1. Introduction
As humankind continues on a trajectory of urbanization, cities have begun to constitute a large and increasing influence on biodiversity distributions and patterns. By 2030, the greater proportion of the world’s human population is predicted to reside in urban areas [
1]. Cities provide heterogeneous environments for biodiversity, with dense, frequent and intense interactions between humans and nature [
2]. Thus, human land use has a persistent impact on urban ecosystems, with changes in urban green space clearly linked with the reduced resilience of cities [
1,
3].
Insects constitute key indicators that enable the monitoring of the impact of urbanization on biodiversity, responding sensitively to changes in habitat extent and quality and to altered management practices associated with urbanization [
4,
5]. Insects play key roles in nutrient cycling, organic matter decomposition, pollination and soil aeration in urban ecosystems [
6]. Yet, although arthropod taxa, such as, ants and butterflies, are abundant in urban environments, little is known about how these are distributed within a city environment [
7,
8,
9]. Most urban biodiversity research has tended to focus on large green patches of remnant forests or urban parks [
10,
11]. Yet, there has been increasing research to show the potential for small scattered habitats, such as green roofs, domestic gardens and community gardens, to support rich biodiversity, even in densely populated urban areas [
5,
8,
12].
Domestic gardens—private patches of garden associated with residential homes—are widespread across most urban locations, but tend to be under researched [
13]. Although it is known that domestic gardens can contain rich insect assemblages [
14,
15,
16], the factors related to variation in biodiversity remain poorly understood [
17,
18]. Domestic gardens differ from most urban forests and parks in the fact that they are managed largely at an individual level [
19]. Thus, they can vary greatly in their structure, composition and biodiversity, depending on the often idiosyncratic preferences of home owners and residents, influenced further by their socio-economic and cultural backgrounds and contexts [
20,
21].
Trees and plants in domestic gardens provide critical habitat for small-sized taxa, such as small mammals, birds and insects, and even small changes, such as adding a few plants at a micro-ecological scale, can result in significant increases in insect recruitment [
18]. Further, though individual garden patches are often small and scattered, at a city scale, networks of domestic garden patches can be quite extensive, providing important habitat connectivity to facilitate species movement and exchange in urban contexts [
22] and increasing biodiversity levels in adjacent locations [
23]. They can also occupy a substantial proportion of urban land use in many cities, covering close to 25% of the area in many cities in the UK [
24] and 36% of the area within a city in New Zealand [
25].
This paper assesses the factors influencing the diversity and distribution of insect taxa in the urban domestic gardens of Bangalore city. Bangalore constitutes India’s third largest city, with a population of close to 8.5 million. A rapid increase in population density over the past decade has resulted in substantial expansion of built area and a corresponding decrease in green space [
26]. The city has witnessed a concomitant, slow conversion of many large- and moderate-sized single domestic gardens in the city into small-sized, shared apartment gardens with lower tree density and diversity [
5], which may also indicate a decrease in the levels of biodiversity of other species, such as birds and insects. Previous research has indicated that home owners and residents perceive declines in a variety of urban wildlife visiting domestic gardens, ranging from monkeys to sparrows. Yet, a majority of house owners indicated that they could not support further increases in their garden area, citing reasons of lack of space and difficulties in maintenance, indicating the challenges of the protection of domestic gardens in a continuously expanding city with the constraints of land availability [
5].
This paper assesses the abundance, diversity and distribution of insect taxa in urban domestic gardens in Bangalore and seeks to identify the factors influencing the distribution of insects. Urban domestic gardens remain little studied, and this is especially so in developing regions of the world, as with Bangalore. This research contributes to a long-term program of investigation aimed at providing information on the factors influencing urban ecology and biodiversity in Bangalore, which can greatly help in urban conservation planning and management [
5,
27,
28,
29]. The study adds to the paucity of information currently available on insect diversity in urban environments, as well as providing information on the ecology of urban domestic gardens in tropical developing countries, with implications for land use planning.
2. Methods
The administrative boundary of Bangalore,
i.e., the Bruhat Bengaluru Mahanagara Palike (BBMP) boundary, was used to circumscribe the study area. Within this, sampling was done at the level of wards, which represent the lowest administrative unit of governance within the city. The BBMP boundary consists of 198 administrative sub-units, or wards, within the BBMP, of which 30 wards were randomly selected for study. We assumed that such a ward-based selection enabled us to ensure a wide geographic distribution across the city and to cover a diversity of areas with different land use history and socio-economic distributions, which have been shown to impact plant species composition and density in streets and parks in Bangalore [
28,
29], such that the results can be generalizable to the city scale.
Within a ward, the geographic center was located, and two domestic gardens located nearest to the ward center were selected for sampling, based on a garden size cutoff of at least 100 square feet and the availability of permission from residents to conduct sampling. In five wards, we could not locate any home gardens of the required size—these wards were all located in the center of the city, where homes tend to be congested, with limited garden area. A total of 50 domestic gardens were sampled, therefore, from 25 wards. Sampling was conducted once in each garden in the post-monsoon season, between September and December, 2009. Given the challenges with getting permission from residents to conduct sampling, we were not able to conduct repeated sampling in these gardens at different points in time, during other seasons.
Within a garden, all trees were identified at the species level and their diameter at breast height (DBH) and height recorded. Shrubs, herbs and creepers were also identified at the species level in each garden, except for genera of highly hybridized plants. We did not record the number of individuals belonging to these categories, but only recorded their presence/absence, as they were sometimes planted in dense clumps, making it difficult to accurately record the numbers of individuals. For each garden, we also recorded the total area, the proportion of garden covered by grass, non-grass and concrete surfaces and the presence of ponds and composting pits (if any). We conducted brief interviews with residents with the help of a brief open-ended questionnaire that asked residents about the age of the house, the intensity of weeding, pruning and watering, the use of pesticides and herbicides, the presence of a bird feed and whether any other ecological practices were observed (such as the use of neem cakes, the application of firewood ash, etc.). Insect sampling was conducted using a combination of pitfall traps and light traps, which were placed at the same garden once during the sampling period. These traps were chosen after conducting a trial study of different sampling techniques in urban gardens in Bangalore. We selected a set of traps that were safe to be installed in domestic gardens where there are pets and curious children and that residents were tolerant of, providing minimum disturbance to their gardens. Disposable plastic containers, of 110 mm height and 90 mm diameter at the rim, were used for pitfall traps. Two pitfall traps were buried in the garden, in areas of exposed soil (as residents did not permit their lawns and vegetated areas to be disturbed during sampling), with the rim of the container placed at ground level. Each trap contained 100 mL of 50% propylene glycol solution (we avoided using ethylene glycol, as these traps were placed in domestic gardens, due to the risk of being found by pets or children). Each trap was covered with a plastic cover, positioned at a gap of 20 mm above the rim of the trap to provide some protection from wind and rain, while permitting insects to enter. Both traps were set up on the same evening, located at the maximum distance possible given the garden layout, and left for a period of 24 hours on the sampling site. The insects trapped in the container were collected and preserved in 50% propylene glycol and transported to the laboratory for identification. Information reported from each garden for pitfall traps is based on a summation of insects collected from both traps.
Portable light traps were assembled using locally available materials. Each light trap used an 11-watt, 12-volt Philips CFL (compact fluorescent lamp) tube, powered by a 12-volt sealed battery. The light source was placed on an aluminum frame and fitted onto a plastic funnel (200 mm in diameter) to collect the insects attracted to the light into a plastic container (1 liter beaker) containing 200 mL of 50% propylene glycol. One trap was placed in each garden. The light was switched on at 18:00, with a capacity of 8 hours of power. The insects trapped in the container were collected and preserved in 50% propylene glycol and transported to the laboratory for identification. Light traps and pitfall traps were set out at the same time, shortly after dusk, and positioned as far apart as possible given the layout of the garden. Insects were identified at the order level. Diversity was assessed based on total order richness (the number of insect orders per location). After removing outliers, we conducted a multiple regression to assess the impact of independent variables on insect abundance and insect order richness.
3. Results
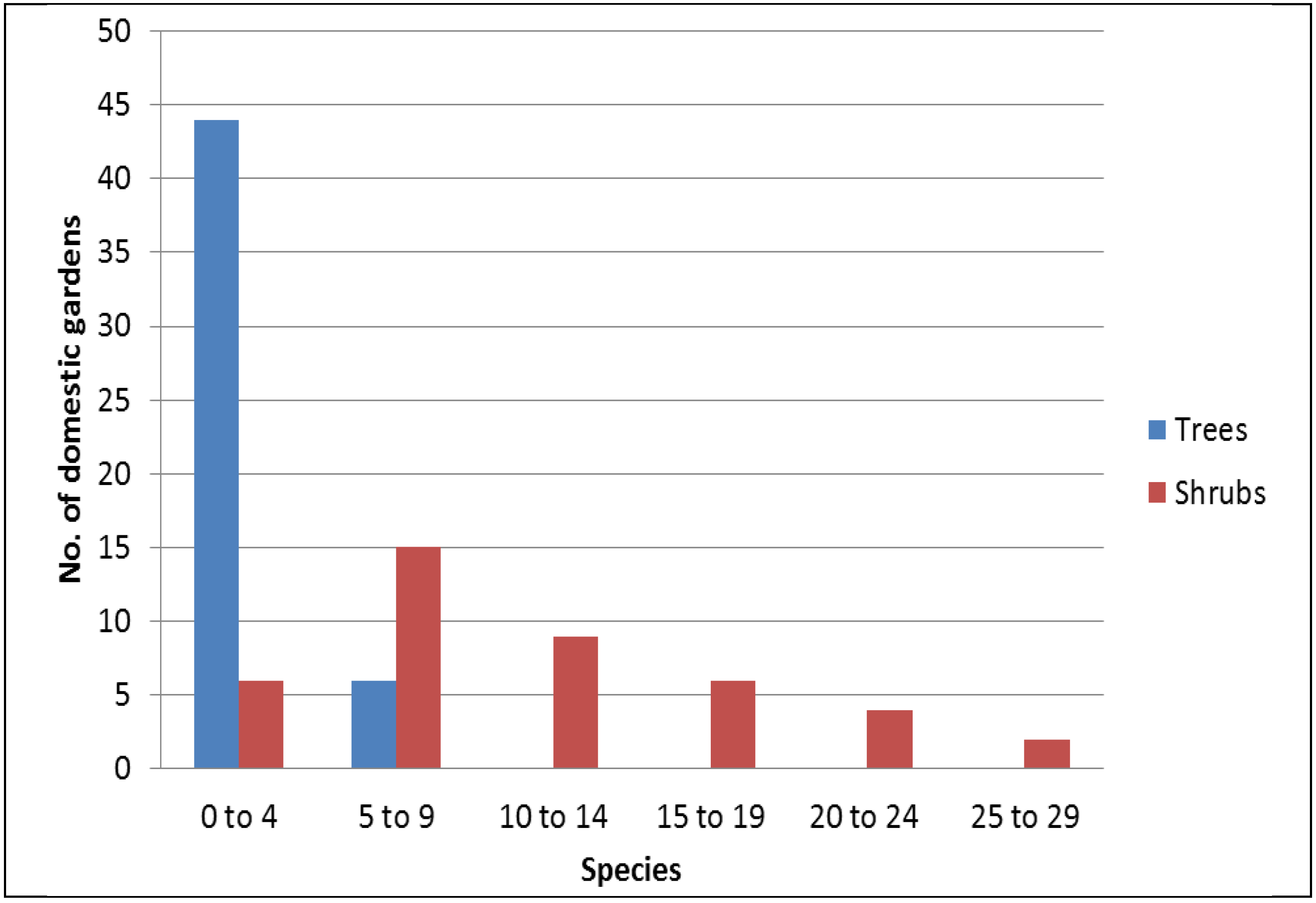
A total of 160 trees from 25 species, as well as 117 species of herbs and shrubs, were encountered in 50 households of the sampled area. We encountered an average of three trees and two tree species per garden, and about 90% of the houses sampled had less than five tree species (
Figure 1). A greater diversity of shrub species was observed, with an average of 11 shrub species per garden. The most common trees, found in more than 30 percent of the home gardens, are
Mangifera indica (mango),
Polyalthia longifolia and
Cocos nucifera (coconut). The most common shrubs found in more than 30 percent of the home gardens are
Rosa spp.,
Bergera koenigii,
Codiaeum variegatum,
Jasminum spp.,
Chrysalidocarpus lutescens,
Hibiscus rosa-sinensis and
Ocimum tenuiflorum.
Figure 1.
Frequency distribution of tree and shrub species across 50 domestic gardens in Bangalore.
Figure 1.
Frequency distribution of tree and shrub species across 50 domestic gardens in Bangalore.
We recorded a total of 2,185 insects from 10 orders; of these 1,072 insects belonging to eight orders (
Table 1)—Coleoptera, Dermaptera, Diptera, Hemiptera, Hymenoptera, Lepidoptera, Neuroptera and Orthoptera—were captured using the light traps, while 1,173 insects belonging to nine orders—Blattodea, Coleoptera, Dermaptera, Diptera, Hemiptera, Hymenoptera, Isopoda, Neuroptera, and Orthoptera—were captured in pitfall traps. Insects belonging to order Lepidoptera were only encountered in light traps, while insects belonging to the orders, Blattodea and Isopoda, were encountered solely in pitfall traps.
Table 1.
Insects belonging to different orders encountered in pitfall and light traps across 50 domestic gardens in Bangalore.
Table 1.
Insects belonging to different orders encountered in pitfall and light traps across 50 domestic gardens in Bangalore.
| Order | minimum | maximum | median | mean | No. of individuals |
|---|
| pitfall traps |
| Blattodea | 1 | 3 | 2.0 | 1.6 | 28 |
| Coleoptera | 1 | 21 | 4.0 | 4.8 | 152 |
| Dermaptera | 1 | 9 | 4.0 | 4.0 | 28 |
| Diptera | 2 | 9 | 6.0 | 5.8 | 23 |
| Hemiptera | 1 | 9 | 4.0 | 3.7 | 146 |
| Hymenoptera | 2 | 68 | 11.5 | 13.2 | 659 |
| Isopoda | 1 | 9 | 3.5 | 3.3 | 39 |
| Neuroptera | 2 | 6 | 4.5 | 4.5 | 27 |
| Orthoptera | 1 | 3 | 2.0 | 1.8 | 11 |
| light traps |
| Coleoptera | 1 | 10 | 2.0 | 3.0 | 69 |
| Dermaptera | 1 | 4 | 3.0 | 2.5 | 33 |
| Diptera | 1 | 9 | 4.0 | 4.2 | 89 |
| Hemiptera | 1 | 11 | 3.5 | 4.0 | 103 |
| Homoptera | 4 | 11 | 5.0 | 7.2 | 36 |
| Hymenoptera | 3 | 65 | 12.0 | 13.5 | 663 |
| Lepidoptera | 1 | 7 | 2.0 | 2.9 | 46 |
| Neuroptera | 1 | 4 | 1.5 | 2.2 | 13 |
Four orders—beetles (Coleoptera), flies (Diptera), bugs (Hemiptera) and ants (Hymenoptera)—were widespread and abundant, collectively providing over 87.13% of total captures in pitfall and light traps. Hymenoptera was the most common order encountered in the sampled gardens (n = 50, light trap = 98%, pitfall trap = 100%) followed by Hemiptera (n = 50, light trap = 52%, pitfall trap = 78%) and Coleoptera (n = 50, light trap = 46%, pitfall trap = 64%). The garden with the most number of insects was located at the periphery of the city, with 120 insects belonging to five orders, while the garden with the least insect order richness had a recorded 12 individuals of five orders, closer to the city center. We removed two outlier gardens with very high numbers of insects (ants) recorded in pitfall traps.
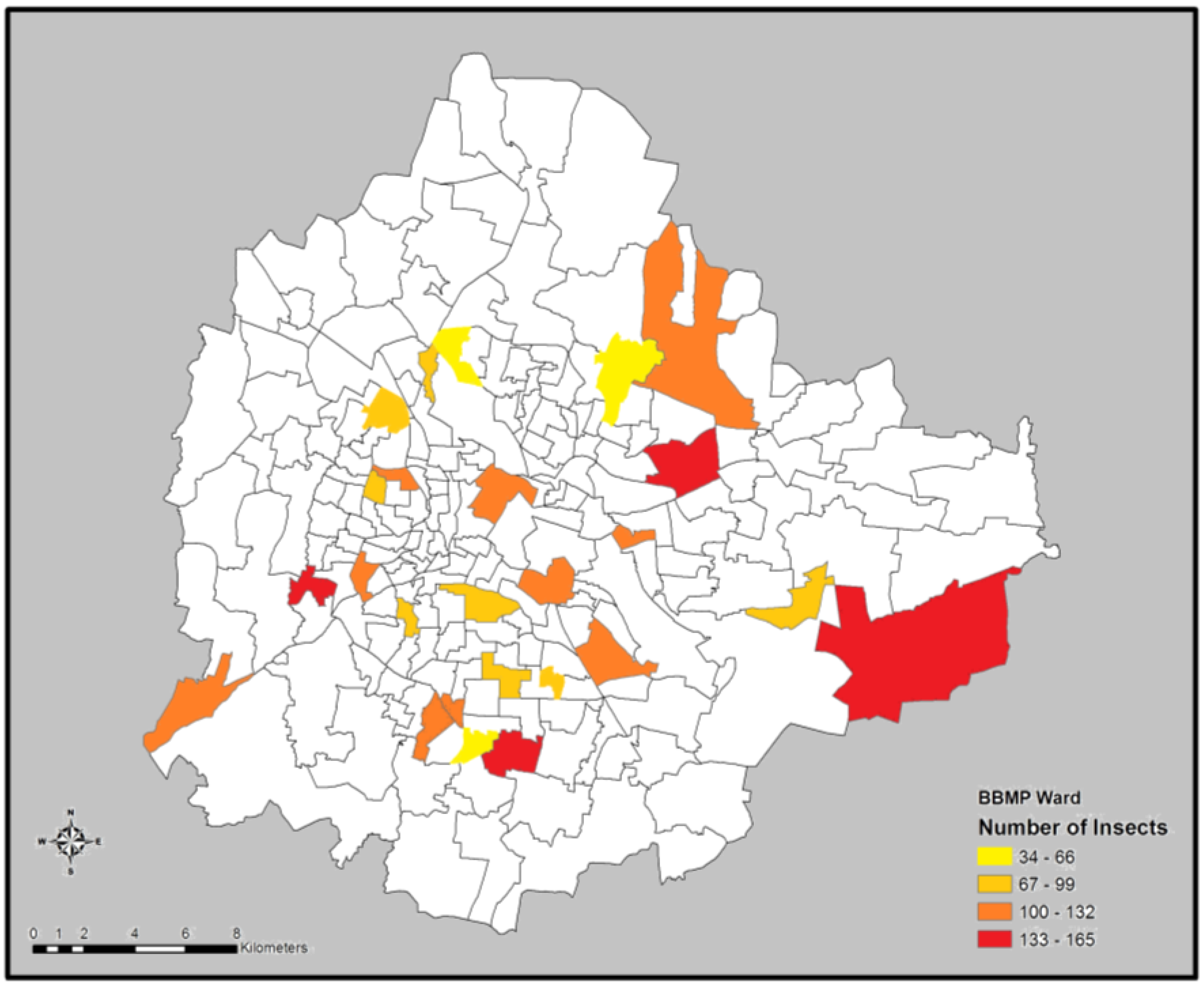
We found an overall trend towards the increase in the number of insects and orders from the center of the city to the periphery (
Figure 2), although this was not statistically significant.
Figure 2.
Number of insects encountered in two domestic gardens located in each of the 25 randomly identified wards in Bangalore.
Figure 2.
Number of insects encountered in two domestic gardens located in each of the 25 randomly identified wards in Bangalore.
Based on the information obtained from residents, the age of the home gardens ranged from two years to 50 years, with an average age of 18 years. The size of the garden area ranged from 100 square feet to 5,000 square feet, with an average area of 1,490 square feet. Gardens had an average of 25% grass cover, 42% bare soil and 30% concreted area. Of the 50 gardens sampled, only four gardens had a pond, while six had a composting area, and seven had a bird feeder—all practices known to be associated with insects. The majority of residents, forty-four respondents in total, indicated that they watered their gardens regularly, with most residents providing water either daily or once in two days. Twenty-one residents weeded their garden regularly (at least once in six months), and 14 residents pruned their gardens regularly (at least once in six months). Most gardens were maintained using insect-friendly practices, with only 10 out of 50 respondents indicating the application of pesticides and eight out of 50 indicating the application of herbicides. Many residents indicated that they did not use chemicals in their garden, because of concerns about health and, in particular, the impacts on their children and pets.
The date of sampling was not significantly correlated with the number of insects or with order richness (Pearson’s r, p > 0.05). After initial exploratory analysis, we conducted a multiple regression analysis with the remaining dataset of 48 gardens (minus outliers) using Excel, to assess the role of the following three continuous variables: total plant (tree and shrub) species richness, size of garden, percentage of grass cover and one dichotomous variable—the practice of weeding—on insect abundance and order richness. Data from light traps and pitfall traps were analyzed separately, as these were expected to relate to specific types of insects influenced by different criteria.
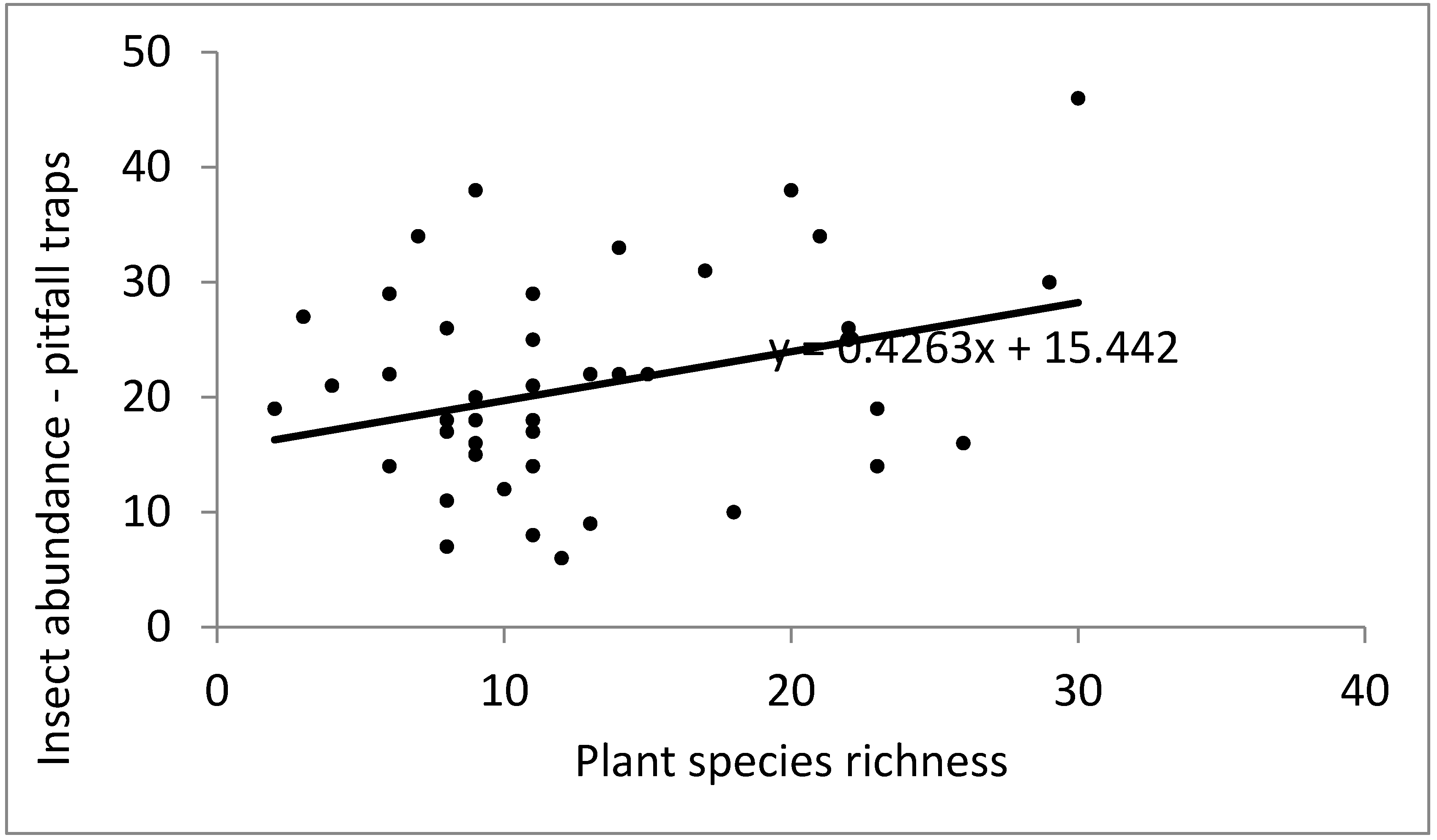
Plant species richness and total garden area were significant predictors of insect abundance in pitfall traps (
Figure 3), while the practice of weeding significantly influenced insect abundance in light traps (
Table 2). Insect order richness in light traps was significantly influenced by plant species richness, total garden area and the percent of grass cover.
Figure 3.
Correlation of insect abundance in pitfall traps against plant species richness.
Figure 3.
Correlation of insect abundance in pitfall traps against plant species richness.
Table 2.
Outputs of a multiple regression assessing the influence of garden variables on insect abundance and order richness.
Table 2.
Outputs of a multiple regression assessing the influence of garden variables on insect abundance and order richness.
| | Insect Abundance | Insect Order Richness |
|---|
| Pitfall Traps | Light Traps | Pitfall Traps | Light Traps |
|---|
| Plant Species Richness | p | 0.015** | 0.615 | 0.172 | 0.038** |
| SE | 0.203 | 0.264 | 0.020 | 0.024 |
| Total Garden Area | p | 0.046** | 0.898 | 0.322 | 0.068* |
| SE | 0.001 | 0.002 | 0.000 | 0.000 |
| Percent Grass Cover | p | 0.157 | 0.277 | 0.821 | 0.091* |
| SE | 0.039 | 0.050 | 0.004 | 0.005 |
| Practice of Weeding | p | 0.244 | 0.024** | 0.942 | 0.301 |
| SE | 2.627 | 3.409 | 0.263 | 0.306 |
| Intercept | Coefficient | 13.48 | 20.64 | 2.92 | 2.40 |
| p | <0.001*** | <0.001*** | <0.001*** | <0.001*** |
| SE | 3.368 | 4.371 | 0.337 | 0.392 |
| Model | Adjusted r2 | 0.165 | 0.065 | -0.011 | 0.199 |
| SE | 8.004 | 10.387 | 0.801 | 0.932 |
4. Discussion
Domestic gardens in Bangalore have a rich diversity of plants, with our study recording an impressive total of 25 species of trees and 117 species of herbs and plants, from 50 houses. Many of these species are planted for their culinary, sacred and/or medicinal properties, as also indicated by previous research on urban domestic gardens in Bangalore [
5,
30]. Domestic gardens also seem to be an important source of support for insects, with our investigations recording over 2,000 insects from 10 orders in these 50 houses, with ants, bugs, beetles and flies being the most commonly encountered. The number of insect orders appears to increase as the number of species of trees, herbs and shrubs increases, indicating that simple practices, such as planting a greater variety of plants in one’s domestic garden, can be very important in supporting insect diversity.
Garden management practices influenced the abundance and diversity of insects, with greater insect abundance in pitfall traps encountered in larger gardens with more plant species, while insect abundance in light traps was related to the practice of weeding. Thus, multiple factors appear to be responsible for shaping the abundance and diversity of insects in Bangalore’s home gardens, as also observed in other urban ecological studies, e.g., of the drivers of herbivore distribution in cities in California [
31]. Since Hymenoptera constitutes by far the most abundant insect order encountered, the preponderance of this order may be driving many of the relationships we encounter between insect abundance and garden variables; thus we suggest that further research be conducted specifically focusing on Hymenoptera. Insect order richness encountered from light traps was significantly related to plant species richness, garden size and the percentage of the area under grass cover. However, greater taxonomic resolution is required to provide additional insights, as pointed out by previous studies [
32].
Culture and aesthetics can play a major role in Bangalore, as in other cities, in influencing the landscaping of domestic gardens. For instance, a survey of students from the University of Alabama indicated that those with a background in wildlife studies or those participating in environmental activities were more likely to select a more “natural” landscaping, with less intensive management, such as pruning and weeding [
21]. Urbanization can further accelerate these changes. For instance, Perry and Nawaz [
33] found a steady increase in impervious cover within domestic gardens in Leeds over time, and Verbeeck
et al. [
34] indicate similar findings in a study in Flanders.
Similarly, in Bangalore, our personal observations indicate that many older residents of the city tend to prefer gardens with a less manicured look and a greater diversity of plant species, while residents of apartments tend to prefer trees and plants that fit with the aesthetic of a manicured garden, with a greater preference for ornamental plants. Most residents avoid the practices of the application of pesticides and herbicides, largely because of health concerns, and we found insect abundance to be significantly reduced in gardens with pesticide application. Coupled with our findings that increased plant diversity and lawn area lead to an increase in ground (pitfall trap sensitive) insect numbers, this indicates that cultural preferences concomitant with older domestic gardens in Bangalore may support a greater associated insect population. The increased conversion of single homes to shared apartment gardens in Bangalore, and the shrinking of available garden space over time [
5], also poses challenges for insect conservation in the city.
The “second urban transition” is taking place at accelerated rates in Asia and Africa, with these areas projected to contain over 80% of the world’s urban population by 2030 [
35,
36]. Urban ecology is a relatively new field of research in most developing countries, and the information available has tended to have a predominant focus on plants [
28,
37,
38]. Yet, it is critical to understand the factors shaping the abundance and diversity of insects, which provide a range of supporting ecosystem functions in urban ecosystems [
6,
39], support other, insectivorous taxa, such as birds and bats [
40], and constitute sensitive indicators of changes in management practices and habitat characteristics impacting overall biodiversity [
4].
The research presented corroborates other studies that point to the significant levels of biodiversity support provided by small, local-scale patches of green space, even in congested, densely populated urban neighborhoods [
8,
41,
42,
43]. Our findings also corroborate research conducted in Toronto [
18] that suggests that simple steps taken to increase the micro-scale diversity of domestic gardens, such as adding more plants and increasing micro-scale diversity, can lead to significant improvement, certainly in invertebrate abundance and, possibly, additionally in diversity. This research helps to lay the foundation for further investigations into the human factors shaping the distribution of faunal and insect diversity in urban ecosystems in the developing world, an area about which little is known, particularly at local scales [
35,
42,
43].