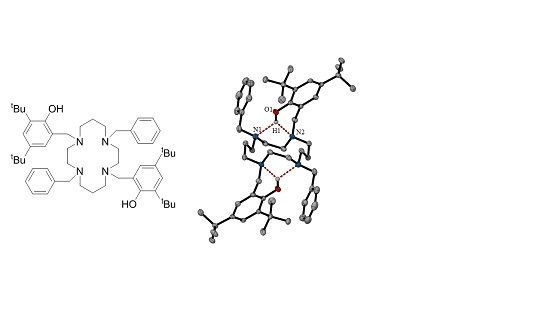
1,8-bis(2-hydroxy-3,5-di-tert-butylbenzyl)-4,11-dibenzyl-1,4,8,11-tetraazacyclotetradecane
Abstract
:1. Introduction
2. Results and Discussion
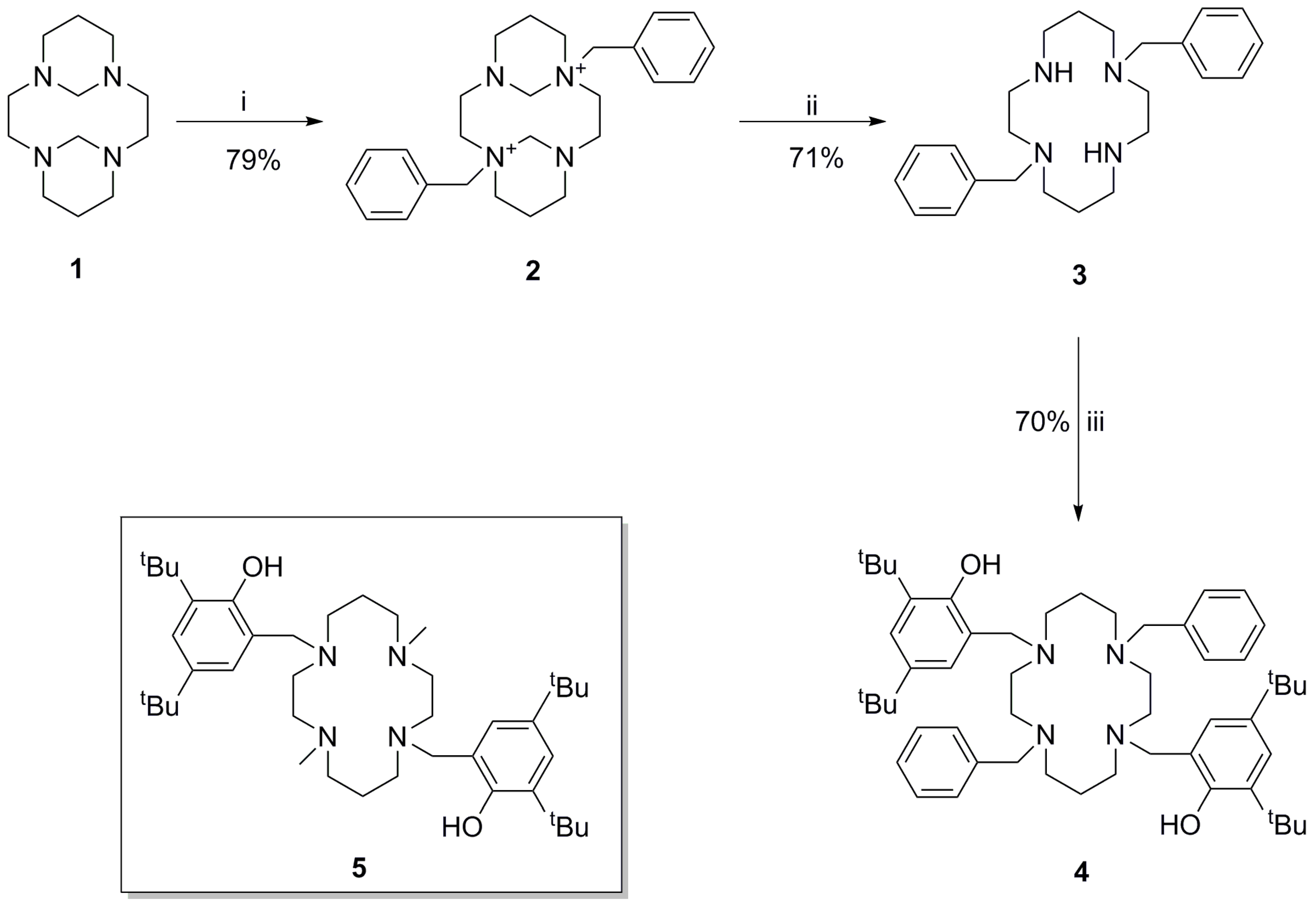
2.1. Synthesis and Characterisation of the Title Compound
2.1.1. NMR Analysis
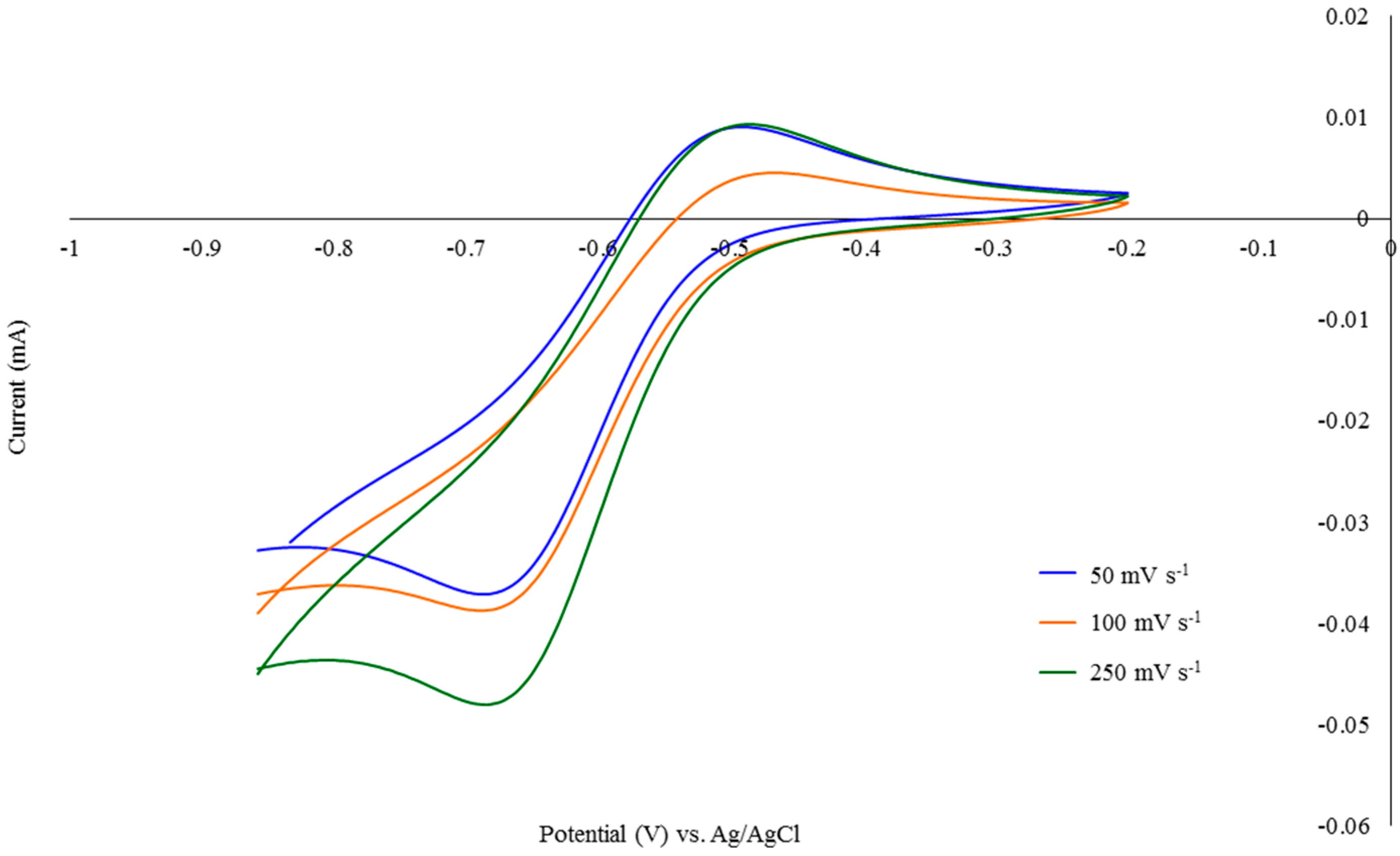
2.1.2. Cyclic Voltammetry Analysis
2.1.3. X-ray Diffraction Analysis
3. Materials and Methods
3.1. General Methods and Physical Measurements
3.2. Synthesis of 1,8-Bis(2-hydroxy-3,5-di-tert-butylbenzyl)-4,11-dibenzyl-1,4,8,11-tetraazacyclotetradecane
3.3. Single Crystal X-ray Diffraction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fricker, S.P. Physiology and Pharmacology of Plerixafor. Transfus. Med. Hemother. 2013, 40, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Poty, S.; Desogere, P.; Goze, C.; Boschetti, F.; D’Huys, T.; Schols, D.; Cawthorne, C.; Archibald, S.J.; Maecke, H.R.; Denat, F. New AMD3100 derivatives for CXCR4 chemokine receptor targeted molecular imaging studies: Synthesis, anti-HIV-1 evaluation and binding affinities. Dalton Trans. 2015, 44, 5004–5016. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.; Choi, J.-H.; Hunter, T.M.; Pannecouque, C.; Moggach, S.A.; Parsons, S.; De Clercq, E.; Sadler, P.J. Zinc(II) complexes of constrained antiviral macrocycles. Dalton Trans. 2012, 41, 6408–6418. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Huskens, D.; Daelemans, D.; Mewis, R.E.; Garcia, C.D.; Cain, A.N.; Freeman, T.N.C.; Pannecouque, C.; De Clercq, E.; Schols, D.; et al. CXCR4 chemokine receptor antagonists: Nickel(II) complexes of configurationally restricted macrocycles. Dalton Trans. 2012, 41, 11369–11377. [Google Scholar] [CrossRef] [PubMed]
- Blahut, J.; Hermann, P.; Galisova, A.; Herynek, V.; Cisarova, I.; Tosner, Z.; Kotek, J. Nickel(II) complexes of N-CH2CF3 cyclam derivatives as contrast agents for F-19 magnetic resonance imaging. Dalton Trans. 2016, 45, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Poty, S.; Gourni, E.; Desogere, P.; Boschetti, F.; Goze, C.; Maecke, H.R.; Denat, F. AMD3100: A Versatile Platform for CXCR4 Targeting Ga-68-Based Radiopharmaceuticals. Bioconjugate Chem. 2016, 27, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Boros, E.; Holland, J.P.; Kenton, N.; Rotile, N.; Caravan, P. Macrocycle-Based Hydroxamate Ligands for Complexation and Immunoconjugation of 89Zirconium for Positron Emission Tomography (PET) Imaging. Chempluschem 2016, 81, 274–281. [Google Scholar] [CrossRef] [PubMed]
- David, T.; Kubicek, V.; Gutten, O.; Lubal, P.; Kotek, J.; Pietzsch, H.-J.; Rulisek, L.; Hermann, P. Cyclam Derivatives with a Bis(phosphinate) or a Phosphinato-Phosphonate Pendant Arm: Ligands for Fast and Efficient Copper(II) Complexation for Nuclear Medical Applications. Inorg. Chem. 2015, 54, 11751–11766. [Google Scholar] [CrossRef] [PubMed]
- Halime, Z.; Frindel, M.; Camus, N.; Orain, P.-Y.; Lacombe, M.; Cherel, M.; Gestin, J.-F.; Faivre-Chauvet, A.; Tripier, R. New synthesis of phenyl-isothiocyanate C-functionalised cyclams. Bioconjugation and Cu-64 phenotypic PET imaging studies of multiple myeloma with the te2a derivative. Org. Biomol. Chem. 2015, 13, 11302–11314. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, Y.; Lv, K.; Zhong, W.; Wang, H.; Wu, Z.; Yi, P.; Jiang, J. Cyclam-functionalized carbon dots sensor for sensitive and selective detection of copper(II) ion and sulfide anion in aqueous media and its imaging in live cells. Sens. Actuators B 2016, 224, 298–306. [Google Scholar] [CrossRef]
- Feier, B.; Fizesan, I.; Meriadec, C.; Girard, S.A.; Cristea, C.; Sandulescu, R.; Geneste, F. Influence of the electrografting method on the performances of a flow electrochemical sensor using modified electrodes for trace analysis of copper (II). J. Electroanal. Chem. 2015, 744, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jasmin, J.-P.; Ouhenia-Ouadahi, K.; Miserque, F.; Dumas, E.; Cannizzo, C.; Chausse, A. Straightforward grafting approach for cyclam-functionalized screen-printed electrodes for selective Cu(II) determination. Electrochim. Acta 2016, 200, 115–122. [Google Scholar] [CrossRef]
- Pandya, D.N.; Bhatt, N.; An, G.I.; Ha, Y.S.; Soni, N.; Lee, H.; Lee, Y.J.; Kim, J.Y.; Lee, W.; Ahn, H.; Yoo, J. Propylene Cross-Bridged Macrocyclic Bifunctional Chelator: A New Design for Facile Bioconjugation and Robust 64Cu Complex Stability. J. Med. Chem. 2014, 57, 7234–7243. [Google Scholar] [CrossRef] [PubMed]
- Maria, L.; Santos, I.C.; Alves, L.G.; Marçalo, J.; Martins, A.M. Rare earth metal complexes anchored on a new dianionic bis(phenolate)dimethylamineCyclam ligand. J. Organomet. Chem. 2013, 728, 57–67. [Google Scholar] [CrossRef]
- Maria, L.; Santos, I.C.; Sousa, V.R.; Marçalo, J. Uranium(III) Redox Chemistry Assisted by a Hemilabile Bis(phenolate) Cyclam Ligand: Uranium–Nitrogen Multiple Bond Formation Comprising a trans-{RN═U(VI)═NR}2+ Complex. Inorg. Chem. 2015, 54, 9115–9126. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.A.; Fanwick, P.E.; Welch, M.J. Synthesis, characterization, and solid-state structure of a new hexachelating ligand and its complex with gallium(III). Inorg. Chem. 1989, 28, 1504–1506. [Google Scholar] [CrossRef]
- Auerbach, U.; Eckert, U.; Wieghardt, K.; Nuber, B.; Weiss, J. Synthesis and coordination chemistry of the hexadentate ligands 1,4,7-tris(2-hydroxybenzyl)-1,4,7-triazacyclononane (H3L1) and 1,4,7-tris(3-tert-butyl-2-hydroxybenzyl)-1,4,7-triazacyclononane (H3L2). Crystal structures of [HL1CuII] and [L2FeIII]acacH. Inorg. Chem. 1990, 29, 938–944. [Google Scholar] [CrossRef]
- Kimura, S.; Bill, E.; Bothe, E.; Weyhermüller, T.; Wieghardt, K. Phenylthiyl Radical Complexes of Gallium(III), Iron(III), and Cobalt(III) and Comparison with Their Phenoxyl Analogues. J. Am. Chem. Soc. 2001, 123, 6025–6039. [Google Scholar] [CrossRef] [PubMed]
- Pujols-Ayala, I.; Barry, B.A. Tyrosyl radicals in Photosystem II. Biochim. Biophys. Acta Bioenerg. 2004, 1655, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.S.; Dooley, D.M. Copper-tyrosyl radical enzymes. Curr. Opin. Chem. Biol. 2003, 7, 189–196. [Google Scholar] [CrossRef]
- Royal, G.; Dahaoui-Gindrey, V.; Dahaoui, S.; Tabard, A.; Guilard, R.; Pullumbi, P.; Lecomte, C. New Synthesis of trans-Disubstituted Cyclam Macrocycles—Elucidation of the Disubstitution Mechanism on the Basis of X-ray Data and Molecular Modeling. Eur. J. Org. Chem. 1998, 1998, 1971–1975. [Google Scholar] [CrossRef]
- Alves, L.G.; Duarte, M.T.; Martins, A.M. Structural features of neutral and cationic cyclams. J. Mol. Struct. 2015, 1098, 277–288. [Google Scholar] [CrossRef]
- Dale, J. Exploratory Calculations of Medium and Large Rings. Part 1. Conformational Minima of Cycloalkanes. Acta Chem. Scand. 1973, 27, 1115–1129. [Google Scholar] [CrossRef]
- Meyer, M.; Dahaoui-Gindrey, V.; Lecomte, C.; Guilard, R. Conformations and coordination schemes of carboxylate and carbamoyl derivatives of the tetraazamacrocycles cyclen and cyclam, and the relation to their protonation states. Coord. Chem. Rev. 1998, 178, 1313–1405. [Google Scholar] [CrossRef]
- Bosnich, B.; Poon, C.K.; Tobe, M.L. Complexes of Cobalt(III) with a Cyclic Tetradentate Secondary Amine. Inorg. Chem. 1965, 4, 1102–1108. [Google Scholar] [CrossRef]
- Maria, L.; Sousa, V.R.; Santos, I.C.; Mora, E.; Marçalo, J. Synthesis and structural characterization of polynuclear divalent ytterbium complexes supported by a bis(phenolate) cyclam ligand. Polyhedron 2016, 119, 277–285. [Google Scholar] [CrossRef]
- CrysAlis P R O. Agilent Technologies, Yarnton, England; RC Clark, JS Reid. Acta Crystallogr. 1995, 51, 887. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
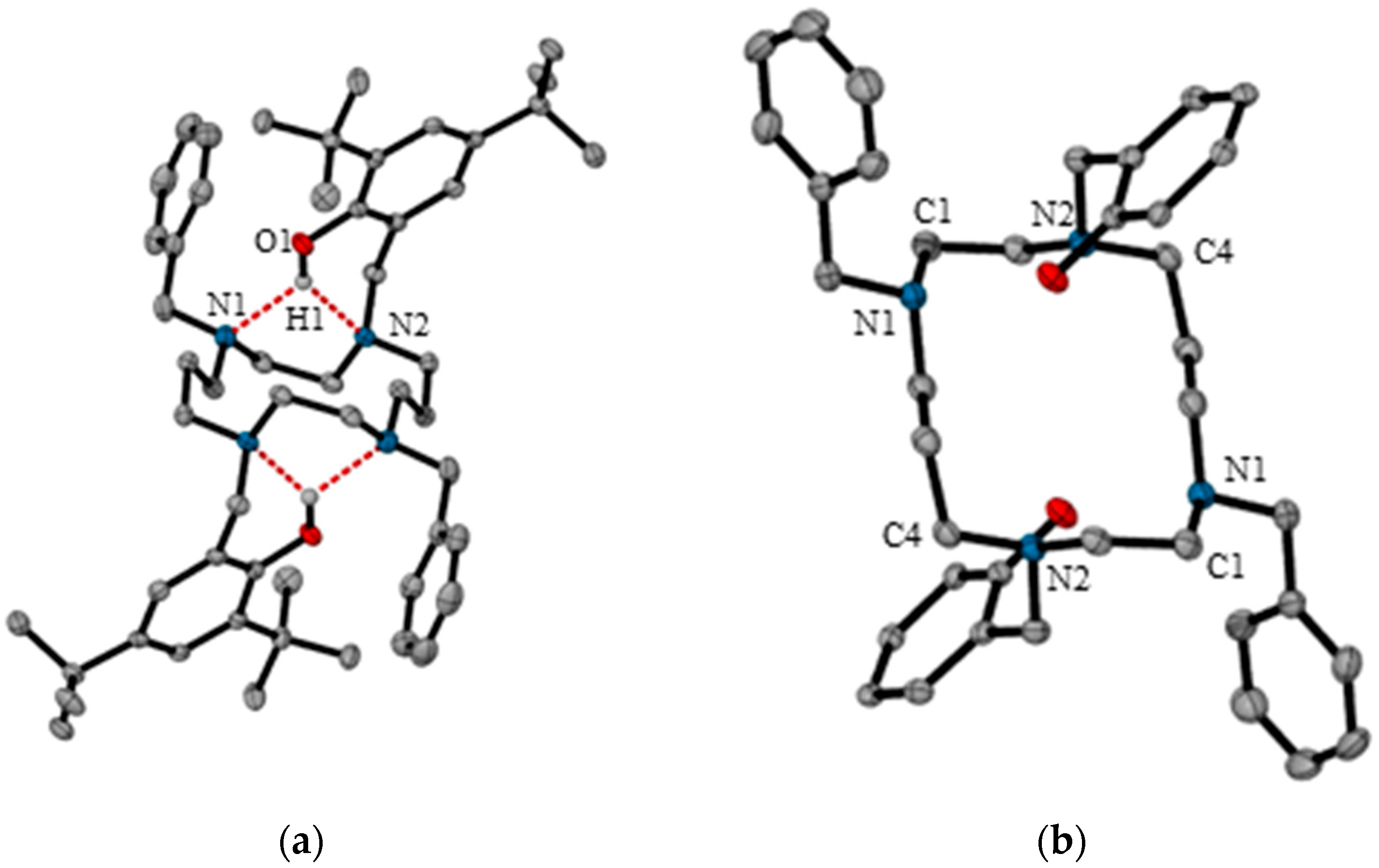
| D–H···A | D–H | H···A | D···A | D–H···A |
|---|---|---|---|---|
| O1–H1···N2 | 0.948 (10) | 2.08 (2) | 2.869 (2) | 139 (3) |
| O1–H1···N11 | 0.948 (10) | 2.28 (2) | 3.055 (2) | 138 (3) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranburu Leiva, A.I.; Kaur, M.; Benjamin, S.L.; Jones, A.M.; Langley, S.K.; Mewis, R.E. 1,8-bis(2-hydroxy-3,5-di-tert-butylbenzyl)-4,11-dibenzyl-1,4,8,11-tetraazacyclotetradecane. Molbank 2017, 2017, M963. https://doi.org/10.3390/M963
Aranburu Leiva AI, Kaur M, Benjamin SL, Jones AM, Langley SK, Mewis RE. 1,8-bis(2-hydroxy-3,5-di-tert-butylbenzyl)-4,11-dibenzyl-1,4,8,11-tetraazacyclotetradecane. Molbank. 2017; 2017(4):M963. https://doi.org/10.3390/M963
Chicago/Turabian StyleAranburu Leiva, Ane I., Mandeep Kaur, Sophie L. Benjamin, Alan M. Jones, Stuart K. Langley, and Ryan E. Mewis. 2017. "1,8-bis(2-hydroxy-3,5-di-tert-butylbenzyl)-4,11-dibenzyl-1,4,8,11-tetraazacyclotetradecane" Molbank 2017, no. 4: M963. https://doi.org/10.3390/M963