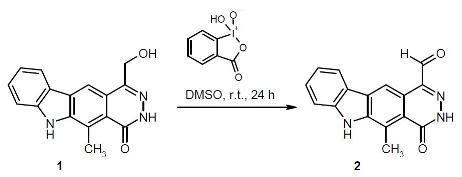
5-Methyl-4-oxo-4,6-dihydro-3H-pyridazino[4,5-b]carbazole-1-carbaldehyde
Abstract
:
Experimental
5-Methyl-4-oxo-4,6-dihydro-3H-pyridazino[4,5-b]carbazole-1-carbaldehyde (2)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgments
References and Notes
- Haider, N. Pyridazine-Fused Carbazoles: Synthesis, Reactivity, and Antitumor Activity. J. Heterocyclic Chem. 2002, 39, 511–521. [Google Scholar] [CrossRef]
- Haider, N.; Kabicher, T.; Käferböck, J.; Plenk, A. Synthesis and in-vitro antitumor activity of 1-[3-(indol-1-yl)prop-1-yn-1-yl]phthalazines and related compounds. Molecules 2007, 12, 1900–1909. [Google Scholar] [CrossRef] [PubMed]
- Haider, N.; Jbara, R.; Käferböck, J.; Traar, U. Synthesis of tetra- and pentacyclic carbazole-fused imides as potential antitumor agents. Arkivoc 2009, 38–47. [Google Scholar]
- Pongprom, N.; Mueller, G.; Schmidt, P.; Holzer, W.; Spreitzer, H. Carbinol derivatives of azanaphthoquinone annelated pyrrols. Monatsh. Chem. 2009, 140, 309–313. [Google Scholar] [CrossRef]
- Shanab, K.; Pongprom, N.; Wulz, E.; Holzer, W.; Spreitzer, H.; Schmidt, P.; Aicher, B.; Mueller, G.; Günther, E. Synthesis and biological evaluation of novel cytotoxic azanaphthoquinone annelated pyrrolo oximes. Bioorg. Med. Chem. Lett. 2007, 17, 6091–6095. [Google Scholar] [CrossRef] [PubMed]
- Spreitzer, H.; Puschmann, C. Dual function antitumor agents based on bioreduction and DNA alkylation. Monatsh. Chem. 2007, 138, 517–522. [Google Scholar] [CrossRef]
- Haider, N.; Sotelo, E. 1,5-Dimethyl-6H-pyridazino[4,5-b]carbazole, a 3-aza bioisoster of the antitumor alkaloid olivacine. Chem. Pharm. Bull. 2002, 50, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, M.; Santagostino, M.; Sputore, S.; Palmisano, G. Oxidation of Alcohols with o-Iodoxy-benzoic Acid (IBX) in DMSO: a New Insight into an Old Hypervalent Iodine Reagent. J. Org. Chem. 1995, 60, 7272–7276. [Google Scholar] [CrossRef]
- Frigerio, M.; Santagostino, M.; Sputore, S. A User-Friendly Entry to 2-Iodoxybenzoic Acid (IBX). J. Org. Chem. 1999, 64, 4537–4538. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Emich, M.; Haider, N. 5-Methyl-4-oxo-4,6-dihydro-3H-pyridazino[4,5-b]carbazole-1-carbaldehyde. Molbank 2009, 2009, M623. https://doi.org/10.3390/M623
Emich M, Haider N. 5-Methyl-4-oxo-4,6-dihydro-3H-pyridazino[4,5-b]carbazole-1-carbaldehyde. Molbank. 2009; 2009(4):M623. https://doi.org/10.3390/M623
Chicago/Turabian StyleEmich, Miriam, and Norbert Haider. 2009. "5-Methyl-4-oxo-4,6-dihydro-3H-pyridazino[4,5-b]carbazole-1-carbaldehyde" Molbank 2009, no. 4: M623. https://doi.org/10.3390/M623