3-tert-butoxycarbonylamino-pyridine-2-carboxylic Acid
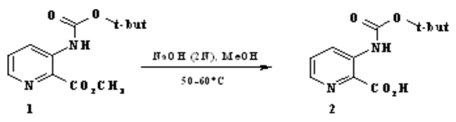
Supplementary materials
Supplementary File 1Supplementary File 2Supplementary File 3Acknowledgements
© 2007 by MDPI (http://www.mdpi.org/). Reproduction is permitted for noncommercial purposes.
Share and Cite
Mamouni, R.; El Haddad, M.; Akssira, M. 3-tert-butoxycarbonylamino-pyridine-2-carboxylic Acid. Molbank 2007, 2007, M546. https://doi.org/10.3390/M546
Mamouni R, El Haddad M, Akssira M. 3-tert-butoxycarbonylamino-pyridine-2-carboxylic Acid. Molbank. 2007; 2007(3):M546. https://doi.org/10.3390/M546
Chicago/Turabian StyleMamouni, Rachid, Mohammadine El Haddad, and Mohamed Akssira. 2007. "3-tert-butoxycarbonylamino-pyridine-2-carboxylic Acid" Molbank 2007, no. 3: M546. https://doi.org/10.3390/M546



