A New Flavonoid Glycoside from Salix denticulata Aerial Parts
Abstract
:1. Introduction
2. Results and discussion
3. Experimental Section
3.1. General
3.2. Plant material
3.3. Extraction and isolation
3.4. 5-Hydroxy-2-(2-hydroxy-4-methoxyphenyl)-4-oxo-4H-chromen-7-yl β-d-glucopyranoside (1)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
References and Notes
- Gaur, R.D. Flora of Garhwal North West Himalaya. Trans Media: Srinagar Garhwal, India, 1999; p. 186. [Google Scholar]
- Zheng, S.; Wang, J.; Lu, J.; Shen, T.; Sun, L.; Shen, X. Two new acyclic diterpene-γ-lactones from Salix matsudan. Planta Med. 2000, 66, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.L.; Nonaka, G.I.; Nishioka, I. Acylated flavanols and procyanidins from Salix sieboldiana. Phytochemistry 1985, 24, 2089–2091. [Google Scholar] [CrossRef]
- Lee, H.; Watanabe, N.; Sasaya, T.; Ozawa, S. Extractives of short-rotation hardwood species. I. Phenolics of the wood of Salix sachalinensis Fr. Schm. Mokuzai Gakkaishi 1993, 39, 1409–1414. [Google Scholar]
- Shelyuto, V.L.; Bondarenko, V.G. Flavonoids of Salix acutifolia. Khim. Prir. Soedin. 1985, 4, 567–568. [Google Scholar]
- Kompantsev, V.A. Polyphenols of the leaves of Salix pantosericea and Salix pentandroides. Khim. Prir. Soedin. 1980, 5, 654–656. [Google Scholar]
- Shao, Y.; Lahloub, M. F.; Meier, B.; Sticher, O. Isolation of phenolic compounds from the bark of Salix pentandra. Planta Med. 1989, 55, 617–620. [Google Scholar] [CrossRef]
- Sati, O.P.; Pant, G. Steroidal constituents of Agave cantala Roxb. (Rootstaks). Pharmazie 1983, 38, 353. [Google Scholar]
- Hikino, H.; Konno, C.; Takemoto, T. Structure of pleoside from Pleopeltis thunbergiana. Yakugaku Zasshi 1969, 89, 372–374. [Google Scholar] [PubMed]
- Mizuno, M.; Kato, M.; Misu, C.; Iinuma, M.; Tanaka, T. Chaenomeloidin: A phenolic glucoside from leaves of Salix chaenomeloides. J. Nat. Prod. 1991, 54, 1447–1450. [Google Scholar] [CrossRef]
- Montoro, P.; Braca, A.; Pizza, C.; De Tommasi, N. Structure antioxidant activity relationships of flavonoids isolated from different plant species. Food Chem. 2005, 92, 349–355. [Google Scholar] [CrossRef]
- Chiruvella, K. K.; Mohammed, A.; Dampuri, G.; Ghanta, R. G.; Raghavan, S. C. Phytochemical and antimicrobial studies of methyl angolensate and luteolin-7-O-glucoside isolated from callus cultures of Soymida febrifuga. Int. J. Biomed. Sci. 2007, 3, 269–278. [Google Scholar] [PubMed]
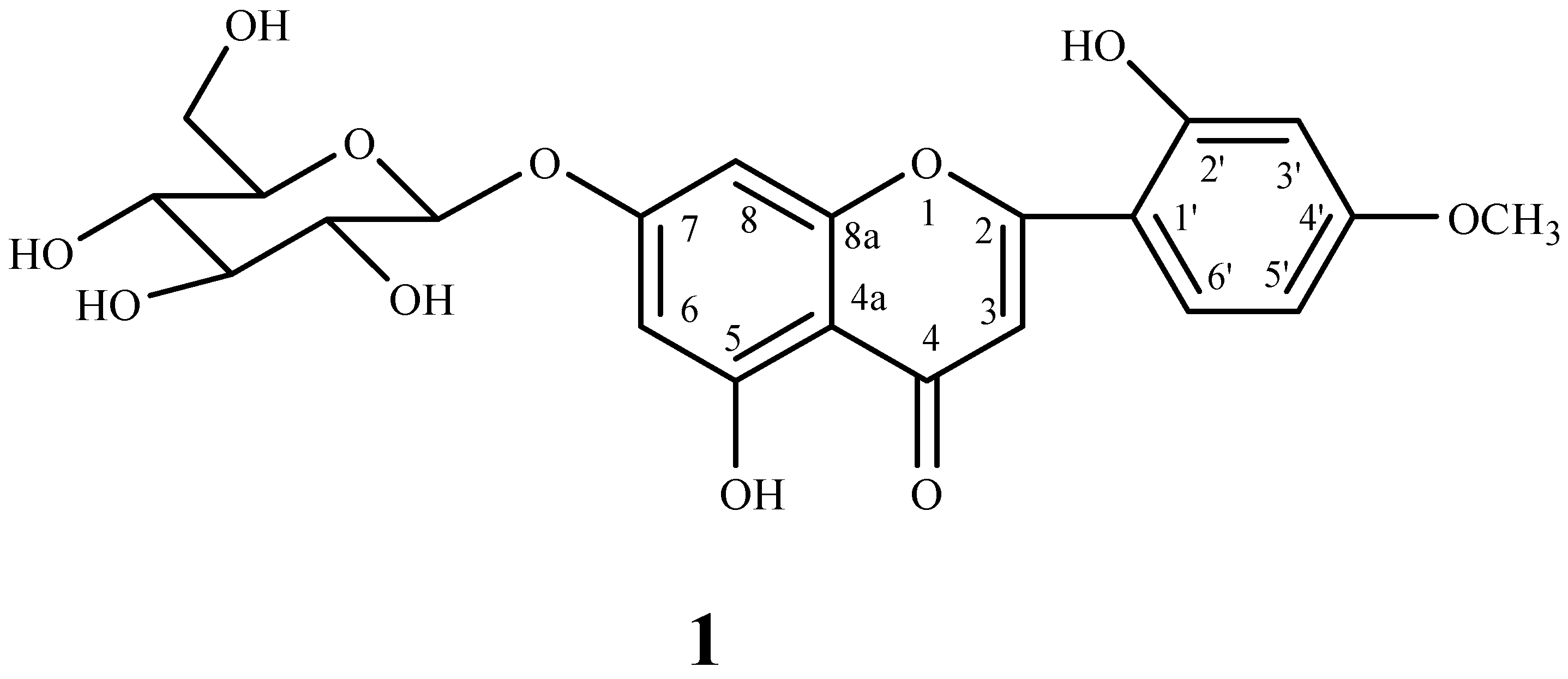

| Position | δC ppm | δH ppm (J Hz) | HSQC | HMBC |
|---|---|---|---|---|
| 2 | 161.16 | - | -C- | - |
| 3 | 103.20 | 6.79, s | -CH- | 4, 4a, 1’ |
| 4 | 181.93 | - | -C- | - |
| 4a | 105.37 | - | -C- | - |
| 5 | 164.50 | - | -C- | - |
| 6 | 99.57 | 6.46 (d, 1.8) | -CH- | 4a, 5, 7, 8 |
| 7 | 162.98 | - | -C- | - |
| 8 | 97.76 | 6.76 (d, 1.8) | -CH- | 6, 7, 4a, 8a |
| 8a | 156.98 | - | -C- | - |
| 1’ | 121.42 | - | -C- | - |
| 2’ | 149.95 | - | -C- | - |
| 3’ | 113.59 | 7.12 (d, 3.4) | -CH- | 4’, 5’ |
| 4’ | 145.81 | - | -C- | - |
| 5’ | 116.02 | 7.44 (dd, 3.4, 8.4) | -CH- | 1’, 3’, 4’, 6’ |
| 6’ | 119.21 | 6.92 (d, 8.4) | -CH- | 1’, 2’, 4’, 5’ |
| 1” | 99.92 | 5.09 (d, 7.2) | -CH- | 7 |
| 2” | 70.36 | 3.29 (d, 8.8) | -C- | - |
| 3” | 73.15 | 3.34 (t, 8.8) | -CH- | - |
| 4” | 76.42 | 3.32, m | -CH- | - |
| 5” | 77.19 | 3.49, m | -CH- | - |
| 6” | 60.65 | 3.73, m | -CH2- | - |
| OCH3-4’ | 55.82 | 3.61, s | -CH3 | 4’ |
| OH-5 | - | 12.9 | - | - |
| OH-2’ | - | 9.5 | - | - |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rawat, U.; Semwal, S.; Semwal, D.K.; Badoni, R.; Bamola, A. A New Flavonoid Glycoside from Salix denticulata Aerial Parts. Molbank 2009, 2009, M622. https://doi.org/10.3390/M622
Rawat U, Semwal S, Semwal DK, Badoni R, Bamola A. A New Flavonoid Glycoside from Salix denticulata Aerial Parts. Molbank. 2009; 2009(3):M622. https://doi.org/10.3390/M622
Chicago/Turabian StyleRawat, Usha, Sushma Semwal, Deepak Kumar Semwal, Ruchi Badoni, and Amita Bamola. 2009. "A New Flavonoid Glycoside from Salix denticulata Aerial Parts" Molbank 2009, no. 3: M622. https://doi.org/10.3390/M622




