Novel Endophytic Trichoderma spp. Isolated from Healthy Coffea arabica Roots are Capable of Controlling Coffee Tracheomycosis
Abstract
:1. Introduction
2. Methods
2.1. Root Samples and Isolation of Endophytic Fungi
2.2. DNA Extraction and PCR Amplifications
2.3. DNA Barcoding
| TUCIM No. (C.P.K) | GenBank Acc. No | Identification | ||||
|---|---|---|---|---|---|---|
| % similarity/% query coverage | Phylum | Putative identification | The most similar NCBI GenBank entry | |||
| taxon | Acc. No | |||||
| 3484 | FJ827622 | 100/100 | Ascomyc. | Aspergillus | Aspergillus flavus | AM745114 |
| 3485 | FJ827626 | 100/94 | Cladosporum | Cladosporium cladosporioides | EF577236 | |
| 3478 | FJ827623 | 100/98 | Rhizopycins | Rhizopycnis sp. IBL 03177 | DQ682600 | |
| 3078 | FJ827619 | 99/94 | Penicillium | Penicillium sp. RCEF3398 | EF570358 | |
| 3084 | FJ827620 | 99/95 | ||||
| 3468 | FJ827620 | 99/92 | Phomopsis | Phomopsis columnaris | AF439625 | |
| 3471 | FJ827629 | 99/92 | ||||
| 3473 | FJ827628 | 99/93 | ||||
| 3512 | FJ827630 | 94/98 | Dipodascaceae | Dipodascus australiensis | AF157596 | |
| 3474 | FJ827625 | 99/98 | Macrophomina | Macrophomina phaseolina | EU250575 | |
| 3486 | FJ827624 | 100/97 | Pleosporales | Pleosporales sp. IBL 03175 | DQ682598 | |
| 3337 | FJ827616 | 99/99 | Fusarium equiseti | F. equiseti isolate 45/1.2.1 | DQ854855 | |
| 3469 | FJ827615 | 99/ 98 | ||||
| 3514 | FJ840530 | 99/98 | ||||
| 3466 | FJ827613 | 99/98 | ||||
| 3472 | FJ827614 | 99/98 | ||||
| 3513 | n/a | 99/98 | ||||
| 3465 | FJ840527 | 99/98 | ||||
| 3480 | FJ827612 | 93/99 | ||||
| 3470 | FJ840525 | 93/98 | F. equiseti isolate SAT73 | DQ465946 | ||
| 3479 | FJ827610 | 93/99 | F. equiseti isolate 21/1.2 | DQ854851 | ||
| 3332 | FJ840526 | 99/98 | ||||
| 3509 | FJ827611 | 99/98 | F. solani | Fusarium sp. NRRL 43704 FSSC | EF453029 | |
| 3510 | FJ840531 | 99/98 | ||||
| 3511 | FJ840532 | 99/98 | ||||
| 3330 | FJ840533 | 99/98 | ||||
| 3515 | FJ840529 | 99/99 | F. oxysporum | F. oxysporum isolate SAT77 | DQ465933 | |
| 3476 | FJ827617 | 99/99 | ||||
| 3475 | FJ827618 | 98/100 | Basidiomyc. | Trametes | Trametes versicolor | FJ608587 |
| TUCIM No. (C.P.K) | NCBI GenBank | TUCIM No. (C.P.K) | NCBI GenBank | TUCIM No. (C.P.K) | NCBI GenBank | ||||
|---|---|---|---|---|---|---|---|---|---|
| ITS1 and 2 | tef1 | ITS1 and 2 | tef1 | ITS1 and 2 | tef1 | ||||
| Section Trichoderma | Harzianum-Catoptron Clade | Section Longibrachiatum | |||||||
| Trichoderma hamatum | Hypocrea ‘pseudoharzianumʼ nom. prov. | T. flagellatum | |||||||
| 3331 | FJ461539 | n/a | 3440 | FJ461557 | FJ763164 | 3334 | FJ461542 | FJ763149 | |
| 3333 | FJ461540 | FJ763148 | 3441 | FJ461558 | FJ763165 | 3345 | FJ461551 | FJ763158 | |
| 3335 | FJ461541 | n/a | 3442 | FJ461559 | FJ763166 | 3350 | FJ461556 | FJ763163 | |
| 3336 | FJ461543 | FJ763150 | 3443 | FJ461560 | FJ763167 | 3496 | FJ461568 | FJ763174 | |
| 3338 | FJ461544 | FJ763151 | 3501 | FJ461573 | FJ763177 | 3503 | FJ461575 | FJ763179 | |
| 3342 | FJ461548 | FJ763155 | 3502 | FJ461574 | FJ763178 | 3504 | FJ461576 | FJ763180 | |
| 3343 | FJ461549 | FJ763156 | 3506 | FJ461578 | FJ763181 | 3517 | FJ461580 | FJ763182 | |
| 3346 | FJ461552 | FJ763159 | T. sp. C.P.K. 1812 | 3522 | FJ461585 | n/a | |||
| 3347 | FJ461553 | FJ763160 | 3328 | FJ461537 | FJ763146 | 3523 | FJ461586 | n/a | |
| 3348 | FJ461554 | FJ763161 | 3329 | FJ461538 | FJ763147 | 3524 | FJ461587 | FJ763183 | |
| TUCIM No. (C.P.K) | NCBI GenBank | TUCIM No. (C.P.K) | NCBI GenBank | ||
|---|---|---|---|---|---|
| ITS1 and 2 | tef1 | ITS1 and 2 | tef1 | ||
| Section Trichoderma | Harzianum-Catoptron Clade | ||||
| Trichoderma hamatum | Hypocrea ‘pseudoharzianumʼ nom. prov. | ||||
| 3491 | FJ461563 | n/a | 3444 | FJ461561 | FJ763168 |
| 3492 | FJ461564 | FJ763170 | 3490 | FJ461562 | FJ763169 |
| 3493 | FJ461565 | FJ763171 | 3494 | FJ461566 | FJ763172 |
| 3497 | FJ461569 | n/a | 3495 | FJ461567 | FJ763173 |
| 3505 | FJ461577 | n/a | 3498 | FJ461570 | n/a |
| 3507 | FJ461579 | n/a | 3499 | FJ461571 | FJ763175 |
| 3518 | FJ461581 | n/a | 3500 | FJ461572 | FJ763176 |
| 3519 | FJ461582 | n/a | T. sp. C.P.K. 1833 | ||
| 3520 | FJ461583 | n/a | 3339 | FJ461545 | FJ763152 |
| 3521 | FJ461584 | n/a | 3341 | FJ461547 | FJ763154 |
2.4. Phylogenetic Analyses
2.5. Screening for Antifungal Activity
3. Results
3.1. The Endophytic Fungal Community in Roots of C. arabica
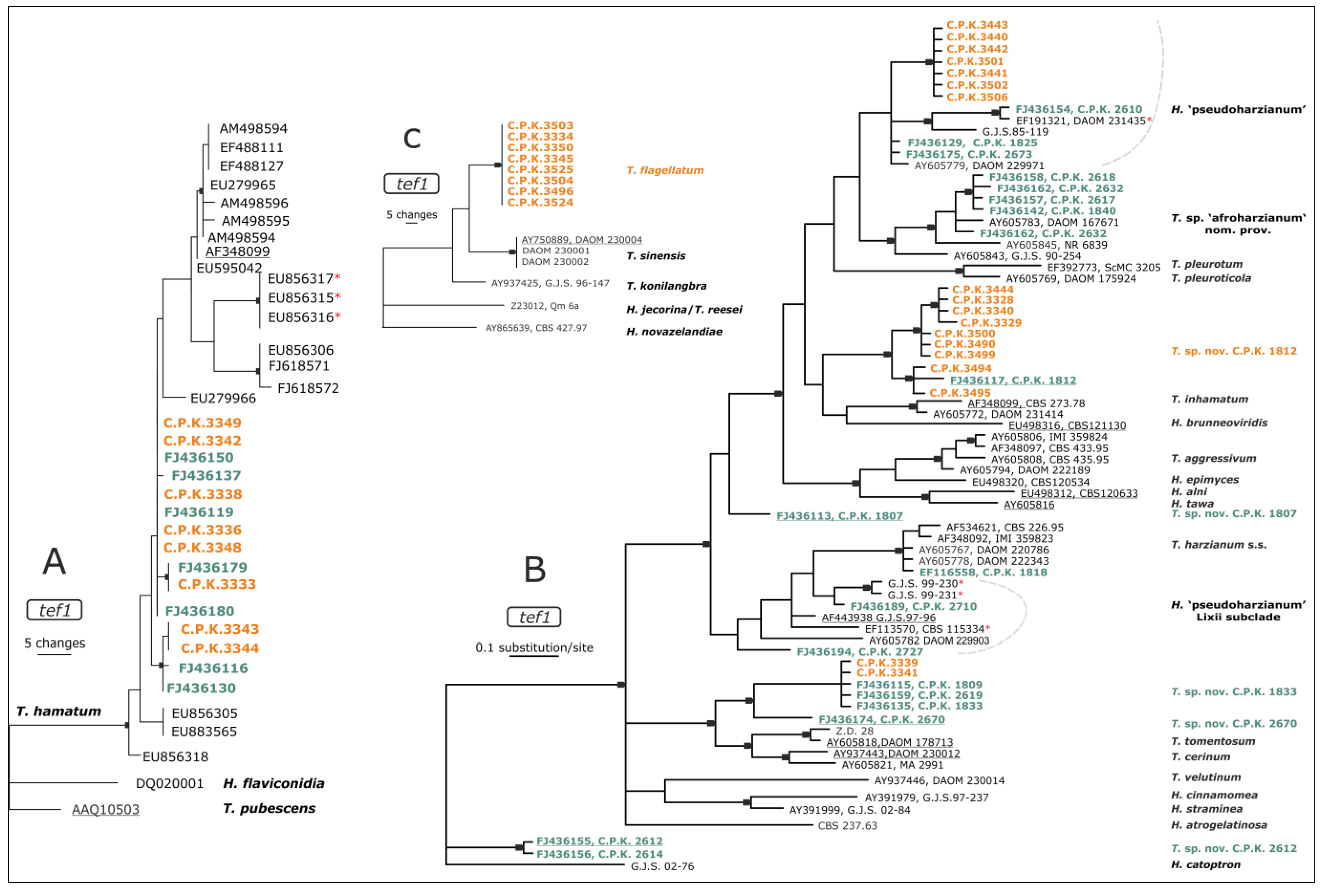
3.2. Diversity of Endophytic Trichoderma
3.3. Diversity of Endophytic Fusarium Isolates
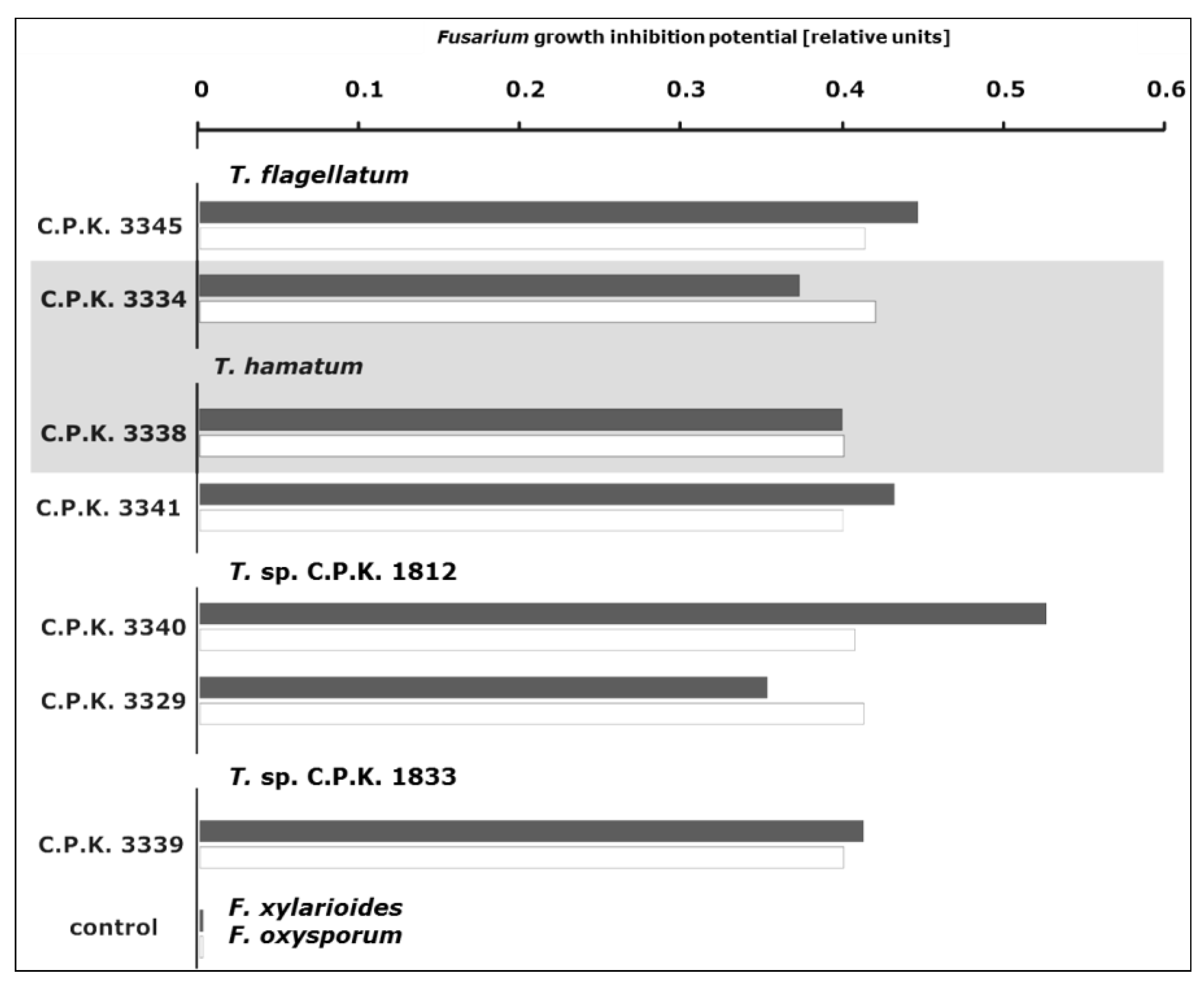
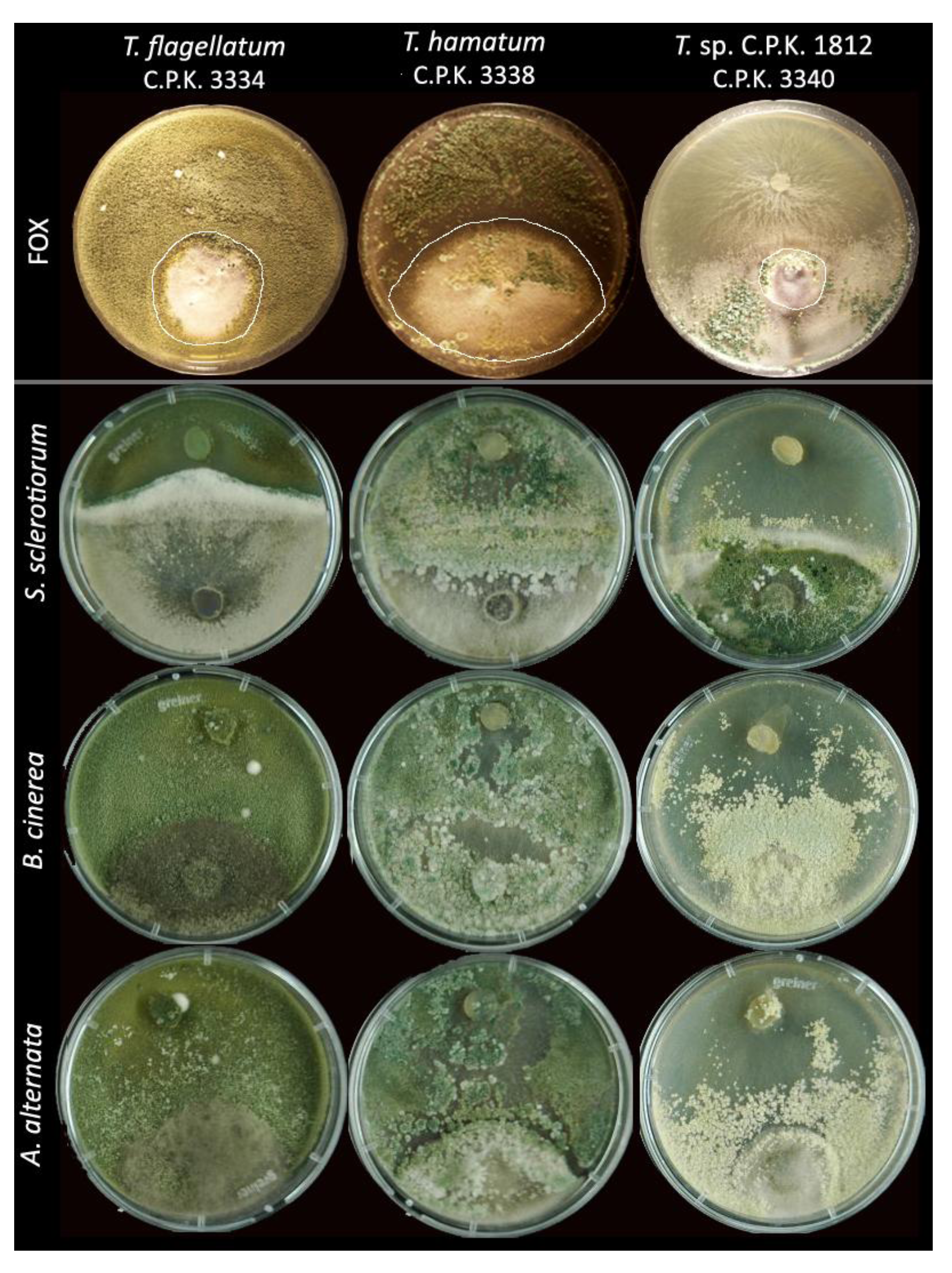
3.4. Antifungal Activity of Trichoderma Species
 | Inhibition of preys (% *) | % of strains capable to overgrow the prey | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Per prey species | For 3 preys | For 3 preys | ||||||||||||||||||
| Ss | SD | Ss | ||||||||||||||||||
| SD | ||||||||||||||||||||
| Endophytic strains from roots of C. arabica | ||||||||||||||||||||
| T. hamatum | 0.65 | 17.66 | 89.5 | 84.2 | ||||||||||||||||
| Control (saprotrophic strains) | ||||||||||||||||||||
| T. hamatum | 8.47 | 0.95 | 17.12 | 75.5 | 82.3 | |||||||||||||||
4. Discussion
4.1. The Narrow Ecological Niche inside Coffee Roots Favors Diverse Community of Endophytic Fungi
4.2. Diversity of Endophytic Trichoderma in Roots of C. arabica is Unique
4.3. G. xylarioides is not Present in the Community of Endophytic Fungi from Healthy Coffee Plants
4.4. Endophytic Trichoderma Strains Exhibit High Antifungal Potential
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Bills, G.F. Isolation and Analysis of Endophytic Fungal Communities from Woody Plants. In Systematics, Ecology and Evolution of Endophytic Fungi in Grasses and Woody Plants; Redlin, S., Carris, L.M., Eds.; APS Press: St. Paul, MN, USA, 1996; pp. 31–65. [Google Scholar]
- Larran, S.; Perello, A.; Simon, M.R.; Moreno, V. Isolation and analysis of endophytic microorganisms in wheat (Triticum aestivum L.) leaves. World J. Microb. Biot. 2002, 18, 683–686. [Google Scholar] [CrossRef]
- Redlin, S.C.; Carris, L.M. Endophytic Fungi in Grasses and Woody Plants. In Systematics, Ecology and Evolution of Endophytic Fungi in Grasses and Woody Plants; Redlin, S., Carris, L.M., Eds.; APS press: St. Paul, MN, USA, 1996; p. 216. [Google Scholar]
- Marshall, D.; Tunali, B.; Nelson, L.R. Occurrence of fungal endophytes in species of wild Triticum. Crop Sci. 1996, 39, 1507–1512. [Google Scholar] [CrossRef]
- Huang, Y.; Jianfeng, W.G.L.; Zhonghui, Z.; Wenjin, S. Anti tumor and antifungalactivities in endophytic fungi isolated from pharmaceutical plants Taxus mairei, Cephalataxus fortunei and Torreya grandis. FEMS Immunol. Med. Microbiol. 2001, 31, 163–167. [Google Scholar] [CrossRef]
- Arnold, A.E.; Mejía, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal endophytes limit pathogen damage in a tropical tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar]
- Niere, B.; Gold, C.S.; Coyne, D. Can fungal endophytes control soilborne pests in banana? Bull. OILB/SROP 2004, 27, 203–209. [Google Scholar]
- Santamaría, J.; Bayman, P. Fungal epiphytes and endophytes of Coffee Leaves (Coffea arabica). Microb. Ecol. 2005, 50, 1–8. [Google Scholar] [CrossRef]
- Sylvain, P. Ethiopian coffee—its significance to world coffee problems. Econ. Bot. 1958, 12, 111–139. [Google Scholar] [CrossRef]
- Aga, E.; Bryngelsson, T.; Bekele, E.; Salomon, B. Genetic diversity of forest arabica coffee (Coffea arabica L.) in Ethiopia as revealed by random amplified polymorphic DNA (RAPD) analysis. Hereditas 2003, 138, 36–46. [Google Scholar] [CrossRef]
- Geiser, D.M.; Ivey, M.L.L.; Hakiza, G.; Juba, J.H.; Miller, S.A. Gibberella xylarioides (anamorph: Fusarium xylarioides), a causative agent of coffee wilt disease in Africa, is a previously unrecognized member of the G. fujikuroi species complex. Mycologia 2005, 97, 191–201. [Google Scholar] [CrossRef]
- Girma, A.; Hulluka, M.; Hindorf, H. Incidence of tracheomycosis, Gibberella xylarioides (Fusarium xylarioides), on Arabica coffee in Ethiopia. Zeitschrift fur Pflanzenkrankheiten und Pflanzenschutz 2001, 108, 136–142. [Google Scholar]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species: Opportunistic avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The genomics of opportunistic success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef]
- Zhang, C.L.; Druzhinina, I.S.; Kubicek, C.P.; Xu, T. Biodiversity of Trichoderma in China: Evidence for a North to South difference of species distribution in East Asia. FEMS Microbiol. Letts. 2005, 251, 251–257. [Google Scholar] [CrossRef]
- Hanada, R.E.; de Jorge Souza, T.; Pomella, A.W.; Hebbar, K.P.; Pereira, J.O.; Ismaiel, A.; Samuels, G.J. Trichoderma martiale sp. nov., a new endophyte from sapwood of Theobroma cacao with a potential for biological control. Mycol. Res. 2008, 112, 1335–1343. [Google Scholar] [CrossRef]
- Samuels, G.J.; Suarez, C.; Solis, K.; Holmes, K.A.; Thomas, S.E.; Ismaiel, A.; Evans, H.C. Trichoderma theobromicola and T. paucisporum: Two new species isolated from cacao in South America. Mycol. Res. 2006, 110, 381–392. [Google Scholar] [CrossRef]
- Samuels, G.J.; Ismaiel, A. Trichoderma evansii and T. lieckfeldtiae: Two new T. hamatum-like species. Mycologia 2009, 101, 142–156. [Google Scholar] [CrossRef]
- Mulaw, T.B.; Kubicek, C.P.; Druzhinina, I.S. The distinguished diversity of Trichoderma is associated with rhizosphere of Coffea arabica in highland forests of Ethiopia. Diversity 2010, 2, 527–549. [Google Scholar] [CrossRef]
- Mysore, V.T.; Basavanna, M.; Monnanda, S.N.; Harishchandra, S.P.; Kukkundoor, R.K.; Ven, S.; Hunthrike, S.S. Endophytic fungal assemblages from inner and twig of Terminalia arjuna W. and A. (Combretaceae). World J. Microbiol. Biotechnol. 2005, 21, 1535–1540. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Kullnig-Gradinger, C.M.; Szakacs, G.; Kubicek, C.P. Phylogeny and evolution of the fungal genus Trichoderma—a multigene approach. Mycol. Res. 2002, 106, 757–767. [Google Scholar] [CrossRef]
- Chaverri, P.; Castlebury, L.A.; Samuels, G.J.; Geiser, D. Multilocus phylogenetic structure within the Trichoderma/Hypocrea lixii complex. Mol. Phylogenet. Evol. 2003, 27, 302–313. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Komoń-Zelazowska, M.; Kredics, L.; Hatvani, L.; Antal, Z.; Belayneh, T.; Kubicek, C.P. Alternative reproductive strategies of Hypocrea orientalis and genetically close but clonal Trichoderma longibrachiatum, both capable of causing invasive mycoses of humans. Microbiology 2008, 154, 3447–3459. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar]
- Druzhinina, S.I.; Kopchinskiy, G.A.; Komon, M.; Bissett, J.; Szakacs, G.; Kubicek, P.C. An oligonucleotide barcode for species identification in Trichoderma and Hypocrea. Fungal Genet. Biol. 2005, 42, 813–828. [Google Scholar] [CrossRef]
- Kopchinskiy, A.; Komon, M.; Kubicek, C.P.; Druzhinina, I.S. TRICHOBLAST: A multilocus database for Trichoderma and Hypocrea identifications. Mycol. Res. 2005, 109, 658–660. [Google Scholar] [CrossRef]
- O’Donnell, K. Molecular phylogeny of the Nectria haematococca-Fusarium solani species complex. Mycologia 2000, 92, 919–938. [Google Scholar] [CrossRef]
- Geiser, D.M.; Jiménez-Gasco, M.; Kang, S.; Makalowska, I.; Veeraraghavan, N.; Ward, T.J.; Zhang, N.; Kuldau, G.A.; O’Donnell, K. FUSARIUM-ID v.1.0: A DNA Sequence Database for Identifying Fusarium. Eur. J. Plant Pathol. 2004, 110, 473–479. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The ClustalX windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Nicholas, K.B.; Nicholas, H.B., Jr.; Deerfield, D.W., II. GeneDoc: Analysis and Visualization of Genetic Variation, EMBNEW.NEWS 1997, 4:14. Available online: http://www.psc.edu/biomed/genedoc (accessed on 28 August 2013).
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Tavaré, S. Some Probabilistic and Statistical Problems in the Analysis of DNA Sequences. In Some Mathematical Questions in Biology—DNA Sequence Analysis; Miura, R.M., Ed.; American Mathematical Society: Providence, RI, USA, 1986; pp. 57–86. [Google Scholar]
- Atanasova, L.; Jaklitsch, W.M.; Komon-Zelazowska, M.; Kubicek, C.P.; Druzhinina, I.S. Clonal species Trichoderma parareesei sp. nov. likely resembles the ancestor of the cellulase producer Hypocrea jecorina/T. reesei. Appl. Environ. Microbiol. 2010, 76, 7259–7267. [Google Scholar] [CrossRef]
- Leaché, A.D.; Reeder, T.W. Molecular systematics of the Eastern Fence lizard (Sceloporus undulatus): A comparison of parsimony, likelihood and Bayesian approaches. Syst. Biol. 2002, 51, 44–68. [Google Scholar] [CrossRef]
- Ortiz, A.; Orduz, S. In vitro evaluation of Trichoderma and Gliocladium antagonism against the symbiotic fungus of the leaf-cutting ant Atta cephalotes. Mycopathologia 2001, 150, 53–60. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Kubicek, C.P.; Komon-Zelazowska, M.; Mulaw, T.B.; Bissett, J. The Trichoderma harzianum demon: Complex speciation history resulting in coexistence of hypothetical biological species, recent agamospecies and numerous relict lineages. BMC Evol. Biol. 2010. [Google Scholar] [CrossRef]
- Bisset, J.; Szakacs, G.; Nolan, C.A.; Druzhinina, S.I.; Gradinger, C.M.; Kubicek, C.P. New species of Trichoderma from Asia. Can. J. Bot. 2003, 81, 570–586. [Google Scholar] [CrossRef]
- Samuels, G.J.; Ismaiel, A.; Mulaw, T.B.; Szakacs, G.; Druzhinina, I.S.; Kubicek, C.P.; Jaklitsch, W.M. The Longibrachiatum Clade of Trichoderma: A revision with new species. Fungal Divers. 2012, 55, 77–108. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Komoń-Zelazowska, M.; Ismaiel, A.; Jaklitsch, W.; Mullaw, T.; Samuels, G.J.; Kubicek, C.P. Molecular phylogeny and species delimitation in the section Longibrachiatum of Trichoderma. Fungal Genet. Biol. 2012, 49, 358–368. [Google Scholar] [CrossRef]
- Vega, F.E.; Pava-Ripoll, M.; Posada, F.; Buyer, J.S. Endophytic bacteria in Coffea arabica L. J. Basic Microbiol. 2005, 45, 371–380. [Google Scholar] [CrossRef]
- Vega, F.E.; Posada, F.; Peterson, S.W.; Gianfagna, T.J.; Chaves, F. Penicillium species endophytic in coffee plants and ochratoxin A production. Mycologia 2006, 98, 31–42. [Google Scholar] [CrossRef]
- Bailey, B.A.; Strem, M.D.; Wood, D. Trichoderma species form endophytic associations within Theobroma cacao trichomes. Mycol. Res. 2009, 113, 1365–1376. [Google Scholar] [CrossRef]
- Holmes, K.A.; Schroers, H.-J.; Thomas, S.E.; Evans, H.C.; Samuels, G.J. Taxonomy and biocontrol potential of a new species of Trichoderma from the Amazon basin of South America. Mycol. Prog. 2004, 3, 199–210. [Google Scholar] [CrossRef]
- Samuels, G.J.; Pardo-Schultheiss, R.; Hebbar, K.P.; Lumsden, R.D.; Bastos, C.N.; Costa, J.C.; Bezerra, J.L. Trichoderma stromaticum sp. nov., a parasite of the cacao witches broom pathogen. Mycol. Res. 2000, 104, 760–764. [Google Scholar] [CrossRef]
- Samuels, G.J.; Dodd, S.L.; Lu, B.-S.; Petrini, O.; Schroers, H.-J.; Druzhinina, I.S. The Trichoderma koningii aggregate species. Stud. Mycol. 2006, 56, 67–133. [Google Scholar] [CrossRef]
- Zhang, C.L.; Liu, S.P.; Lin, F.C.; Kubicek, C.P.; Druzhinina, I.S. Trichoderma taxi sp. nov., an endophytic fungus from Chinese yew Taxus mairei. FEMS Microbiol. Lett. 2007, 270, 90–96. [Google Scholar] [CrossRef]
- Masumbuko, L.I.; Bryngelsson, T.; Mneney, E.E.; Salomon, B. Genetic diversity in Tanzanian Arabica coffee using random amplified polymorphic DNA (RAPD) markers. Hereditas 2003, 139, 56–63. [Google Scholar] [CrossRef]
- Booth, C. The Genus Fusarium; Commonwealth Mycological Institute: Surrey, UK, 1971. [Google Scholar]
- Zhang, N.; O’Donnell, K.; Sutton, D.A.; Nalim, F.A.; Summerbell, R.C.; Padhye, A.A.; Geiser, D.M. Members of the Fusariuim solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microbiol. 2006, 44, 2186–2190. [Google Scholar] [CrossRef]
- Goswami, R.S.; Dong, Y.; Punja, Z.K. Host rang and mycotoxin production by Fusarium equiseti isolates originating from Ginseng fields. Can. J. Plant. Pathol. 2008, 30, 155–160. [Google Scholar] [CrossRef]
- Bae, H.; Sicher, R.C.; Kim, M.S.; Kim, S.H.; Strem, M.D.; Melnick, R.L.; Bailey, B.A. The beneficial endophyte Trichoderma hamatum isolate DIS 219b promotes growth and delays the onset of the drought response in Theobroma cacao. J. Exp. Bot. 2009, 60, 3279–3295. [Google Scholar] [CrossRef]
- Bae, H.; Roberts, D.P.; Lim, H.S.; Strem, M.D.; Park, S.C.; Ryu, C.M.; Melnick, R.L.; Bailey, B.A. Endophytic Trichoderma isolates from tropical environments delay disease onset and induce resistance against Phytophthora capsici in hot pepper using multiple mechanisms. Mol. Plant Microbe. Interact. 2011, 24, 336–351. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mulaw, T.B.; Druzhinina, I.S.; Kubicek, C.P.; Atanasova, L. Novel Endophytic Trichoderma spp. Isolated from Healthy Coffea arabica Roots are Capable of Controlling Coffee Tracheomycosis. Diversity 2013, 5, 750-766. https://doi.org/10.3390/d5040750
Mulaw TB, Druzhinina IS, Kubicek CP, Atanasova L. Novel Endophytic Trichoderma spp. Isolated from Healthy Coffea arabica Roots are Capable of Controlling Coffee Tracheomycosis. Diversity. 2013; 5(4):750-766. https://doi.org/10.3390/d5040750
Chicago/Turabian StyleMulaw, Temesgen Belayneh, Irina S. Druzhinina, Christian P. Kubicek, and Lea Atanasova. 2013. "Novel Endophytic Trichoderma spp. Isolated from Healthy Coffea arabica Roots are Capable of Controlling Coffee Tracheomycosis" Diversity 5, no. 4: 750-766. https://doi.org/10.3390/d5040750
APA StyleMulaw, T. B., Druzhinina, I. S., Kubicek, C. P., & Atanasova, L. (2013). Novel Endophytic Trichoderma spp. Isolated from Healthy Coffea arabica Roots are Capable of Controlling Coffee Tracheomycosis. Diversity, 5(4), 750-766. https://doi.org/10.3390/d5040750




