilson, S.K.; Depczynski, M.; Fisher, R.; Holmes, T.H.; O’Leary, R.A.; Tinkler, P. Habitat associations of juvenile fish at Ningaloo Reef, Western Australia: The importance of coral and algae. PLoS ONE 2010, 5, e15185. [Google Scholar] [CrossRef] [PubMed]
- Lecchini, D.; Osenberg, C.W.; Shima, J.S.; St Mary, C.M.; Galzin, R. Ontogenetic changes in habitat selection during settlement in a coral reef fish: Ecological determinants and sensory mechanisms. Coral Reefs 2007, 26, 423–432. [Google Scholar] [CrossRef]
- Evans, R.D.; Wilson, S.K.; Field, S.N.; Moore, J.A.Y. Importance of macroalgal fields as coral reef fish nursery habitat in north-west Australia. Mar. Biol. 2014, 161, 599–607. [Google Scholar] [CrossRef]
- Hoey, A.S.; Brandl, S.J.; Bellwood, D.R. Diet and cross-shelf distribution of rabbitfishes (f. Siganidae) on the northern Great Barrier Reef: Implications for ecosystem function. Coral Reefs 2013, 32, 973–984. [Google Scholar] [CrossRef]
- Chong-Seng, K.M.; Mannering, T.D.; Pratchett, M.S.; Bellwood, D.R.; Graham, N.A.J. The influence of coral reef benthic condition on associated fish assemblages. PLoS ONE 2012, 7, e42167. [Google Scholar] [CrossRef] [PubMed]
- Chaves, L.T.C.; Pereira, P.H.C.; Feitosa, J.L.L. Coral reef fish association with macroalgal beds on a tropical reef system in North-eastern Brazil. Mar. Freshw. Res. 2013, 64, 1101–1111. [Google Scholar] [CrossRef]
- Hoey, A.S.; Bellwood, D.R. Cross-shelf variation in browsing intensity on the Great Barrier Reef. Coral Reefs 2010, 29, 499–508. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Jennings, S.; MacNeil, M.A.; Mouillot, D.; Wilson, S.K. Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 2015, 518, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.K.; Fulton, C.J.; Depczynski, M.; Holmes, T.H.; Noble, M.M.; Radford, B.; Tinkler, P. Seasonal changes in habitat structure underpin shifts in macroalgae-associated tropical fish communities. Mar. Biol. 2014, 161, 2597–2607. [Google Scholar] [CrossRef]
- Hoey, A.S.; Bellwood, D.R. Limited functional redundancy in a high diversity system: Single species dominates key ecological process on coral reefs. Ecosystems 2009, 12, 1316–1328. [Google Scholar] [CrossRef]
- Cole, A.J.; Lawton, R.J.; Pratchett, M.S.; Wilson, S.K. Chronic coral consumption by butterflyfishes. Coral Reefs 2011, 30, 85–93. [Google Scholar] [CrossRef]
- Cole, A.J.; Lawton, R.J.; Wilson, S.K.; Pratchett, M.S. Consumption of tabular acroporid corals by reef fishes: a comparison with plant–herbivore interactions. Funct. Ecol. 2012, 26, 307–316. [Google Scholar] [CrossRef]
- Kennedy, E.V.; Perry, C.T.; Halloran, P.R.; Iglesias-Prieto, R.; Schönberg, C.H.; Wisshak, M.; Form, A.U.; Carricart-Ganivet, J.P.; Fine, M.; Eakin, C.M.; et al. Avoiding coral reef functional collapse requires local and global action. Curr. Biol. 2013, 23, 912–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoey, A.S.; Howells, E.; Johansen, J.L.; Hobbs, J.-P.A.; Messmer, V.; McCowan, D.M.; Wilson, S.K.; Pratchett, M.S. Recent Advances in Understanding the Effects of Climate Change on Coral Reefs. Diversity 2016, 8, 12. https://doi.org/10.3390/d8020012
Hoey AS, Howells E, Johansen JL, Hobbs J-PA, Messmer V, McCowan DM, Wilson SK, Pratchett MS. Recent Advances in Understanding the Effects of Climate Change on Coral Reefs. Diversity. 2016; 8(2):12. https://doi.org/10.3390/d8020012
Chicago/Turabian StyleHoey, Andrew S., Emily Howells, Jacob L. Johansen, Jean-Paul A. Hobbs, Vanessa Messmer, Dominique M. McCowan, Shaun K. Wilson, and Morgan S. Pratchett. 2016. "Recent Advances in Understanding the Effects of Climate Change on Coral Reefs" Diversity 8, no. 2: 12. https://doi.org/10.3390/d8020012





