The Impact of Nesting Socotra Cormorants on Soil Chemistry and Vegetation in a Large Colony in the United Arab Emirates
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Area
2.2. Approach
2.3. Procedure
2.4. Statistical Analyses
3. Results
3.1. Vegetation Attributes to Discriminate Nesting Areas
| Steps | Variables | 2011 vs. 2012 | 2011 vs. Control | 2012 vs. Control |
|---|---|---|---|---|
| 1 | Percent Droppings | 0.09 | 0.001 | 0.004 |
| 2 | Percent Droppings and Other | 0.001 | 0.001 | 0.008 |
- 2011 Nesting Area: 0.070 % × Droppings + 1.040 × Other − 4.676
- 2012 Nesting Area: 0.054 % × Droppings + 1.57 × Other − 5.668
- Control Area: 0.032 % × Droppings + 1.388 × Other − 4.084
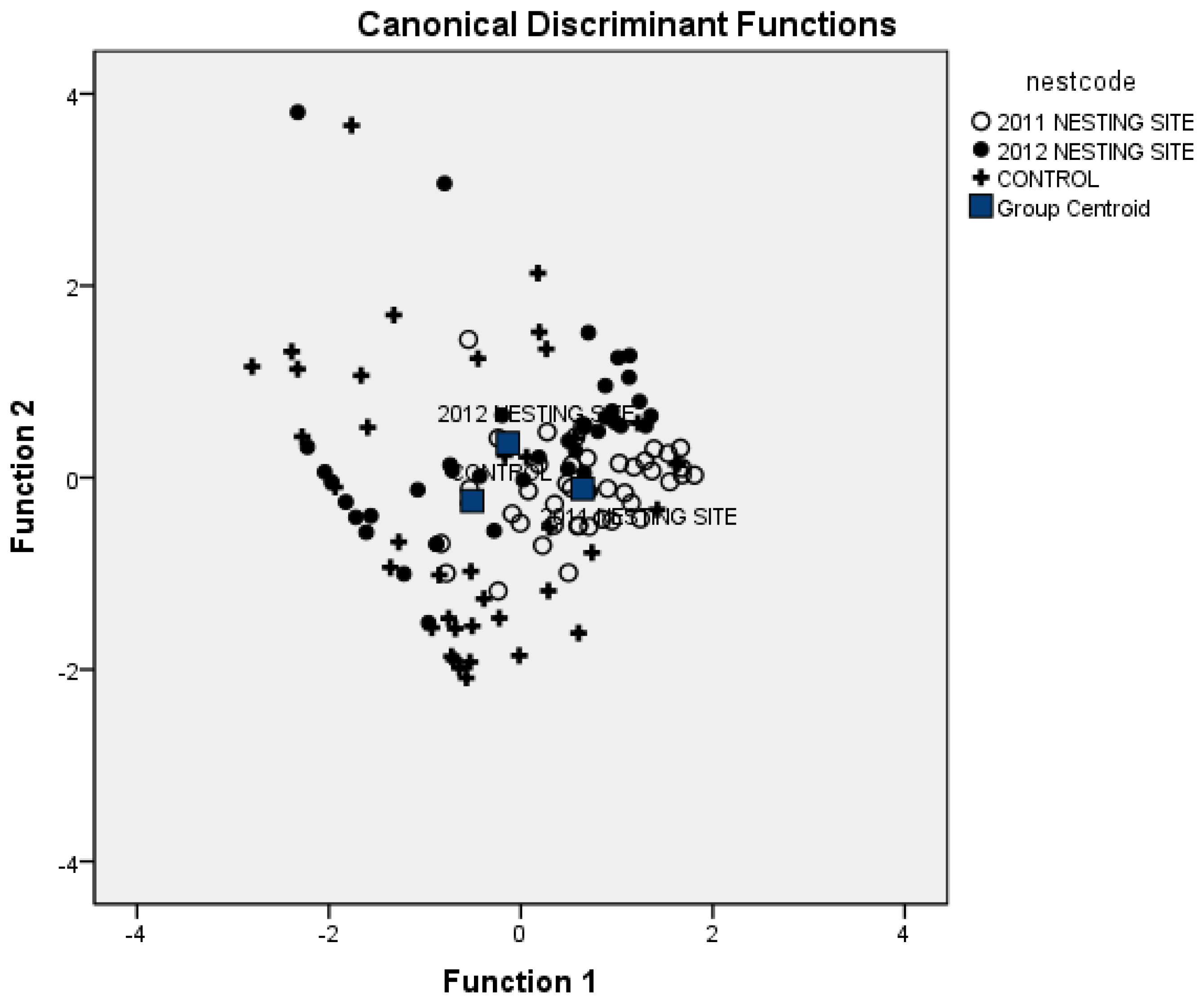
| Sampled Sites | 2011 Nesting Site | 2012 Nesting Site | Control Site |
|---|---|---|---|
| 2011 Nesting Site | 73.2 | 12.2 | 14.6 |
| 2012 Nesting Site | 46.3 | 14.6 | 39.0 |
| Control Site | 22.0 | 22.0 | 56.0 |
3.2. Soil Attributes to Discriminate Nesting Areas
| Soil Elements | Function 1 | Function 2 |
|---|---|---|
| Al (ppm) | 1.115 | 1.066 |
| Ca (ppm) | 0.688 | 0.108 |
| Cr (ppm) | −0.861 | 1.084 |
| Cu (ppm) | 0.494 | −0.689 |
| Fe (ppm) | 1.906 | −0.248 |
| Mg (ppm) | −1.157 | 0.532 |
| Mn (ppm) | −1.723 | −0.852 |
| Na (ppm) | 0.475 | 0.129 |
| P (ppm) | 0.058 | 0.470 |
| V (ppm) | −0.103 | −0.596 |
| Soil Elements | 2011 Nesting Site | 2012 Nesting Site | Control Site |
|---|---|---|---|
| Al (ppm) | 0.039 | 0.022 | 0.013 |
| Cr (ppm) | −0.048 | 0.077 | 0.037 |
| Cu (ppm) | 1.903 | −0.937 | 0.060 |
| Fe (ppm) | 0.017 | −0.012 | −0.013 |
| Mn (ppm) | −0.709 | −0.110 | 0.072 |
| V (ppm) | −0.193 | −0.291 | −44.764 |
| (Constant) | −76.434 | −52.684 | −44.764 |

| Sampled Sites | 2011 Nesting Site | 2012 Nesting Site | Control Site |
|---|---|---|---|
| 2011 Nesting Site | 100 | 0 | 0 |
| 2012 Nesting Site | 0 | 80.5 | 19.5 |
| Control Site | 0 | 4.9 | 95.1 |
3.3. Combining Vegetation and Soil Attributes to Discriminate Nest Areas
| Soil Elements | Function 1 | Function 2 |
|---|---|---|
| Al (ppm) | 2.402 | .809 |
| Ca (ppm) | 0.636 | 0.040 |
| Cr (ppm) | −0.101 | 1.003 |
| Cu (ppm) | 0.621 | −0.796 |
| Mg (ppm) | −1.116 | 0.645 |
| Mn (ppm) | −1.454 | −0.834 |
| Na (ppm) | 0.514 | 0.088 |
| P (ppm) | 0.025 | 0.437 |
| V (ppm) | −0.057 | −0.551 |
| Dead cover % | −0.234 | −0.276 |
3.4. Importance of Vegetation and Soil Attributes
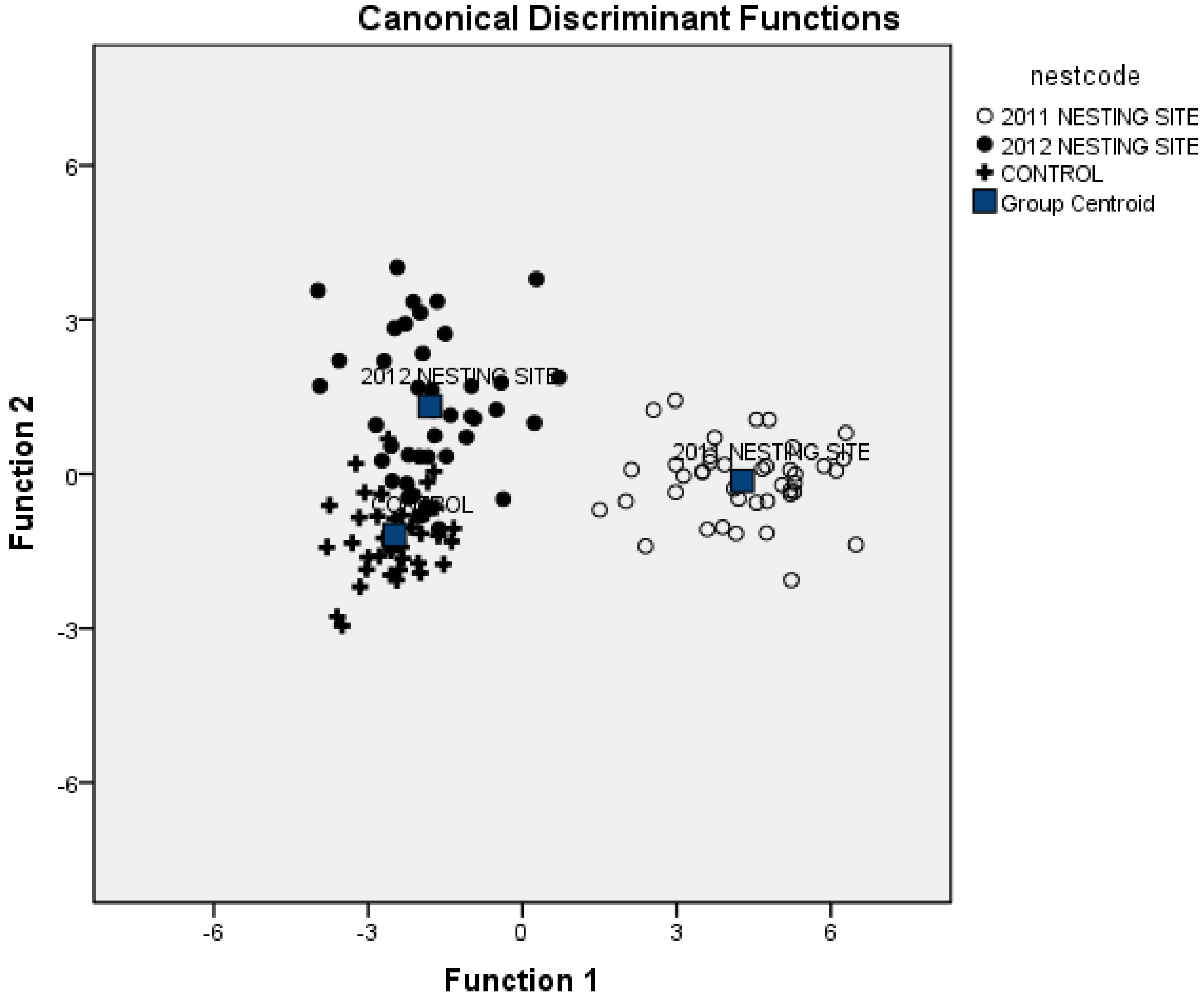
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ellis, J.C. Marine birds on land: A review of plant biomass, species richness, and community composition in seabird colonies. Plant Ecol. 2005, 181, 227–241. [Google Scholar] [CrossRef]
- Ellis, J.C.; Fariña, J.M.; Witman, J.D. Nutrient transfer from sea to land: The case of gulls and cormorants in the Gulf of Maine. J. Anim. Ecol. 2006, 75, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Mulder, C.; Ellis, J. (Eds.) Seabirds as ecosystem engineers: Nutrient inputs and physical disturbance. In Seabird Islands: Ecology, Invasion and Restoration; Oxford University Press: Oxford, UK, 2011; pp. 27–55.
- Mulder, C.; Jones, H.; Kameda, K.; Palmborg, C.; Schmidt, S.; Ellis, J.; Orrock, J.; Wait, A.; Wardle, D.; Yang, L.; et al. (Eds.) Impacts of seabirds on plant and soil properties. In Seabird Islands: Ecology, Invasion and Restoration; Oxford University Press: Oxford, UK, 2011.
- Wait, D.; Aubrey, D.; Anderson, W. Seabird guano influences on desert islands: soil chemistry and herbaceous species richness and productivity. J. Arid Environ. 2005, 60, 681–695. [Google Scholar] [CrossRef]
- Schreiber, E.A.; Burger, J. Biology of Marine Birds; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Steele, J.H. The Structure of Marine Ecosystems; Harvard University Press: Cambridge, MA, USA, 1974. [Google Scholar]
- Sharpley, A.N.; Smith, S.; Bain, W. Nitrogen and phosphorus fate from long-term poultry litter applications to Oklahoma soils. Soil Sci. Soc. Am. J. 1993, 57, 1131–1137. [Google Scholar] [CrossRef]
- Blackall, T.D.; Wilson, L.J.; Theobald, M.R.; Milford, C.; Nemitz, E.; Bull, J.; Bacon, P.J.; Hamer, K.C.; Wanless, S.; Sutton, M.A. Ammonia emissions from seabird colonies. Geophys. Res. Lett. 2007, 34. [Google Scholar] [CrossRef]
- Sun, L.; Xie, Z.; Zhao, J. Palaeoecology: A 3,000-year record of penguin populations. Nature 2000, 407, 858–858. [Google Scholar] [CrossRef] [PubMed]
- Rajakaruna, N.; Pope, N.; Perez-Orozco, J.; Harris, T.B. Ornithocoprophilous plants of Mount Desert Rock, a remote bird-nesting island in the Gulf of Maine, USA. Rhodora 2009, 111, 417–447. [Google Scholar] [CrossRef]
- Garcia, L.V.; Maranón, T.; Ojeda, F.; Clemente, L.; Redondo, R. Seagull influence on soil properties, chenopod shrub distribution, and leaf nutrient status in semi-arid Mediterranean islands. Oikos 2002, 98, 75–86. [Google Scholar] [CrossRef]
- Szpak, P.; Longstaffe, F.J.; Millaire, J.F.; White, C.D. Stable Isotope Biogeochemistry of Seabird Guano Fertilization: Results from Growth Chamber Studies with Maize (Zea mays). PLoS One 2012, 7, e33741. [Google Scholar] [CrossRef] [PubMed]
- Muzaffar, S.B.; Gubiani, R.; Benjamin, S. Reproductive Performance of the Socotra Cormorant Phalacrocorax nigrogularis on Siniya Island, United Arab Emirates: Planted Trees Increase Hatching Success. Waterbirds 2012, 35, 626–630. [Google Scholar] [CrossRef]
- Gubiani, R.; Benjamin, S.; Muzaffar, S.B. First Record of Cannibalism in Socotra Cormorants (Phalacrocorax nigrogularis): Large, Immature Birds Opportunistically Feed on Younger Conspecifics. Waterbirds 2012, 35, 338–341. [Google Scholar] [CrossRef]
- Jennings, M.C.; Krupp, F.; others. Fauna of Arabia. Atlas of the Breeding Birds of Arabia; King Abdulaziz City for Science and Technology: Ryadh, KSA, 2010; Volume 25. [Google Scholar]
- Symens, P.; Kinzelbach, R.; Suhaibani, A.; Werner, M. A review of the status, distribution and conservation of the Socotra Cormorant, Phalacrocorax nigrogularis. Zool. Middle East 1993, 8, 17–30. [Google Scholar] [CrossRef]
- Muzaffar, S.B.; Gubiani, R.; Benjamin, S. Nest location influences hatching success in the Socotra Cormorant (Phalacrocorax nigrogularis) on Siniya Island, United Arab Emirates. Wildl. Res. 2014, in press. [Google Scholar]
- Breuning-Madsen, H.; Ehlers, C.B.; Borggaard, O.K. The impact of perennial cormorant colonies on soil phosphorus status. Geoderma 2008, 148, 51–54. [Google Scholar] [CrossRef]
- Landgrebe, D.; Biehl, L. An introduction to MultiSpec; School of Electrical Engineering, Purdue University: West Lafayette, IN, USA, 1994. [Google Scholar]
- Bettinelli, M.; Beone, G.; Spezia, S.; Baffi, C. Determination of heavy metals in soils and sediments by microwave-assisted digestion and inductively coupled plasma optical emission spectrometry analysis. Anal. Chim. Acta 2000, 424, 289–296. [Google Scholar] [CrossRef]
- Arbuckle, J.L. IBM SPSS ® Amos 19 User’s Guide; Amos Development Corporation: Crawfordville, FL, USA, 2010. [Google Scholar]
- Burger, J.; Gochfeld, M. Colony and habitat selection of six Kelp Gull Larus dominicanus colonies in South Africa. Ibis 1981, 123, 298–310. [Google Scholar] [CrossRef]
- Storey, A.E.; Montevecchi, W.A.; Andrews, H.F.; Sims, N.; others. Constraints on nest site selection: A comparison of predator and flood avoidance in four species of marsh-nesting birds (genera: Catoptrophorus, Larus, Rallus, and Sterna). J. Comp. Psychol. (Washington, DC: 1983) 1988, 102, 14. [Google Scholar] [CrossRef]
- Willis, H.H.; MacDonald Gibson, J.; Shih, R.A.; Geschwind, S.; Olmstead, S.; Hu, J.; Curtright, A.E.; Cecchine, G.; Moore, M. Prioritizing environmental health risks in the UAE. Risk Anal. 2010, 30, 1842–1856. [Google Scholar]
- Alyazouri, A.; Jewsbury, R.; Tayim, H.; Humphreys, P.; Al-Sayah, M.H. Applicability of heavy-metal phytoextraction in United Arab Emirates: an investigation of candidate species. Soil Sediment Contamin. 2014, 23, 557–570. [Google Scholar] [CrossRef]
- Hawke, D.J. Soil P in a forested seabird colony: Inventories, parent material contributions, and N:P stoichiometry. Soil Res. 2006, 43, 957–962. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ksiksi, T.S.; Muzaffar, S.B.; Gubiani, R.; Alshihi, R.M. The Impact of Nesting Socotra Cormorants on Soil Chemistry and Vegetation in a Large Colony in the United Arab Emirates. Diversity 2015, 7, 60-73. https://doi.org/10.3390/d7010060
Ksiksi TS, Muzaffar SB, Gubiani R, Alshihi RM. The Impact of Nesting Socotra Cormorants on Soil Chemistry and Vegetation in a Large Colony in the United Arab Emirates. Diversity. 2015; 7(1):60-73. https://doi.org/10.3390/d7010060
Chicago/Turabian StyleKsiksi, Taoufik Saleh, Sabir Bin Muzaffar, Robert Gubiani, and Rashid Mohamed Alshihi. 2015. "The Impact of Nesting Socotra Cormorants on Soil Chemistry and Vegetation in a Large Colony in the United Arab Emirates" Diversity 7, no. 1: 60-73. https://doi.org/10.3390/d7010060
APA StyleKsiksi, T. S., Muzaffar, S. B., Gubiani, R., & Alshihi, R. M. (2015). The Impact of Nesting Socotra Cormorants on Soil Chemistry and Vegetation in a Large Colony in the United Arab Emirates. Diversity, 7(1), 60-73. https://doi.org/10.3390/d7010060







