The Impacts of Recently Established Fish Populations on Zooplankton Communities in a Desert Spring, and Potential Conflicts in Setting Conservation Goals
Abstract
:1. Introduction
2. Experimental Section
2.1. Experimental Setup
| Invertebrate | Average Density (per L ± SE) | W | p-Value | |
|---|---|---|---|---|
| Group | Small Tank (n = 2) | Large Tank (n = 3) | ||
| Rotifers | 19,841.0 (±10,459.0) | 31,516.7 (±2534.5) | 1 | 0.4 |
| Crustacean nauplii | 39.8 (±1.3) | 33.2 (±31.9) | 6 | 0.2 |
| Cladocera | 277.0 (±29.0) | 264.0 (±21.2) | 4 | 0.8 |
| Calanoid copepods | 20.5 (±9.0) | 25.2 (±7.1) | 2 | 0.8 |
| Cyclopoid copepods | 105.0 (±51.0) | 67.2 (±28.2) | 5 | 0.4 |
| Water mites | 12.0 (±2.0) | 4.5 (±1.5) | 6 | 0.2 |
2.2. Zooplankton Sampling
2.3. Statistical Analysis
2.3.1. Univariate Analysis
2.3.2. Multivariate Analysis
3. Results and Discussion
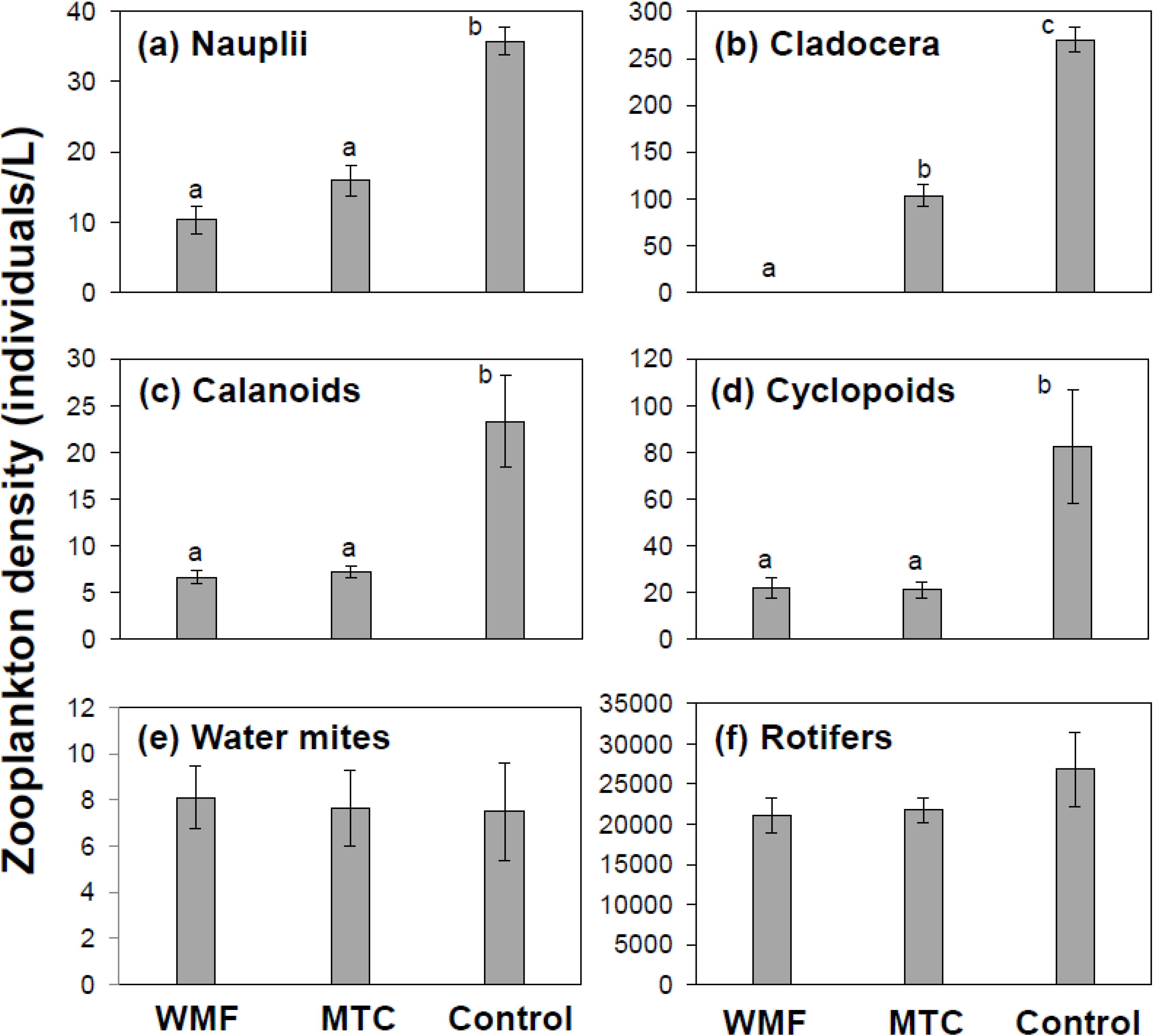
3.1. Univariate Analysis
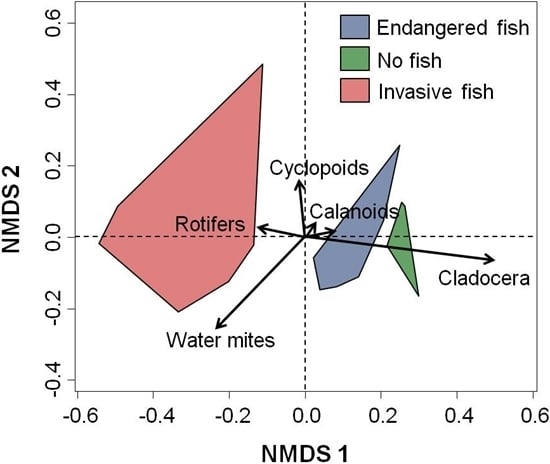
3.2. Multivariate Analysis
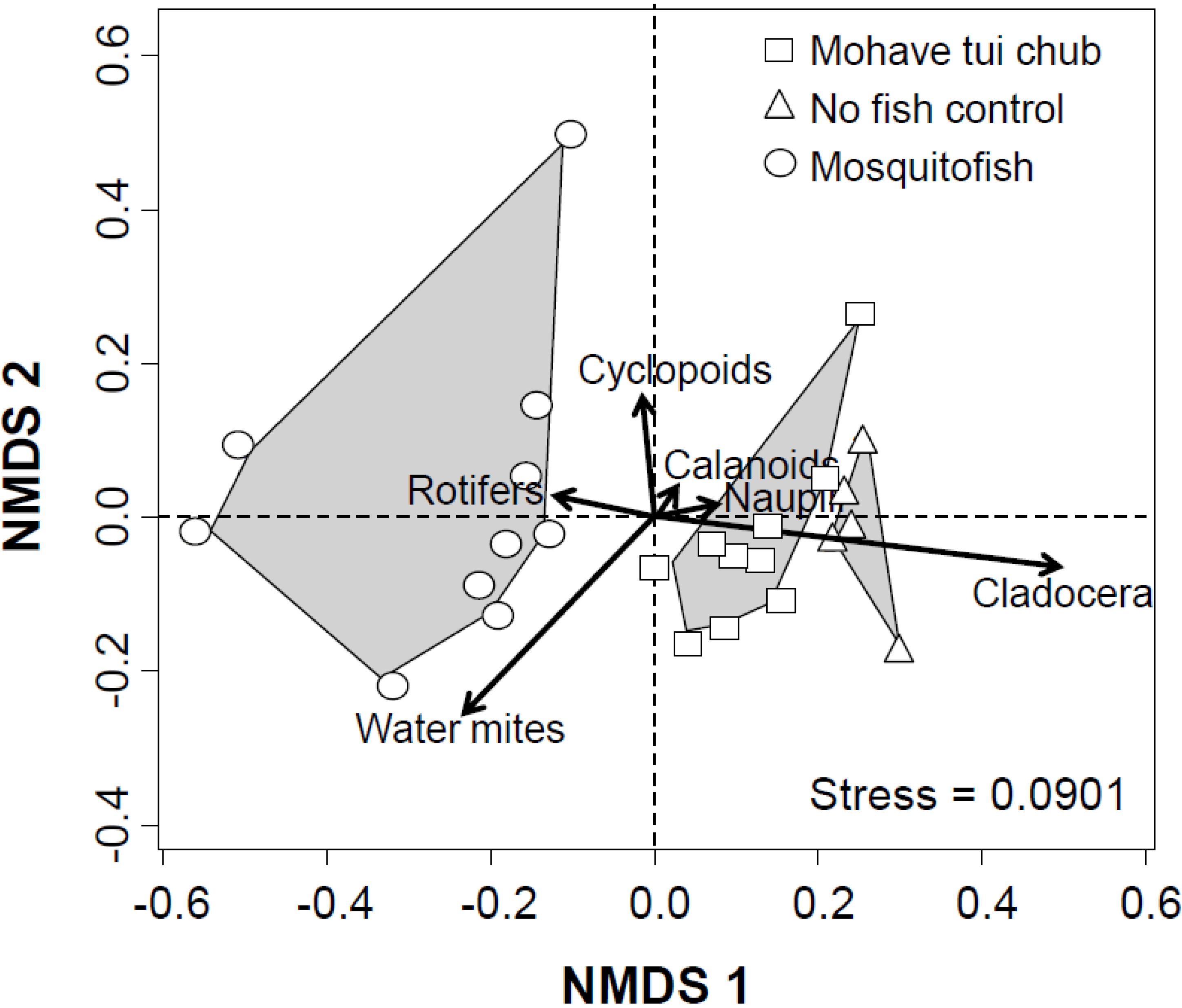
3.3. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Williams, J.E.; Bowman, D.B.; Brooks, J.E.; Echelle, A.A.; Edwards, R.J.; Hendrickson, A.; Landye, J.J. Endangered Aquatic Ecosystems in North American Deserts with a List of Vanishing Fishes of the Region. J. Ariz.-Nev. Acad. Sci. 1985, 20, 1–62. [Google Scholar]
- Stevens, L.E.; Meretsky, V.J. Springs ecosystem ecology and conservation. In Aridland Springs of North America: Ecology and Conservation; Stevens, L.E., Meretsky, V.J., Eds.; Arizona-Sonora Desert Museum Studies in Natural History, The University of Arizona Press: Tucson, AZ, USA, 2008; pp. 3–10. [Google Scholar]
- Shepard, W.D. Desert springs-both rare and endangered. Aquat. Conserv. Mar. Freshw. Ecosyst. 1993, 3, 351–359. [Google Scholar] [CrossRef]
- Hershler, R.; Sada, D.W. Springsnails (Gastropoda: Hydrobiidae) of Ash Meadows, Amargosa Basin, California-Nevada. Proc. Biol. Soc. Wash. 1987, 100, 776–843. [Google Scholar]
- Hershler, R.; Sada, D.W. Biogeography of Great Basin aquatic snails of the genus Pyrgulopsis. In Great Basin Aquatic Systems History; Heshler, R., Madsen, D., Currey, D.R., Eds.; Smithsonian Contributions to the Earth Science: Washington, DC, USA, 2002; pp. 283–298. [Google Scholar]
- Shepard, W.D. Microcylloepus formicoideus (Coleoptera: Elmidae), A new riffle beetle from Death Valley National Monument, California. Entomol. News 1990, 101, 147–153. [Google Scholar]
- Polhemus, D.A.; Polhemus, J.T. Basin and ranges: The biogeography of aquatic true bugs (Insecta: Heteroptera). In Great Basin Aquatic Systems History; Heshler, R., Madsen, D., Currey, D.R., Eds.; Smithsonian Contributions to the Earth Science: Washington, DC, USA, 2002; pp. 235–254. [Google Scholar]
- Hershler, R.; Liu, H.; Landye, J.J. New species and records of spring snails (Caenogastropoda: Cochliopidae: Tryonia) from the Chihuahuan Desert (Mexico and United States), an imperiled biodiversity hotspot. Zootaxa 2011, 3001, 1–32. [Google Scholar]
- Bogan, M.T.; Noriega-Felix, N.; Vidal-Aguilar, S.L.; Gutiérrez-Ruacho, O.G.; Findley, L.T.; Lytle, D.A.; Alvarado-Castro, J.A.; Varela-Romero, A. Biogeography and conservation of aquatic fauna in spring-fed tropical canyons of the southern Sonoran Desert, Mexico. Biodivers. Conserv. 2014, 23, 2705–2748. [Google Scholar] [CrossRef]
- Walsh, E.J.; Schröder, T.; Wallace, R.L.; Ríos-Arana, J.V.; Rico-Martínez, R. Rotifers from selected inland saline waters in the Chihuahuan Desert of Mexico. Saline Syst. 2008. [Google Scholar] [CrossRef]
- Hale, J.R.; Mims, M.C.; Bogan, M.T.; Olden, J.D. Links between two interacting factors, novel habitats and non-native predators, and aquatic invertebrate communities in a dryland environment. Hydrobiologia 2014. [Google Scholar] [CrossRef]
- Suárez-Morales, E.; Gutiérrez-Aguirre, M.A.; Walsh, E.J. Freshwater Copepoda (Crustacea) from the Chihuahuan Desert with Comments on Biogeography. Southwest. Nat. 2010, 55, 525–531. [Google Scholar]
- Unmack, P.J.; Minckley, W.L. The demise of desert springs. In Aridland Springs of North America: Ecology and Conservation; Stevens, L.E., Meretsky, V.J., Eds.; Arizona-Sonora Desert Museum Studies in Natural History, The University of Arizona Press: Tucson, AZ, USA, 2008; pp. 11–34. [Google Scholar]
- Pister, E. Desert fishes and their habitats. Trans. Am. Fish. Soc. 1974, 3, 531–540. [Google Scholar] [CrossRef]
- Meffe, G.K. Predation and species replacement in American southwestern fishes: A case study. Southwest. Nat. 1985, 30, 173–187. [Google Scholar] [CrossRef]
- Deacon, J.E.; Williams, C.D. Ash Meadows and the legacy of the Devils Hole pupfish. In Battle Against Extinction: Native Fish Management in the American West; Minckley, W.L., Deacon, J.E., Eds.; The University of Arizona Press: Tucson, AZ, USA, 1991; pp. 69–87. [Google Scholar]
- Soltz, D.L.; Naiman, R.J. The Natural History of Native Fishes in the Death Valley System; Science Series 30; Natural History Museum of Los Angeles County: Los Angeles, CA, USA, 1978. [Google Scholar]
- Hendrickson, D.A.; Brooks, J.E. Transplanting short-lived fishes in North American deserts: Review, assessment, and recommendations. In Battle Against Extinction: Native Fish Management in the American West; Minckley, W.L., Deacon, J.E., Eds.; The University of Arizona Press: Tucson, AZ, USA, 1991; pp. 283–298. [Google Scholar]
- Minckley, W.L. Translocation as a tool for conserving imperiled fishes: Experiences in western United States. Biol. Conserv. 1995, 72, 297–309. [Google Scholar] [CrossRef]
- Hoover, F.; Amant, J.A., St. Results of Mohave tui chub (Gila bicolor mohavensis) relocations in California and Nevada. Calif. Fish Game 1983, 69, 54–56. [Google Scholar]
- U.S. Fish & Wildlife Service. Recovery Plan Pahrump Killifish; U.S. Fish & Wildlife Service: Portland, OR, USA, 1980.
- Johnson, J.E.; Hubbs, C. Status and Conservation of Poeciliid Fishes. In Ecology and Evolution of Livebearing Fishes (Poeciliidae); Meffe, G.K., Snelson, F.F., Jr., Eds.; Prentice Hall Advanced Reference Series; Prentice Hall Inc.: Upper Saddle River, NJ, USA, 1989; pp. 301–317. [Google Scholar]
- Roemer, G.W.; Wayne, R.K. Conservation in conflict: The tale of two endangered species. Conserv. Biol. 2003, 17, 1251–1260. [Google Scholar] [CrossRef]
- Gumm, J.M.; Sneckser, J.L.; Itzkowitz, M. Conservation and conflict between endangered desert fishes. Biol. Lett. 2008, 4, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Gumm, J.M.; Sneckser, J.L.; Leesa, J.M.; Little, K.P.; Leiser, J.K.; Imhoff, V.E.; Westrick, B.; Itzkowitz, M. Management of interactions between endangered species using habitat restoration. Biol. Conserv. 2011, 144, 2171–2176. [Google Scholar] [CrossRef]
- Hurlbert, S.H.; Zedler, J.; Fairbanks, D. Ecosystem alteration by mosquitofish (Gambusia affinis) predation. Science 1972, 175, 639–641. [Google Scholar] [CrossRef] [PubMed]
- Angeler, D.G.; Álvarez-Cobelas, M.; Sánchez-Carrillo, S.; Rodrigo, M.A. Assessment of exotic fish impacts on water quality and zooplankton in a degraded semi-arid floodplain wetland. Aquat. Sci.-Res. Bound. 2002, 64, 76–86. [Google Scholar] [CrossRef]
- Stockwell, C.A.; Henkanaththegedara, S.M. Evolutionary Conservation Biology of Poeciliids. In Ecology and Evolution of Poeciliid Fishes; Evan, J., Pilastro, A., Schlupp, I., Eds.; University of Chicago Press: Chicago, IL, USA, 2011; pp. 128–141. [Google Scholar]
- Leyse, K.E.; Lawler, S.P.; Strange, T. Effects of alien fish, Gambusia affinis, on an endemic California fairy shrimp, Linderiella occidentalis: Implications for conservation of diversity in fishless waters. Biol. Conserv. 2004, 118, 57–65. [Google Scholar] [CrossRef]
- Moyle, P.B. Fish introductions in California: History and impact on native fishes. Biol. Conserv. 1976, 9, 101–118. [Google Scholar] [CrossRef]
- Miller, R.R. Records of some native freshwater fishes transplanted in to various waters of California, Baja California and Nevada. Calif. Fish Game 1968, 54, 170–179. [Google Scholar]
- U.S. Fish & Wildlife Service. Recovery Plan for the Mohave Tui Chub; Gila bicolor mohavensis, United States Fish and Wildlife Service: Portland, OR, USA, 1984; pp. 1–56.
- Henkanaththegedara, S.M.; Stockwell, C.A. Melanism in Endangered Mohave Tui Chub Siphateles Bicolor Mohavensis Snyder 1918 (Cypriniformes: Cyprinidae). West. North Am. Nat. 2011, 71, 127–130. [Google Scholar] [CrossRef]
- Henkanaththegedara, S.; Longwood University, Farmville, VA, USA. Unpublished work. 2011.
- Henkanaththegedara, S.M.; Stockwell, C.A. Intraguild predation may facilitate coexistence of native and non-native fish. J. Appl. Ecol. 2014, 51, 1057–1065. [Google Scholar] [CrossRef]
- Lind, O. Handbook of Common Methods in Limnology, 2nd ed.; Kendall/Hunt Publishing Company: Dubuque, IA, USA, 1985; p. 33. [Google Scholar]
- Pennak, R.W. Fresh-Water Invertebrates of the United States: Protozoa to Mollusca, 3rd ed.; Wiley-Interscience: New York, NY, USA, 1989; pp. 1–656. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2010. Available online: http://www.R-project.org (accessed on 12 August 2012).
- Zar, J.H. Biostatistical Analysis, 5th ed.; Prentice Hall Inc.: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Rice, W.R. Analyzing tables of statistical tests. Evolution 1989, 43, 223–225. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Henry, M.; Stevens, H.; et al. Vegan: Community Ecology Package. R package version 2.0–4. 2011. Available online: http://CRAN.R-project.org/package=vegan2011 (accessed on 12 August 2012).
- Oksanen, J. Multivariate analysis of ecological communities in R: Vegan tutorial. 2011. Available online: http://cc.oulu.fi/~jarioksa/opetus/metodi/vegantutor.pdf (accessed on 12 August 2012).
- McCune, B.; Grace, J.B. Analysis of Ecological Communities; MjM Software Design: Gleneden Beach, OR, USA, 2002; pp. 125–142. [Google Scholar]
- Hurlbert, S.H.; Mulla, M.S. Impacts of mosquitofish (Gambusia affinis) predation on plankton communities. Hydrobiologia 1981, 83, 125–151. [Google Scholar] [CrossRef]
- Preston, D.L.; Henderson, J.S.; Johnson, P.T.J. Community ecology of invasions: Direct and indirect effects of multiple invasive species on aquatic communities. Ecology 2012, 93, 1254–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, J.L.; Dodson, S.I. Body Size, and Composition of Plankton. Science 1965, 150, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Kerfoot, W.C. A question of taste: Crypsis and warning coloration in fresh water zooplankton communities. Ecology 1982, 63, 538–554. [Google Scholar] [CrossRef]
- Hunter, M.L. Climate Change and Moving Species: Furthering the Debate on Assisted Colonization. Conserv. Biol. 2007, 21, 1356–1358. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, J.H.; Hellmann, J.J.; Schwartz, M.W. A Framework for Debate of Assisted Migration in an Era of Climate Change. Conserv. Biol. 2007, 21, 297–302. [Google Scholar]
- Hoegh-Guldberg, O.; Hughes, L.; McIntyre, S.; Lindenmayer, D.B.; Parmesan, C.; Possingham, H.P.; Thomas, C.D. Assisted Colonization and Rapid Climate Change. Science 2008, 321, 345–346. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Riggs, M.R. Potential effects of fish predation on Wetland invertebrates: A comparison of wetlands with and without fathead minnows. Wetlands 1995, 15, 167–175. [Google Scholar] [CrossRef]
- Zimmer, K.D.; Hanson, M.A.; Butler, M.G. Effects of Fathead Minnow Colonization and Removal on a Prairie Wetland Ecosystem. Ecosystems 2001, 4, 346–357. [Google Scholar] [CrossRef]
- Gilinsky, E. The Role of Fish Predation and Spatial Heterogeneity in Determining Benthic Community Structure. Ecology 1984, 65, 455–468. [Google Scholar] [CrossRef]
- Henkanaththegedara, S.M. Ecological Complexity of Non-Native Species Impacts in Desert Aquatic Systems. Ph.D. Thesis, North Dakota State University, Fargo, ND, USA, 2011. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henkanaththegedara, S.M.; Fisher, J.D.L.; McEwen, D.C.; Stockwell, C.A. The Impacts of Recently Established Fish Populations on Zooplankton Communities in a Desert Spring, and Potential Conflicts in Setting Conservation Goals. Diversity 2015, 7, 3-15. https://doi.org/10.3390/d7010003
Henkanaththegedara SM, Fisher JDL, McEwen DC, Stockwell CA. The Impacts of Recently Established Fish Populations on Zooplankton Communities in a Desert Spring, and Potential Conflicts in Setting Conservation Goals. Diversity. 2015; 7(1):3-15. https://doi.org/10.3390/d7010003
Chicago/Turabian StyleHenkanaththegedara, Sujan M., Justin D. L. Fisher, Daniel C. McEwen, and Craig A. Stockwell. 2015. "The Impacts of Recently Established Fish Populations on Zooplankton Communities in a Desert Spring, and Potential Conflicts in Setting Conservation Goals" Diversity 7, no. 1: 3-15. https://doi.org/10.3390/d7010003