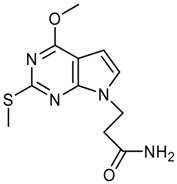
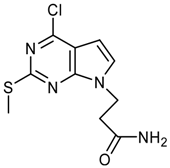
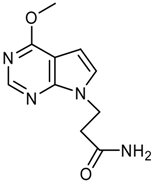
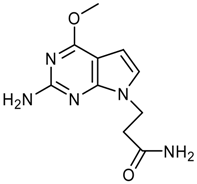
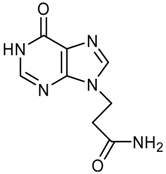
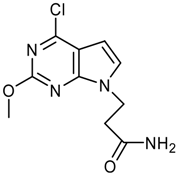
Synthesis of 3-(4-Chloro-2-methoxy-7H-pyrrolo[2,3-d]-pyrimidin-7-yl)propanoic acid – An N(9)-Functionalized 7-Deazapurine
Abstract
:1. Introduction
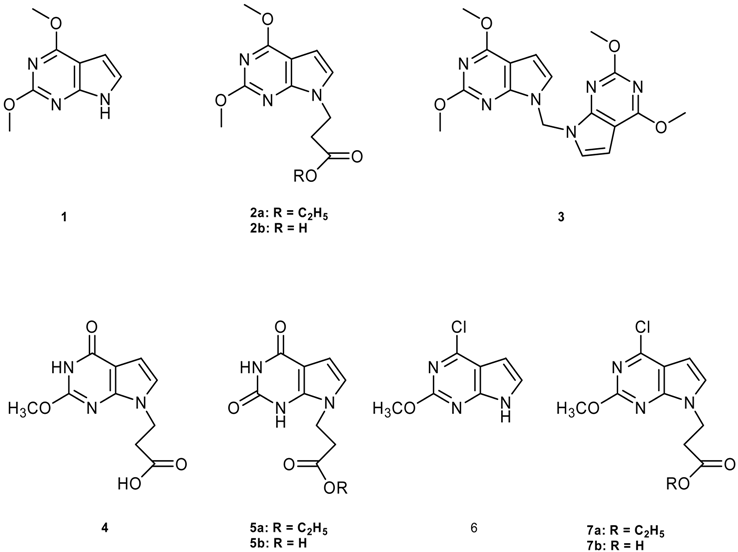
2. Experimental
General
7,7’-Methanediylbis(2,4-dimethoxy-7H-pyrrolo[2,3-d]pyrimidine) (3)
3-(4-Chloro-2-methoxy-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propanoic acid (7b)
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3References and Notes
- Suhadolnik, R.J. Nucleosides As Biological Probes; John Wiley & Sons: New York, 1979; pp. 158–166. [Google Scholar]
- Revankar, G.R.; Robins, R.K. Chemistry of Nucleosides and Nucleotides; Townsend, L.B., Ed.; Plenum Press: New York, 1991; pp. 200–247, and literature cited therein. [Google Scholar]
- Seela, F.; Peng, X.; Budow, S. Advances in the synthesis of 7-deazapurine – pyrrolo[2,3-d]pyrimidine – 2’-deoxyribonucleosides including D- and L-enantiomers, fluoro derivatives and 2’,3’-dideoxyribonucleosides. Curr. Org. Chem. 2007, 11, 427–462, and literature cited therein. [Google Scholar] [CrossRef]
- Mueller, C.E.; Geis, U.; Grahner, B.; Lanzner, W.; Eger, K. Chiral pyrrolo[2,3-d]pyrimidine and pyrimido[4,5-b]indole derivatives: structure-activity relationships of potent, highly stereoselective A-adenosine receptor antagonists. J. Med. Chem. 1996, 39, 2482–2491, and literature cited therein. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wu, T.Y.H.; Brinker, A.; Peters, E.C.; Hur, W.; Gray, N.S.; Schultz, P.G. Synthetic small molecules that control stem cell fate. Proc. Natl. Acad. Sci. 2003, 100, 7632–7637. [Google Scholar] [CrossRef] [PubMed]
- Rosemeyer, H.; Kaiser, K.; Seela, F. Dextran-linked 7-deazaguanine – a polymer-bound inhibitor of xanthine oxidase. Int. J. Biol. Macromol. 1987, 9, 205–210. [Google Scholar] [CrossRef]
- Ahlers, M.; Ringsdorf, H.; Rosemeyer, H.; Seela, F. Orientation, recognition, and photoreaction of nucleolipids in model membranes. Colloid. Polym. Sci. 1990, 268, 132–142. [Google Scholar] [CrossRef]
- Rosemeyer, H.; Ahlers, M.; Schmidt, B.; Seela, F. A nucleolipid with antiviral acycloguanosine as head group – synthesis and liposome formation. Angew. Chem. Int. Ed. 1985, 24, 501–502. [Google Scholar] [CrossRef]
- Rosemeyer, H.; Stürenberg, E.M.; Herdewijn, P. Nucleolipids as potential organogelators. Nucleos. Nucleot. Nucleic. Acids 2007, 26, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Lee, Y.S.; Kim, B.H. Chem. Commun. 2003, 2912–2913.
- Rosemeyer, H. Synthesis of 3-amino(4-amino-7H-pyrrolo[2,3-d]pyrimidin-7-yl)propanoic acid – a functionalized base derivative of the nucleoside antibiotic Tubercidin. Molbank 2007, M555. [Google Scholar] [CrossRef]
- Rosemeyer, H.; Kaiser, K.; Seela, F. Spontaneous hydroxylation of a cyclization intermediate of allopurinol. J. Org. Chem. 1985, 50, 1847–1852. [Google Scholar] [CrossRef]
- Rosemeyer, H.; Kaiser, K.; Seela, F.; Zabel, V.; Saenger, W. Spontane Öffnung des Pyrimidinringes in Pyrrolo[2,3-d]pyrimidinen nach intramolekularer Acylierung. Helv. Chim. Acta 1985, 68, 534–544. [Google Scholar] [CrossRef]
- Rosemeyer, H.; Seela, F. Hydrolytic cleavage of 6-oxopurines after intramolecular acylation at N-3. Heterocycles 1985, 23, 2669–2676. [Google Scholar]
- Rosemeyer, H.; Kretschmer, U.; Seela, F. Cyclization of 7-deazaxanthine-9-propionic acid to an active-site-directed, irreversibly acting inhibitor of xanthine oxidase. Helv. Chim. Acta 1985, 68, 2165–2172. [Google Scholar] [CrossRef]
- logP Values were calculated using the program suite ChemSketch (version 11.0, provided by Advanced Chemistry Developments Inc. Toronto, Canada; http://www.acdlabs.com).
- Seela, F.; Liman, U. ara-7-Deazaxanthosin – ein Xanthin-Nucleosid mit stabiler N-glycosylischer Bindung. Liebigs. Ann. Chem. 1984, 273–282. [Google Scholar] [CrossRef]
- For commercial sources of compounds 1 and 6 see: http://www.cas.org/expertise/cascontent/chemcats.html.
- Rosemeyer, H.; Anders, M.; Seela, F. Symmetrically and unsymmetrically bridged methylenebis (allopurinols): synthesis of dimeric potential anti-gout drugs. Molecules 2007, 12, 563–575. [Google Scholar] [CrossRef] [PubMed]
 logP = -0.02 +/- 0.83 | 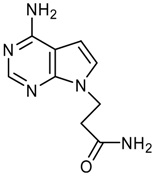 logP = -0.42 +/- 0.36 |  logP = -0.56 +/- 0.93 | 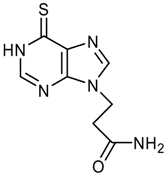 logP = -0.61 +/- 0.43 |
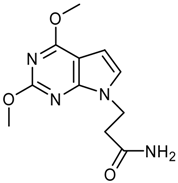 logP = -0.70 +/- 1.28 | 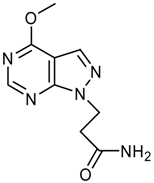 logP = -0.85 +/- 1.15 | 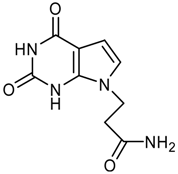 logP = -1.09 +/- 0.42 | 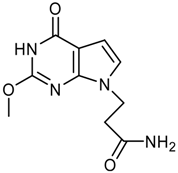 logP = -1.14 +/- 0.92 |
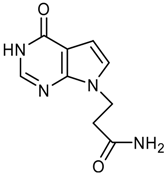 logP = -1.54 +/- 0.81 | 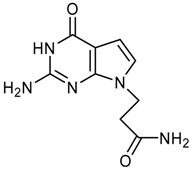 logP = -1.71 +/- 0.81 |  logP = -1.78 +/- 1.07 |  logP = -1.77 +/- 0.54 |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rosemeyer, H. Synthesis of 3-(4-Chloro-2-methoxy-7H-pyrrolo[2,3-d]-pyrimidin-7-yl)propanoic acid – An N(9)-Functionalized 7-Deazapurine. Molbank 2009, 2009, M590. https://doi.org/10.3390/M590
Rosemeyer H. Synthesis of 3-(4-Chloro-2-methoxy-7H-pyrrolo[2,3-d]-pyrimidin-7-yl)propanoic acid – An N(9)-Functionalized 7-Deazapurine. Molbank. 2009; 2009(1):M590. https://doi.org/10.3390/M590
Chicago/Turabian StyleRosemeyer, Helmut. 2009. "Synthesis of 3-(4-Chloro-2-methoxy-7H-pyrrolo[2,3-d]-pyrimidin-7-yl)propanoic acid – An N(9)-Functionalized 7-Deazapurine" Molbank 2009, no. 1: M590. https://doi.org/10.3390/M590