Abstract
A new compound, 1,3,5-tris-(2,3-dihydro-1H-1,5-benzodiazepin-4-yl)-1,2,3,4,5,6-hexahydro-s-triazine was obtained by the reaction of ortho-phenylenediamine 1 with 1,3,5-triacryl-1,2,3,4,5,6-hexahydro-1,3,5-triazine 2. Spectroscopic (IR, 1H-NMR, 13C-NMR and MS) data are supplied to support the proposed structure for the title compound.
A method for the preparation of 1,5-benzodiazepine derivatives corresponds to the condensation reaction between aromatic o-diamines with 1,3-dielectrophilic compounds such as acrylic acid, acrylic esters and acrylamides. This approach is quite versatile, which makes it appropriate for synthetic purposes [1,2,3,4,5,6]. Benzodiazepine moieties are well-known due to their wide range of pharmacological activities [7,8]. Previous outcomes reported by us have shown that other systems based on the diazepine core, introduced special properties against different cancer types; turning them into promising pharmacophores [9,10].
On the other hand, the (1,3,5-triacryl)-s-triazine fragments have been used as cross-linking agents for improving the fixation of dyes on polyamide fibers [11].
Synthesis of 1,3,5-tris-(2,3-dihydro-1H-1,5-benzodiazepin-4-yl)-1,2,3,4,5,6-hexahydro-s-triazine (3)
A mixture of ortho-phenylenediamine 1 (345 mg, 3.2 mmol) and 1,3,5-triacryl-1,2,3,4,5,6-hexahydro-1,3,5-triazine 2 (248 mg, 1 mmol) in methanol (10 mL) with triethylamine (1 mL) was refluxed for 16 h (see Scheme 1). The precipitate formed was filtered off and then purified by column chromatography on silica gel, using CH2Cl2:MeOH (20:1) as eluent.
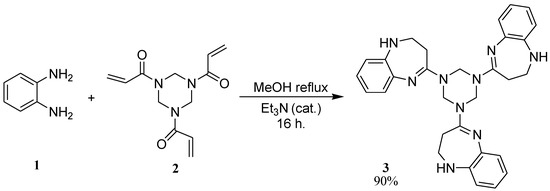
Scheme 1.
Synthesis of 1,3,5-tris-(2,3-dihydro-1H-1,5-benzodiazepin-4-yl)-1,2,3,4,5,6-hexahydro-s-triazine 3.
The 1,3,5-tris-(2,3-dihydro-1H-1,5-benzodiazepin-4-yl)-1,2,3,4,5,6-hexahydro-s-triazine 3 was obtained as a pale-yellow solid (yield: 467 mg, 90%), this compound showed high solubility in solvents such as CHCl3, CH2Cl2, DMF and DMSO. Studies on the generality of this procedure to obtain new analogues and on some biological properties of compound 3 are in progress.
Melting point: >350 °C.
IR (KBr, cm-1): 3385–3338 (NH), 1647 (C=N), 1508 (C=C).
1H-NMR (CDCl3, 400 MHz): δ = 6.82-6.78 (m, 3H, Ar-H); 6.69-6.66 (m, 9H, Ar-H); 5.23 (s, 6H, N-CH2-N); 3.45 (t, J = 6.0 Hz, 6H, CH2); 3.44 (bs, 3H, NH); 2.86 (bs, 6H, CH2) ppm.
13C-NMR (CDCl3, 100 MHz): δ = 171.4, 137.2, 135.4, 120.8, 119.6, 116.9, 112.9, 56.1, 39.7, 32.3 ppm.
EI-MS (m/z, %): 519.2 (M+, 100%), 374.5 (21), 230.1 (16), 145.3 (49),107.3 (35).
Elemental Analysis: Calculated for C30H33N9: C, 69.34%, H, 6.40%, N, 24.26%. Found: C, 69.30%, H, 6.37%, N, 24.24%.
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
The authors are grateful to Colciencias, Universidad del Valle (Colombia) and Universidad de Jaén (Spain) for financial support.
References and Notes
- Orlov, V.D.; Quiroga, J.; Kolos, N.N. Aromatic derivatives of 1H-2,3-dihydropyrazolo[4,5-b]-1,5-diazepine. Khim. Geterotsikl. Soedin. 1987, 363–369, Chem. Abstr. 1987, 107, 217603. [Google Scholar] [CrossRef]
- Insuasty, B.; Abonía, R.; Quiroga, J. The reaction of ketones with ortho-diamines. I. The reaction of aromatic α,β-unsaturated ketones with 4,5-dimethyl-1,2-phenylenediamine. An. Quim. 1992, 88, 718–721. [Google Scholar]
- Orlov, V.D.; Kolos, N.N.; Quiroga, J.; Kaluski, Z.; Figas, E.; Potekhin, A. Reaction of substituted 4,5-diaminopyrazoles with chalcones and acetylarenes. Chem. Heterocycl. Compd. 1992, 506–510. [Google Scholar]
- Insuasty, B.; Ramos, M.; Quiroga, J.; Sánchez, A.; Nogueras, M.; Hanold, N.; Meier, H. The reaction of aromatic, α,β-unsaturated ketones with 4,5-diamino-1,6-dihydropyrimidin-6-ones. J. Heterocycl. Chem. 1994, 31, 61–64. [Google Scholar] [CrossRef]
- Insuasty, B.; Ramos, M.; Moreno, R.; Quiroga, J.; Sánchez, A.; Nogueras, M.; Hanold, N.; Meier, H. Reaction of 4,5-diamino-1,6-dihydropyrimidin-6-ones with two equivalents of chalcones. J. Heterocycl. Chem. 1995, 32, 1229–1233. [Google Scholar] [CrossRef]
- Insuasty, B.; Abonía, R.; Quiroga, J.; Meier, H. Cyclocondensation reaction of 1,2-diamino-4-methylbenzene and p-substituted acetophenones. J. Heterocycl. Chem. 1993, 30, 229–231. [Google Scholar] [CrossRef]
- Landquist, J.K. Comprehensive Heterocyclic Chemistry; Katritzky, A.R., Rees, C.W., Eds.; Pergamon: Oxford, UK, 1984; p. 166. [Google Scholar]
- Schutz, H. Benzodiazepines; Springer: Heidelberg, Germany, 1982. [Google Scholar]
- Insuasty, B.; Orozco, F.; Lizarazo, C.; Quiroga, J.; Abonía, R.; Hursthouse, M.; Nogueras, M.; Cobo, J. Synthesis of new indeno[1,2-e]pyrimido[4,5-b][1,4]diazepine-5,11-diones as potential antitumor agents. Bioorg. Med. Chem. 2008, 16, 8492–8500. [Google Scholar] [CrossRef] [PubMed]
- Insuasty, B.; Orozco, F.; Quiroga, J.; Abonía, R.; Nogueras, M.; Cobo, J. Microwave induced synthesis of novel 8,9-dihydro-7H-pyrimido[4,5-b][1,4]diazepines as potential antitumor agents. Eur. J. Med. Chem. 2008, 43, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.M.; Ho, Y.C. Improved fixation of dyes on polyamide fibres. Part 1: Using 1,3,5-triacroylamino-hexahydro-s-triazine as a crosslinking agent. Dyes Pigm. 1995, 28, 171–192. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).