Effect of Site Attributes and Matrix Composition on Neotropical Primate Species Richness and Functional Traits: A Comparison Among Regions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Review
2.2. Geographic Information System (GIS) Analysis
2.3. Primate Variables
2.4. Data Analysis
3. Results
3.1. Matrix Differences Among Neotropical Sub-Regions
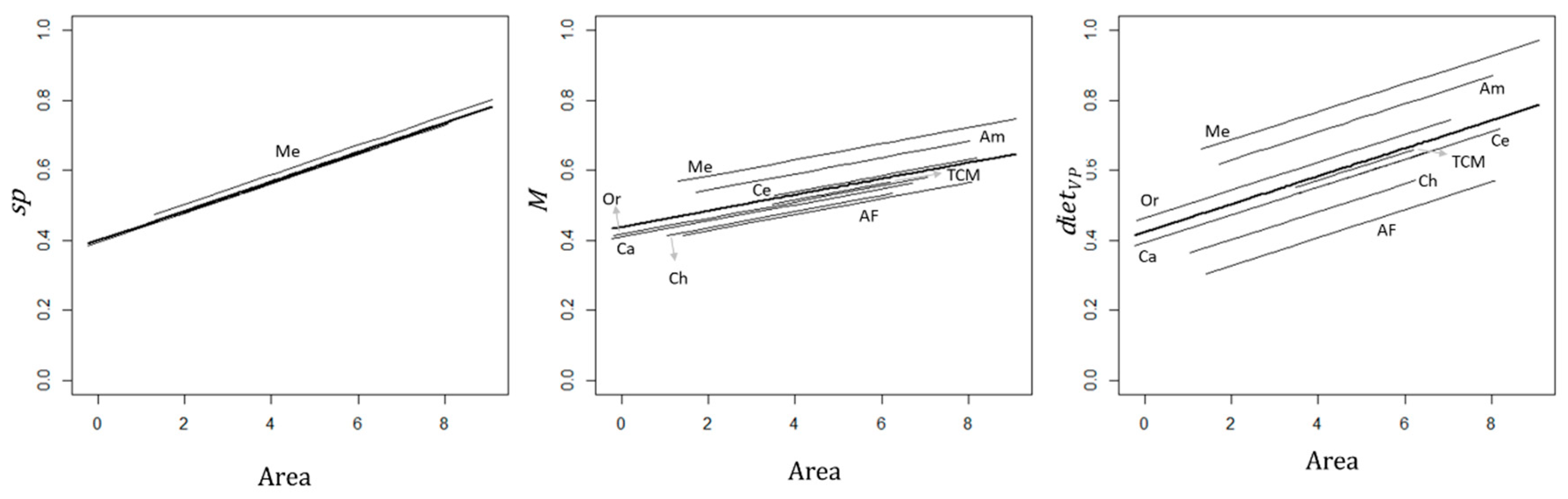
3.2. Effects of Matrix Components, Environmental Conditions, and Site Attributes on Primates
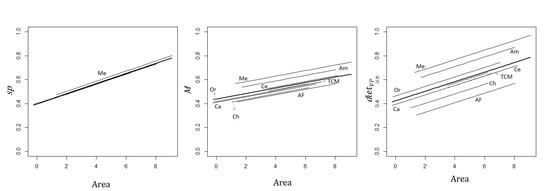
3.3. Effect of Sub-Region on the Relationship between Primates and Site and Matrix Attributes
4. Discussion
4.1. Relationship Between Matrix and Site Attributes and Primate Species Richness and Functional Traits
4.2. Effect of the Neotropical Sub-Region on Primate Species Richness and Functional Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s sixth mass extinction already arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.C.; Potapov, P.V.; Moore, R.; Hancher, M.; Turubanova, S.A.; Tyukavina, A.; Thau, D.; Stehman, S.V.; Goetz, S.J.; Loveland, T.R.; et al. High-resolution global maps of 21st-century forest cover change. Science 2013, 342, 850–853. [Google Scholar] [CrossRef]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D.; et al. Habitat fragmentation and its lasting impact on earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [PubMed]
- Brockerhoff, E.G.; Jactel, H.; Parrotta, J.A.; Quine, C.P.; Sayer, J. Plantation forests and biodiversity: Oxymoron or opportunity? Biodivers. Conserv. 2008, 17, 925–951. [Google Scholar] [CrossRef]
- Laurance, W.F.; Goosem, M.; Laurance, S.G.W. Impacts of roads and linear clearings on tropical forests. Trends Ecol. Evol. 2009, 24, 659–669. [Google Scholar] [CrossRef]
- Benchimol, M.; Venticinque, E.M. Responses of primates to landscape change in Amazonian land-bridge islands—a multi-scale analysis. Biotropica 2014, 46, 470–478. [Google Scholar] [CrossRef]
- da Silva, L.G.; Ribeiro, M.C.; Hasui, E.; da Costa, C.A.; da Cunha, R.G.T. Patch size, functional isolation, visibility and matrix permeability influences Neotropical primate occurrence within highly fragmented landscapes. PLoS ONE 2015, 10, e0114025. [Google Scholar] [CrossRef] [PubMed]
- Borges-Matos, C.; Aragón, S.; da Silva, M.N.F.; Fortin, M.J.; Magnusson, W.E. Importance of the matrix in determining small-mammal assemblages in an Amazonian forest-savanna mosaic. Biol. Conserv. 2016, 204, 417–425. [Google Scholar] [CrossRef]
- Fahrig, L.; Merriam, G. Conservation of fragmented populations. Conserv. Biol. 1994, 8, 50–59. [Google Scholar] [CrossRef]
- Ricketts, T.H. The matrix matters: Effective isolation in fragmented landscapes. Am. Nat. 2001, 158, 87–99. [Google Scholar] [CrossRef]
- Tischendorf, L.; Fahrig, L. On the usage and measurement of landscape connectivity. Oikos 2000, 90, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Arroyo-Rodríguez, V.; Fahrig, L. Why is a landscape perspective important in studies of primates? Am. J. Primatol. 2014, 76, 901–909. [Google Scholar] [CrossRef]
- Dirzo, R.; Young, H.S.; Galetti, M.; Ceballos, G.; Isaac, N.J.B.; Collen, B. Defaunation in the anthropocene. Science 2014, 345, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Asensio, N.; Arroyo-Rodríguez, V.; Dunn, J.C.; Cristóbal-Azkarate, J. Conservation value of landscape supplementation for Howler Monkeys living in forest patches. Biotropica 2009, 41, 768–773. [Google Scholar] [CrossRef]
- Pozo-Montuy, G.; Serio-Silva, J.C.; Chapman, C.A.; Bonilla-Sánchez, Y.M. Resource use in a landscape matrix by an arboreal primate: Evidence of supplementation in Black Howlers (Alouatta pigra). Int. J. Primatol. 2013, 34, 714–731. [Google Scholar] [CrossRef]
- Estrada, A.; Garber, P.A.; Rylands, A.B.; Roos, C.; Fernandez-Duque, E.; DiFiore, A.; Nekaris, K.A.I.; Nijman, V.; Heymann, E.W.; Lambert, J.E.; et al. Impending extinction crisis of the world’s primates: Why primates matter. Sci. Adv. 2017, 3, e1600946. [Google Scholar] [CrossRef] [PubMed]
- de Assumpção, C.T. Cebus apella and Brachyteles arachnoides (Cebidae) as potential pollinators of Mabea fistulifera (Euphorbiaceae). J. Mammal. 1981, 62, 386–388. [Google Scholar] [CrossRef]
- Janson, C.H.; Terborgh, J.; Emmons, L.H. Non-flying mammals as pollinating agents in the Amazonian forest. Biotropica 1981, 13, 1–6. [Google Scholar] [CrossRef]
- Chapman, C.A.; Bonnell, T.R.; Gogarten, J.F.; Lambert, J.E.; Omeja, P.A.; Twinomugisha, D.; Wasserman, M.D.; Rothman, J.M. Are primates ecosystem engineers? Int. J. Primatol. 2013, 34, 1–14. [Google Scholar] [CrossRef]
- Andresen, E.; Arroyo-Rodríguez, V.; Ramos-Robles, M. Primate seed dispersal: Old and new challenges. Int. J. Primatol. 2018, 39, 443–465. [Google Scholar] [CrossRef]
- Stevenson, P.R. The abundance of large Ateline monkeys is positively associated with the diversity of plants regenerating in Neotropical forests. Biotropica 2011, 43, 512–519. [Google Scholar] [CrossRef]
- Cormier, L.A. Preliminary review of Neotropical primates in the subsistence and symbolism of indigenous lowland South American peoples. Ecol. Environ. Anthropol. 2006, 2, 14–32. [Google Scholar]
- Peres, C.A.; Emilio, T.; Schietti, J.; Desmoulière, S.J.M.; Levid, T. Dispersal limitation induces long-term biomass collapse in overhunted Amazonian forests. Proc. Natl. Acad. Sci. USA 2016, 113, 892–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalski, F.; Peres, C.A. Anthropogenic determinants of primate and carnivore local extinctions in a fragmented forest landscape of southern Amazonia. Biol. Conserv. 2005, 124, 383–396. [Google Scholar] [CrossRef]
- Boyle, S.A.; Smith, A.T. Can landscape and species characteristics predict primate presence in forest fragments in the Brazilian Amazon? Biol. Conserv. 2010, 143, 1134–1143. [Google Scholar] [CrossRef]
- Arroyo-Rodríguez, V.; Mandujano, S.; Benítez-Malvido, J. Landscape attributes affecting patch occupancy by Howler Monkeys (Alouatta palliata mexicana) at Los Tuxtlas, Mexico. Am. J. Primatol. 2008, 70, 69–77. [Google Scholar] [CrossRef]
- Carretero-Pinzón, X.; Defler, T.R.; McAlpine, C.A.; Rhodes, J.R. The influence of landscape relative to site and patch variables on primate distributions in the Colombian Llanos. Landsc. Ecol. 2017, 32, 883–896. [Google Scholar] [CrossRef] [Green Version]
- Puig-Lagunes, A.A.; Canales-Espinosa, D.; Rangel-Negrín, A.; Dias, P.A.D. The influence of spatial attributes on fragment occupancy and population structure in the Mexican Mantled Howler (Alouatta palliata mexicana). Int. J. Primatol. 2016, 37, 656–670. [Google Scholar] [CrossRef]
- Nuñez-Iturri, G.; Olsson, O.; Howe, H.F. Hunting reduces recruitment of primate-dispersed trees in Amazonian Peru. Biol. Conserv. 2008, 141, 1536–1546. [Google Scholar] [CrossRef]
- Stevenson, P.R.; Aldana, A.M. Potential effects of Ateline extinction and forest fragmentation on plant diversity and composition in the Western Orinoco basin, Colombia. Int. J. Primatol. 2008, 29, 365–377. [Google Scholar] [CrossRef]
- Calle-Rendón, B.R.; Peck, M.; Bennett, S.E.; Morelos-Juarez, C.; Alfonso, F. Comparison of forest regeneration in two sites with different primate abundances in Northwestern Ecuador. Rev. Biol. Trop. 2016, 64, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, S.F.; Villalobos, F.; Dobrovolski, R.; Beltrão-Mendes, R.; Ferrari, S.F. Forest structure drives global diversity of primates. J. Anim. Ecol. 2014, 83, 1523–1530. [Google Scholar] [CrossRef] [Green Version]
- Millennium Ecosystem Assessment. Ecosystems and Human Well–Being: Biodiversity Synthesis; Island Press: Washington, DC, USA, 2005. [Google Scholar]
- Harcourt, A.H.; Doherty, D.A. Species–area relationships of primates in tropical forest fragments: A global analysis. J. Appl. Ecol. 2005, 42, 630–637. [Google Scholar] [CrossRef]
- Gibbons, M.A.; Harcourt, A.H. Biological correlates of extinction and persistence of primates in small forest fragments: A global analysis. Trop. Conserv. Sci. 2009, 2, 388–403. [Google Scholar] [CrossRef]
- Benchimol, M.; Peres, C.A. Anthropogenic modulators of species–area relationships in Neotropical primates: A continental-scale analysis of fragmented forest landscapes. Divers. Distrib. 2013, 19, 1339–1352. [Google Scholar] [CrossRef]
- Altrichter, M.; Boaglio, G.I. Distribution and relative abundance of peccaries in the Argentine Chaco: Associations with human factors. Biol. Conserv. 2004, 116, 217–225. [Google Scholar] [CrossRef]
- Nagy-Reis, N.B.; Estevo, C.A.; Setz, E.Z.F.; Ribeiro, M.C.; Chiarello, A.G.; Nichols, J.D. Relative importance of anthropogenic landscape characteristics for Neotropical frugivores at multiple scales. Anim. Conserv. 2017, 20, 520–531. [Google Scholar] [CrossRef]
- Carrillo, E.; Wong, G.; Cuarón, A.D. Monitoring mammal populations in Costa Rican protected areas under different hunting restrictions. Conserv. Biol. 2000, 14, 1580–1591. [Google Scholar] [CrossRef]
- Rovero, F.; Mtui, A.; Kitegile, A.; Jacob, P.; Araldi, A.; Tenan, S. Primates decline rapidly in unprotected forests: Evidence from a monitoring program with data constraints. PLoS ONE 2015, 10, e0118330. [Google Scholar] [CrossRef]
- Oklander, L.I.; Kowalewski, M.M.; Corach, D. Genetic consequences of habitat fragmentation in Black-and-Gold Howler (Alouatta caraya) populations from northern Argentina. Int. J. Primatol. 2010, 31, 813–832. [Google Scholar] [CrossRef]
- Chaves, P.B.; Alvarenga, C.S.; Possamai, C.B.; Dias, L.G.; Boubli, J.P.; Strier, K.B.; Mendes, S.L.; Fagundes, V. Genetic diversity and population history of a critically endangered primate, the Northern Muriqui (Brachyteles hypoxanthus). PLoS ONE 2011, 6, e20722. [Google Scholar] [CrossRef]
- Benchimol, M.; Peres, C.A. Predicting primate local extinctions within “real-world” forest fragments: A pan-neotropical analysis. Am. J. Primatol. 2014, 76, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Gascon, C.; Lovejoy, T.E.; Bierregaard, R.O.; Malcolm, J.R.; Stouffer, P.C.; Vasconcelos, H.L.; Laurance, W.F.; Zimmerman, B.; Tocher, M.; Borges, S. Matrix habitat and species richness in tropical forest remnants. Biol. Conserv. 1999, 91, 223–229. [Google Scholar] [CrossRef]
- Uehara-Prado, M.; Fernandes, J.O.; Bello, A.M.; Machado, G.; Santos, A.J.; Vaz-de-Mello, F.Z.; Freitas, A.V.L. Selecting terrestrial arthropods as indicators of small-scale disturbance: A first approach in the Brazilian Atlantic Forest. Biol. Conserv. 2009, 142, 1220–1228. [Google Scholar] [CrossRef]
- Rylands, A.B.; Mittermeier, R.A.; Silva Jr, J.S. Neotropical primates: Taxonomy and recently described species and subspecies. Int. Zoo Yearb. 2012, 46, 11–24. [Google Scholar] [CrossRef]
- Kierulff, M.C.; Santos, G.R.; Canale, G.R.; Guidorizzi, C.E.; Cassano, C.R. The use of camera-traps in a survey of the Buff-Headed Capuchin Monkey, Cebus xanthosternos. Neotrop. Primates 2004, 12, 56–59. [Google Scholar]
- Bowler, M.T.; Tobler, M.W.; Endress, B.A.; Gilmore, M.P.; Anderson, M.J. Estimating mammalian species richness and occupancy in tropical forest canopies with arboreal camera traps. Remote Sens. Ecol. Conserv. 2017, 3, 146–157. [Google Scholar] [CrossRef]
- Brito, D.; Grelle, C.E.V. Estimating minimum area of suitable habitat and viable population size for the Northern Muriqui (Brachyteles hypoxanthus). Biodivers. Conserv. 2006, 15, 4197–4210. [Google Scholar] [CrossRef]
- Gestich, C.C.; Arroyo-Rodríguez, V.; Ribeiro, M.C.; da Cunha, R.G.T.; Setz, E.Z.F. Unraveling the scales of effect of landscape structure on primate species richness and density of Titi Monkeys (Callicebus nigrifrons). Ecol. Res. 2019, 34, 150–159. [Google Scholar] [CrossRef]
- Barbosa, R.I.; Campos, C. Detection and geographical distribution of clearing areas in the savannas (‘lavrado’) of Roraima using Google Earth web tool. J. Geogr. Reg. Plan. 2011, 4, 122–136. [Google Scholar]
- Laurance, W.F. Edge effects in tropical forest fragments: Application of a model for the design of nature reserves. Biol. Conserv. 1991, 57, 205–219. [Google Scholar] [CrossRef]
- CEPF. The Biodiversity Hotspots: Version 2016.1. Critical Ecosystem Partnership Fund. Available online: http://www.cepf.net/resources/hotspots/Pages/default.aspx (accessed on 25 January 2017).
- IBGE. Mapa de Biomas do Brasil: Primeira aproximação. Instituto Brasileiro de Geografia e Estatística. Available online: http://mapas.mma.gov.br/mapas/aplic/probio/datadownload.htm (accessed on 25 January 2017).
- Rodríguez-Mahecha, J.V.; Salaman, P.; Jørgensen, P.; Consiglio, T.; Suárez, L.; Arjona, F.; Bensted-Smith, R. Tumbes-Chocó-Magdalena. In Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions; Mittermeier, R.A., Gil, P.R., Hoffmann, M., Pilgrim, J., Brooks, T., Mittermeier, C.G., Lamoreux, J., da Fonseca, G.A.B., Eds.; CEMEX/Agrupación Sierra Madre: Mexico City, Mexico, 2004; pp. 80–84. [Google Scholar]
- Da Fonseca, G.A.B.; Cavalcanti, R.; Rylands, A.; Paglia, A. Cerrado. In Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial, Ecoregions; Mittermeier, R.A., Gil, P.R., Hoffmann, M., Pilgrim, J., Brooks, T., Mittermeier, C.G., Lamoreux, J., da Fonseca, G.A.B., Eds.; CEMEX/Agrupación Sierra Madre: Mexico City, Mexico, 2004; pp. 93–96. [Google Scholar]
- Roesch, L.F.W.; Vieira, F.C.B.; Pereira, V.A.; Schünemann, A.L.; Teixeira, I.F.; Senna, A.J.T.; Stefenon, V.M. The Brazilian Pampa: A fragile biome. Diversity 2009, 1, 182–198. [Google Scholar] [CrossRef]
- Oliveira, G.; Araújo, M.B.; Rangel, T.F.; Alagador, D.; Diniz-Filho, J.A.F. Conserving the Brazilian semiarid (Caatinga) biome under climate change. Biodivers. Conserv. 2012, 21, 2913–2926. [Google Scholar] [CrossRef]
- Larrotta, L.F.; González, J.F.; Rodríguez, A. Primates en un Paisaje de los Montes de María, Colombia: Distribución y Estado Poblacional de Primates en la Ecorregión de Montes de María, Colombia; Editorial Académica Española: Saarbrücken, Germany, 2016. [Google Scholar]
- Rodríguez-Mahecha, J.V.; Salaman, P.; Jørgensen, P.; Consiglio, T.; Forno, E.; Telesca, A.; Suárez, L.; Arjona, F.; Rojas, F.; Bensted-Smith, R.; et al. Tropical Andes. In Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial, Ecoregions; Mittermeier, R.A., Gil, P.R., Hoffmann, M., Pilgrim, J., Brooks, T., Mittermeier, C.G., Lamoreux, J., da Fonseca, G.A.B., Eds.; CEMEX/Agrupación Sierra Madre: Mexico City, Mexico, 2004; pp. 73–79. [Google Scholar]
- da Fonseca, G.A.B.; Rylands, A.; Paglia, A.; Mittermeier, R.A. Atlantic Forest. In Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial Ecoregions; Mittermeier, R.A., Gil, P.R., Hoffmann, M., Pilgrim, J., Brooks, T., Mittermeier, C.G., Lamoreux, J., da Fonseca, G.A.B., Eds.; CEMEX/Agrupación Sierra Madre: Mexico City, Mexico, 2004; pp. 84–88. [Google Scholar]
- Mittermeier, R.A.; Schipper, J.; Davidse, G.; Koleff, P.; Soberón, J.; Ramírez, M.; Goettsch, B.; Mittermeier, C.G. Mesoamerica. In Hotspots Revisited: Earth’s Biologically Richest and Most Endangered Terrestrial, Ecoregions; Mittermeier, R.A., Gil, P.R., Hoffmann, M., Pilgrim, J., Brooks, T., Mittermeier, C.G., Lamoreux, J., da Fonseca, G.A.B., Eds.; CEMEX/Agrupación Sierra Madre: Mexico City, Mexico, 2004; pp. 103–112. [Google Scholar]
- Daly, D.C.; Mitchell, J.D. Lowland vegetation of tropical South America – an overview. In Imperfect Balance: Landscape Transformations in the Pre-Columbian Americas; Lentz, D., Ed.; Columbia University Press: New York, NY, USA, 2000; pp. 391–454. [Google Scholar]
- Santos-Filho, M.; Peres, C.A.; da Silva, D.J.; Sanaiotti, T.M. Habitat patch and matrix effects on small-mammal persistence in Amazonian forest fragments. Biodivers. Conserv. 2012, 21, 1127–1147. [Google Scholar] [CrossRef]
- Hannibal, W.; da Cunha, N.L.; Figueiredo, V.V.; Rossi, R.F.; Cáceres, N.C.; Ferreira, V.L. Multi-scale approach to disentangle the small mammal composition in a fragmented landscape in central Brazil. J. Mammal. 2018, 99, 1455–1464. [Google Scholar] [CrossRef]
- Muñoz, D.; Estrada, A.; Naranjo, E.; Ochoa, S. Foraging ecology of Howler Monkeys in a cacao (Theobroma cacao) plantation in Comalcalco, Mexico. Am. J. Primatol. 2006, 68, 127–142. [Google Scholar] [CrossRef]
- Zeigler, S.L.; Neel, M.C.; Oliveira, L.; Raboy, B.E.; Fagan, W.F. Conspecific and heterospecific attraction in assessments of functional connectivity. Biodivers. Conserv. 2011, 20, 2779–2796. [Google Scholar] [CrossRef]
- Alba-Mejia, L.; Caillaud, D.; Montenegro, O.L.; Sánchez-Palomino, P.; Crofoot, M.C. Spatiotemporal interactions among three neighboring groups of free-ranging White-Footed Tamarins (Saguinus leucopus) in Colombia. Int. J. Primatol. 2013, 34, 1281–1297. [Google Scholar] [CrossRef]
- Fahrig, L. Rethinking patch size and isolation effects: The habitat amount hypothesis. J. Biogeogr. 2013, 40, 1649–1663. [Google Scholar] [CrossRef]
- Simard, M.; Pinto, N.; Fisher, J.B.; Baccini, A. Mapping forest canopy height globally with spaceborne lidar. J. Geophys. Res. 2011, 116, 1–12. [Google Scholar] [CrossRef]
- NEO. Net Primary Productivity. NASA Earth Observations. Available online: https://neo.sci.gsfc.nasa.gov/view.php?datasetId=MOD17A2_M_PSN (accessed on 7 March 2017).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species: Version 2017-1. Available online: http://www.iucnredlist.org/ (accessed on 22 February 2017).
- Hawes, J.E.; Peres, C.A. Ecological correlates of trophic status and frugivory in Neotropical primates. Oikos 2014, 123, 365–377. [Google Scholar] [CrossRef]
- Peres, C.A.; Janson, C.H. Species coexistence, distribution, and environmental determinants of Neotropical primate richness: A community-level zoogeographic analysis. In Primate Communities; Fleagle, J.G., Janson, C.H., Reed, K.E., Eds.; Cambridge University Press: Cambridge, UK, 1999; pp. 55–74. [Google Scholar]
- AWP. All the World’s Primates. Available online:. Available online: https://alltheworldsprimates.org/Home.aspx (accessed on 16 May 2019).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Heisterkamp, S.; van Willigen, B.; EISPACK; R-core. Package ‘nlme’—Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-137. Available online: https://cran.r-project.org/web/packages/nlme/index.html (accessed on 23 October 2018).
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer Science: New York, NY, USA, 2009. [Google Scholar]
- Bartón, K. Pachage ‘MuMIn’—Multi-Model Inference. R Package Version 1.42.1. Available online: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed on 23 October 2018).
- Bjornstad, O.N.; Cai, J. Package ‘ncf’—Spatial Covariance Functions. R Package Version 1.2-6. Available online: https://cran.r-project.org/web/packages/ncf/index.html (accessed on 23 October 2018).
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.r-project.org/ (accessed on 22 October 2018).
- Keinath, D.A.; Doak, D.F.; Hodges, K.E.; Prugh, L.R.; Fagan, W.; Sekercioglu, C.H.; Buchart, S.H.M.; Kauffman, M. A global analysis of traits predicting species sensitivity to habitat fragmentation. Glob. Ecol. Biogeogr. 2017, 26, 115–127. [Google Scholar] [CrossRef]
- Arroyo-Rodríguez, V.; Mandujano, S. Forest fragmentation modifies habitat quality for Alouatta palliata. Int. J. Primatol. 2006, 27, 1079–1096. [Google Scholar] [CrossRef]
- Laurance, W.F.; Nascimento, H.E.M.; Laurance, S.G.; Andrade, A.; Ribeiro, J.E.L.S.; Giraldo, J.P.; Lovejoy, T.E.; Condit, R.; Chave, J.; Harms, K.E.; et al. Rapid decay of tree-community composition in Amazonian forest fragments. Proc. Nat. Acad. Sci. USA 2006, 103, 19010–19014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laurance, W.F.; Delamônica, P.; Laurance, S.G.; Vasconcelos, H.L.; Lovejoy, T.E. Rainforest fragmentation kills big trees. Nature 2000, 404, 836. [Google Scholar] [CrossRef]
- Laurance, W.F.; Nascimento, H.E.M.; Laurance, S.G.; Andrade, A.C.; Fearnside, P.M.; Ribeiro, J.E.L.; Capretz, R.L. Rain forest fragmentation and the proliferation of successional trees. Ecology 2006, 87, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Ripple, W.J.; Wolf, C.; Newsome, T.M.; Hoffmann, M.; Wirsing, A.J.; McCauleyg, D.J. Extinction risk is most acute for the world’s largest and smallest vertebrates. Proc. Nat. Acad. Sci. USA 2017, 114, 10678–10683. [Google Scholar] [CrossRef]
- Benítez-Malvido, J.; Martínez-Ramos, M. Impact of forest fragmentation on understory plant species richness in Amazonia. Conserv. Biol. 2003, 17, 389–400. [Google Scholar] [CrossRef]
- Geldmann, J.; Barnes, M.; Coad, L.; Craigie, I.D.; Hockings, M.; Burgess, N.D. Effectiveness of terrestrial protected areas in reducing habitat loss and population declines. Biol. Conserv. 2013, 161, 230–238. [Google Scholar] [CrossRef]
- Gray, C.L.; Hill, S.L.L.; Newbold, T.; Hudson, L.N.; Börger, L.; Contu, S.; Hoskins, A.J.; Ferrier, S.; Purvis, A.; Scharlemann, J.P.W. Local biodiversity is higher inside than outside terrestrial protected areas worldwide. Nat. Commun. 2016, 7, 12306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poorter, L.; Bongers, F. Leaf traits are good predictors of plant performance across 53 rain forest species. Ecology 2006, 87, 1733–1743. [Google Scholar] [CrossRef]
- Morellato, L.P.C.; Haddad, C.F.B. Introduction: The Brazilian Atlantic Forest. Biotropica 2000, 32, 786–792. [Google Scholar] [CrossRef]
- Mandujano, S.; Escobedo-Morales, L.A. Population viability analysis of Howler Monkeys (Alouatta palliata mexicana) in a highly fragmented landscape in Los Tuxtlas, Mexico. Trop. Conserv. Sci. 2008, 1, 43–62. [Google Scholar] [CrossRef]
- Pyritz, L.W.; Büntge, A.B.S.; Herzog, S.K.; Kessler, M. Effects of habitat structure and fragmentation on diversity and abundance of primates in tropical deciduous forests in Bolivia. Int. J. Primatol. 2010, 31, 796–812. [Google Scholar] [CrossRef] [PubMed]
- Riba-Hernández, P.; Stoner, K.E. Massive destruction of Symphonia globulifera (Clusiaceae) flowers by Central American Spider Monkeys (Ateles geoffroyi). Biotropica 2005, 37, 274–278. [Google Scholar] [CrossRef]
- Stevenson, P.R.; Guzmán-Caro, D.C. Nutrient transport within and between habitats through seed dispersal processes by Woolly Monkeys in North-Western Amazonia. Am. J. Primatol. 2010, 72, 992–1003. [Google Scholar] [CrossRef]
- Galetti, M.; Bovendorp, R.S.; Guevara, R. Defaunation of large mammals leads to an increase in seed predation in the Atlantic forests. Glob. Ecol. Conserv. 2015, 3, 824–830. [Google Scholar] [CrossRef] [Green Version]
- Schweiger, O.; Maelfait, J.P.; van Wingerden, W.; Hendrickx, F.; Billeter, R.; Speelmans, M.; Augenstein, I.; Aukema, B.; Aviron, S.; Bailey, D.; et al. Quantifying the impact of environmental factors on arthropod communities in agricultural landscapes across organizational levels and spatial scales. J. Appl. Ecol. 2005, 42, 1129–1139. [Google Scholar] [CrossRef]
- Hendrickx, F.; Maelfait, J.P.; van Wingerden, W.; Schweiger, O.; Speelmans, M.; Aviron, S.; Augenstein, I.; Billeter, R.; Bailey, D.; Bukacek, R.; et al. How landscape structure, land-use intensity and habitat diversity affect components of total arthropod diversity in agricultural landscapes. J. Appl. Ecol. 2007, 44, 340–351. [Google Scholar] [CrossRef]
- Gonzalez-Kirchner, J.P.; Sainz, M.M. Primates hunting by Guaymi Amerindians in Costa Rica. Hum. Evol. 1998, 13, 15–19. [Google Scholar] [CrossRef]
- Parathian, H.E.; Maldonado, A.M. Human–nonhuman primate interactions amongst Tikuna people: Perceptions and local initiatives for resource management in Amacayacu in the Colombian Amazon. Am. J. Primatol. 2010, 72, 855–865. [Google Scholar] [CrossRef]
- Ruivo, E.B.; Wormell, D. The international conservation programme for the White-Footed Tamarin Saguinus leucopus in Colombia. Int. Zoo Yearb. 2012, 46, 46–55. [Google Scholar] [CrossRef]
- Canale, G.R.; Kierulff, M.C.M.; Chivers, D.J. A critically endangered capuchin monkey (Sapajus xanthosternos) living in a highly fragmented hotspot. In Primates in Fragments: Complexity and Resilience; Marsh, L.K., Chapman, C.A., Eds.; Springer: New York, NY, USA, 2013; pp. 299–311. [Google Scholar]
- Buss, G.; Queiroz, H.; Melo, F.R.; Talebi, M.; Jerusalinsky, L. Ka’apor Capuchin. In Primates in Peril: The World’s 25 Most Endangered Primates 2016–2018; Schwitzer, C., Mittermeier, R.A., Rylands, A.B., Chiozza, F., Williamson, E.A., Macfie, E.J., Wallis, J., Cotton, A., Eds.; IUCN SSC Primate Specialist Group (PSG), International Primatological Society (IPS), Conservation International (CI), and Bristol Zoological Society: Arlington, VA, USA, 2017; pp. 88–90. [Google Scholar]
- Benchimol, M.; Peres, C.A. Widespread forest vertebrate extinctions induced by a mega hydroelectric dam in lowland Amazonia. PLoS ONE 2015, 10, e0129818. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.L.; Bunnefeld, N.; Jump, A.S.; Peres, C.A.; Dent, D.H. Extinction debt on reservoir land-bridge islands. Biol. Conserv. 2016, 199, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Zilihona, I.J.E.; Niemelä, J.; Nummelin, M. Effects of a hydropower plant on Coleopteran diversity and abundance in the Udzungwa Mountains, Tanzania. Biodivers. Conserv. 2004, 13, 1453–1464. [Google Scholar] [CrossRef] [Green Version]
- Paetzold, A.; Yoshimura, C.; Tockner, K. Riparian arthropod responses to flow regulation and river channelization. J. Appl. Ecol. 2008, 45, 894–903. [Google Scholar] [CrossRef]
- Dray, S.; Pélissier, R.; Couteron, P.; Fortin, M.J.; Legendre, P.; Peres-Neto, P.R.; Bellier, E.; Bivand, R.; Blanchet, F.G.; De Cáceres, M.; et al. Community ecology in the age of multivariate multiscale spatial analysis. Ecol. Monogr. 2012, 82, 257–275. [Google Scholar] [CrossRef]
- Condit, R.; Pitman, N.; Leigh, E.G., Jr.; Chave, J.; Terborgh, J.; Foster, R.B.; Núñez, P.; Aguilar, S.; Valencia, R.; Villa, G.; Muller-Landau, H.C.; et al. Beta-diversity in tropical forest trees. Science 2002, 295, 666–669. [Google Scholar] [CrossRef]
- Gardner, T.A.; Barlow, J.; Chazdon, R.; Ewers, R.M.; Harvey, C.A.; Peres, C.A.; Sodhi, N.S. Prospects for tropical forest biodiversity in a human-modified world. Ecol. Lett. 2009, 12, 561–582. [Google Scholar] [CrossRef] [Green Version]
- Denis, T.; Héraul, B.; Brunaux, O.; Guitet, S.; Richard-Hansen, C. Weak environmental controls on the composition and diversity of medium and large-sized vertebrate assemblages in Neotropical rain forests of the Guiana Shield. Divers. Distrib. 2018, 24, 1545–1559. [Google Scholar] [CrossRef]
- Donati, G.; Santini, L.; Eppley, T.M.; Arrigo-Nelson, S.J.; Balestri, M.; Boinski, S.; Bollen, A.; Bridgeman, L.L.; Campera, M.; Carrai, V.; et al. Low levels of fruit nitrogen as drivers for the evolution of Madagascar’s primate communities. Sci. Rep. 2017, 7, 14406. [Google Scholar] [CrossRef]
- Harcourt, A.H. Latitude and latitudinal extent: A global analysis of the Rapoport effect in a tropical mammalian taxon: Primates. J. Biogeogr. 2000, 27, 1169–1182. [Google Scholar] [CrossRef]
- Purvis, A.; Gittleman, J.L.; Cowlishaw, G.; Mace, G.M. Predicting extinction risk in declining species. Proc. R. Soc. Lond. 2000, 267, 1947–1952. [Google Scholar] [CrossRef] [Green Version]
- Estrada, A.; Coates-Estrada, R. Tropical rain forest fragmentation and wild populations of primates at Los Tuxtlas, Mexico. Int. J. Primatol. 1996, 17, 759–783. [Google Scholar] [CrossRef]
- Palacios-Silva, R.; Mandujano, S. Modelando la dinámica de ocupación de parches de selva por primates en un paisaje fragmentado de Los Tuxtlas, México. In Avances en el Estudio de los Mamíferos de México II; Lorenzo, C., Espinoza, E., Ortega, J., Eds.; Asociación Mexicana de Mastozoología, A.C.: Mexico City, México, 2008; pp. 493–509. [Google Scholar]
- Williams-Guillén, K.; Hagell, S.; Otterstrom, S.; Spehar, S.; Gómez, C. Primate populations in fragmented tropical dry forest landscapes in southwestern Nicaragua. In Primates in Fragments: Complexity and Resilience; Marsh, L.K., Chapman, C.A., Eds.; Springer: New York, NY, USA, 2013; pp. 105–120. [Google Scholar]
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; da Fonseca, G.A.B.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef]
- Olson, D.M.; Dinerstein, E. The global 200: Priority ecoregions for global conservation. Ann. Mo. Bot. Gard. 2002, 89, 199–224. [Google Scholar] [CrossRef]
- Jones, K.E.; Bielby, J.; Cardillo, M.; Fritz, S.A.; O’Dell, J.; Orme, C.D.L.; Safi, K.; Sechrest, W.; Boakes, E.H.; Carbone, C.; et al. PanTHERIA: A species-level database of life history, ecology, and geography of extant and recently extinct mammals. Ecology 2009, 90, 2648. [Google Scholar] [CrossRef]
- Strier, K.B. Diet in one group of Woolly Spider Monkeys, or Muiriquis (Brachyteles arachnoides). Am. J. Primatol. 1991, 23, 113–126. [Google Scholar] [CrossRef]
- Carvalho, O.; Ferrari, S.F.; Strier, K.B. Diet of a Muriqui group (Brachyteles arachnoides) in a continuous primary forest. Primates 2004, 45, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Calle-Rendón, B.R.; Moreno, F.; Hilário, R.R. Vulnerability of mammals to land-use changes in Colombia’s post-conflict era. Nat. Conserv. 2018, 29, 79–92. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species: Version 2018-2. Available online: http://www.iucnredlist.org/. (accessed on 24 November 2018).
- Armenteras, D.; Espelta, J.M.; Rodríguez, N.; Retana, J. Deforestation dynamics and drivers in different forest types in Latin America: Three decades of studies (1980–2010). Glob. Environ. Chang. 2017, 46, 139–147. [Google Scholar] [CrossRef]
- Dinerstein, E.; Olson, D.; Joshi, A.; Vynne, C.; Burgess, N.D.; Wikramanayake, E.; Hahn, N.; Palminteri, S.; Hedao, P.; Noss, R.; et al. An ecoregion-based approach to protecting half the terrestrial realm. BioScience 2017, 67, 534–545. [Google Scholar] [CrossRef] [PubMed]

| Model | mR2 | cR2 | Predictors | p-Value | |||
|---|---|---|---|---|---|---|---|
| a | 0.09 | 0.12 | 0.397 | Area | 0.041 | 0.0005 | 0.041 |
| b | 0.09 | 0.17 | 0.427 | Area | 0.020 | 0.040 | 0.089 |
| DB | −0.009 | 0.055 | |||||
| PA | 0.166 | 0.019 | |||||
| c | 0.09 | 0.09 | 0.364 | Area | 0.044 | 0.0008 | 0.00001 |
| b | 0.09 | 0.28 | 0.402 | Area | 0.037 | 0.001 | 0.147 |
| PA | 0.121 | 0.13 | |||||
| b | 0.12 | 0.12 | 0.590 | DB | −0.022 | 0.045 | 0.000008 |
| Shape | 0.039 | 0.081 | |||||
| Wa | −0.416 | 0.011 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calle-Rendón, B.R.; Hilário, R.R.; de Toledo, J.J. Effect of Site Attributes and Matrix Composition on Neotropical Primate Species Richness and Functional Traits: A Comparison Among Regions. Diversity 2019, 11, 83. https://doi.org/10.3390/d11050083
Calle-Rendón BR, Hilário RR, de Toledo JJ. Effect of Site Attributes and Matrix Composition on Neotropical Primate Species Richness and Functional Traits: A Comparison Among Regions. Diversity. 2019; 11(5):83. https://doi.org/10.3390/d11050083
Chicago/Turabian StyleCalle-Rendón, Bayron R., Renato R. Hilário, and José Julio de Toledo. 2019. "Effect of Site Attributes and Matrix Composition on Neotropical Primate Species Richness and Functional Traits: A Comparison Among Regions" Diversity 11, no. 5: 83. https://doi.org/10.3390/d11050083