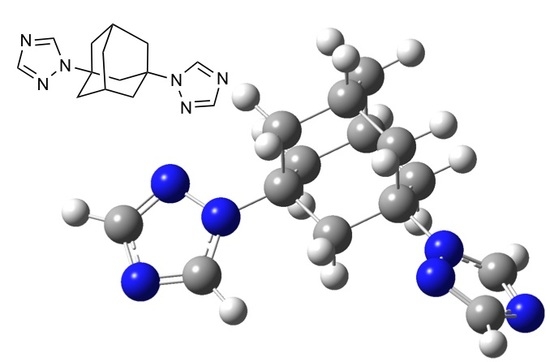
1,3-Bis(1,2,4-triazol-1-yl)adamantane
Abstract
:1. Introduction
2. Results
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Naik, R.; Jeon, C.-O.; Min, H.-Y.; Choi, H.-K.; Min, K.-H.; Lee, K. Synthesis and bioactivity of novel adamantyl derivatives as potent MDR reversal agents. Bull. Korean Chem. Soc. 2011, 32, 4444–4446. [Google Scholar] [CrossRef]
- Zarubaev, V.V.; Golod, E.L.; Anfimov, P.M.; Shtro, A.A.; Saraev, V.V.; Gavrilov, A.S.; Logvinov, A.V.; Kiselev, O.I. Synthesis and anti-viral activity of azolo-adamantanes against influenza A virus. Bioorg. Med. Chem. 2010, 18, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Xia, Y.; Kim, E.K.; Jin, Y.; Kaur, N.; Kim, E.S.; Kim, D.K.; Jung, H.Y.; Choi, Y.; Park, M.-K.; et al. A novel class of highly potent multidrug resistance reversal agents: Disubstituted adamantyl derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 5376–5379. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.E.; Alarcon, B.; Cabildo, P.; Claramunt, R.M.; Sanz, D.; Elguero, J. Synthesis and in vitro antiviral activity of some N-adamantylazoles and benzazoles. Eur. J. Med. Chem. Ther. 1985, 20, 359–362. [Google Scholar]
- Klimochkin, Y.N.; Shiryaev, V.A.; Leonova, M.V. Antiviral properties of cage compounds. New prospects. Russ. Chem. Bull. 2015, 64, 1473–1496. [Google Scholar] [CrossRef]
- Grillaud, M.; Bianco, A. Multifunctional adamantane derivatives as new scaffolds for the multipresentation of bioactive peptides. J. Pept. Sci. 2015, 21, 330–345. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Marx, A. An adamantane-based building block for DNA networks. Chem. Asian J. 2011, 6, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-Q.; Guo, J.-W.; Zhu, D.-Y.; Yang, Z.; Yang, C.-F.; Xian, J.-X.; Li, X. Novel halogen-free flame retardants based on adamantane for polycarbonate. RSC Adv. 2015, 5, 67054–67065. [Google Scholar] [CrossRef]
- Singh, R.; Meena, J.S.; Chang, Y.-C.; Wu, C.-S.; Ko, F.-H. Control of active semiconducting layer packing in organic thin film transistors through synthetic tailoring of dielectric materials. RSC Adv. 2014, 4, 29383–29392. [Google Scholar] [CrossRef]
- Zhou, Y.; Brittain, A.D.; Kong, D.; Xiao, M.; Meng, Y.; Sun, L. Derivatization of diamondoids for functional applications. J. Mater. Chem. C 2015, 3, 6947–6961. [Google Scholar] [CrossRef]
- Tanaka, K.; Hiraoka, T.; Ishiguro, F.; Jeon, J.-H.; Chujo, Y. Adamantane ionic liquids. RSC Adv. 2014, 4, 28107–28110. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.H.; Liu, X.W.; Chen, L.Q.; Wang, S.Q.; Cheng, Y. Synthesis, structure and superoxide dismutase-like activity of two mixed-ligand Cu(II) complexes with N,N′-bis(2-pyridylmethyl)amantadine. Polyhedron 2016, 117, 788–794. [Google Scholar] [CrossRef]
- Natterer, A.; Adhikari, B.; Fyta, M. Complexes of carbene-functionalized diamondoids and metal atoms: Electronic properties. J. Organomet. Chem. 2016, 815–816, 8–15. [Google Scholar] [CrossRef]
- Boldog, I.; Domasevitch, K.V.; Sanchiz, J.; Mayer, P.; Janiak, C. 1,3,5,7-Tetrakis(tetrazol-5-yl)-adamantane: The smallest tetrahedral tetrazole-functionalized ligand and its complexes formed by reaction with anhydrous M(II)Cl2 (M = Mn, Cu, Zn, Cd). Dalton Trans. 2014, 43, 12590–12605. [Google Scholar] [CrossRef] [PubMed]
- Senchyk, G.A.; Lysenko, A.B.; Boldog, I.; Rusanov, E.B.; Chernega, A.N.; Krautscheid, H.; Domasevitch, K.V. 1,2,4-Triazole functionalized adamantanes: A new library of polydentate tectons for designing structures of coordination polymers. Dalton Trans. 2012, 41, 8675–8689. [Google Scholar] [CrossRef] [PubMed]
- Senchyk, G.A.; Lysenko, A.B.; Krautscheid, H.; Rusanov, E.B.; Chernega, A.N.; Krämer, K.W.; Liu, S.X.; Decurtins, S.; Domasevitch, K.V. Functionalized adamantane tectons used in the design of mixed-ligand copper(II) 1,2,4-triazolyl/carboxylate metal-organic frameworks. Inorg. Chem. 2013, 52, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Senchyk, G.A.; Lysenko, A.B.; Rusanov, E.B.; Chernega, A.N.; Krautscheid, H.; Domasevitch, K.V. Polynuclear and polymeric metal complexes based upon 1,2,4-triazolyl functionalized adamantanes. Inorg. Chim. Acta 2009, 362, 4439–4448. [Google Scholar] [CrossRef]
- Senchyk, G.A.; Lysenko, A.B.; Rusanov, E.B.; Chernega, A.N.; Jezierska, J.; Domasevitch, K.V.; Ozarowski, A. Structure and magnetic behavior of CuII MOFs supported by 1,2,4-triazolyl-bifunctionalized adamantane scaffold. Eur. J. Inorg. Chem. 2012, 2012, 5802–5813. [Google Scholar] [CrossRef]
- Maria Claramunt, R.; Cabildo, P.; Forfar, I.; Foces-Foces, C.; Liamas-Saiz, A.L.; Elguero, J. Adamantylation of N-unsubstituted pyrazole derivatives: Mechanistic and structural studies. Heterocycles 1994, 37, 1623–1636. [Google Scholar] [CrossRef]
- Cabildo, P.; Claramunt, R.M.; Elguero, J. Synthesis and reactivity of new l-(1-adamantyl)pyrazoles. J. Heterocycl. Chem. 1984, 21, 249–251. [Google Scholar] [CrossRef]
- Cabildo, P.; Claramunt, R.M.; Sanz, D.; Foces-Foces, M.C.; Hernandez Cano, F.; Catalan, J.; Elguero, J. Structure of 1-(1-adamantyl)pyrazoles. Tetrahedron 1985, 41, 473–478. [Google Scholar] [CrossRef]
- Cabildo, P.; Claramunt, R.M.; Forfar, I.; Elguero, J. Regioselective adamantylation of N-unsubstituted pyrazole derivatives. Tetrahedron Lett. 1994, 35, 183–184. [Google Scholar] [CrossRef]
- Wei, Z.; Li, J.; Wang, N.; Zhang, Q.; Shi, D.; Sun, K. Solvent-free and direct C(sp3)-H amination of adamantanes by grinding. Tetrahedron 2014, 70, 1395–1400. [Google Scholar] [CrossRef]
- Zatonskaya, L.V.; Schepetkin, I.A.; Petrenko, T.V.; Ogorodnikov, V.D.; Khlebnikov, A.I.; Potapov, A.S. Synthesis and cytotoxicity of bis(pyrazol-1-yl)-alkane derivatives with Polymethylene Linkers and Related Mono- and Dipyrazolium Salts. Chem. Heterocycl. Compd. 2016, 52, 388–401. [Google Scholar] [CrossRef]
- Barsukova, M.O.; Samsonenko, D.G.; Goncharova, T.V.; Potapov, A.S.; Sapchenko, S.A.; Dybtsev, D.N.; Fedin, V.P. Coordination polymers with adjustable dimensionality based on CuII and bis-imidazolyl bridging ligand. Russ. Chem. Bull. 2016, 65, 2914–2919. [Google Scholar] [CrossRef]
- Domina, G.A.; Potapov, A.S.; Khlebnikov, A.I.; Ogorodnikov, V.D. Synthesis of 1,8-di(pyrazol-1-yl)- 3,6-dioxaoctane and its derivatives. Russ. J. Org. Chem. 2009, 45, 1224–1228. [Google Scholar] [CrossRef]
- Potapov, A.S.; Nudnova, E.A.; Khlebnikov, A.I.; Ogorodnikov, V.D.; Petrenko, T.V. Synthesis of new polydentate pyrazolyl-ethene ligands by interaction of 1H-pyrazole and 1,1,2,2-tetrabromoethane in a superbasic medium. J. Heterocycl. Chem. 2011, 48, 645–651. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchenko, R.; Potapov, A. 1,3-Bis(1,2,4-triazol-1-yl)adamantane. Molbank 2017, 2017, M968. https://doi.org/10.3390/M968
Marchenko R, Potapov A. 1,3-Bis(1,2,4-triazol-1-yl)adamantane. Molbank. 2017; 2017(4):M968. https://doi.org/10.3390/M968
Chicago/Turabian StyleMarchenko, Roman, and Andrei Potapov. 2017. "1,3-Bis(1,2,4-triazol-1-yl)adamantane" Molbank 2017, no. 4: M968. https://doi.org/10.3390/M968