Synthetic Efforts for Stereo Structure Determination of Cytotoxic Marine Natural Product Pericosines as Metabolites of Periconia sp. from Sea Hare
Abstract
:1. Introduction
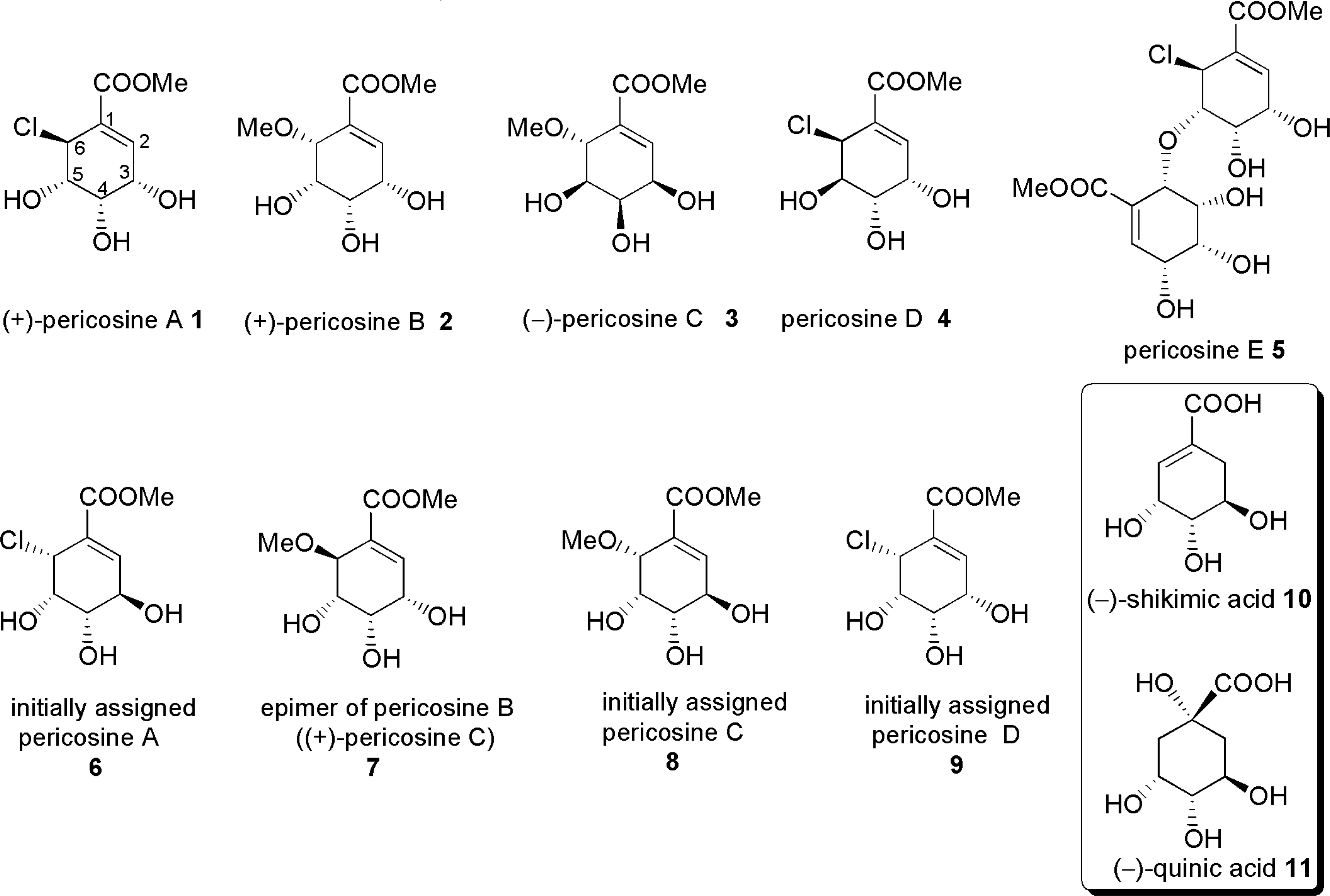
2. Isolation of Pericosines from Periconia byssoides [54, 55]
3. Synthetic Efforts for Pericosines
3.1. Total synthesis of pericosine B [58]
3.1.1. Donohoe’s approach
3.1.2. Okamura’s approach [59]
3.1.3. Usami’s approach [60]
3.1.4. Garcia Ruano’s approach [61]
3.2. Total synthesis of epimer of pericosine B: Synthesis of pericosine C [63]
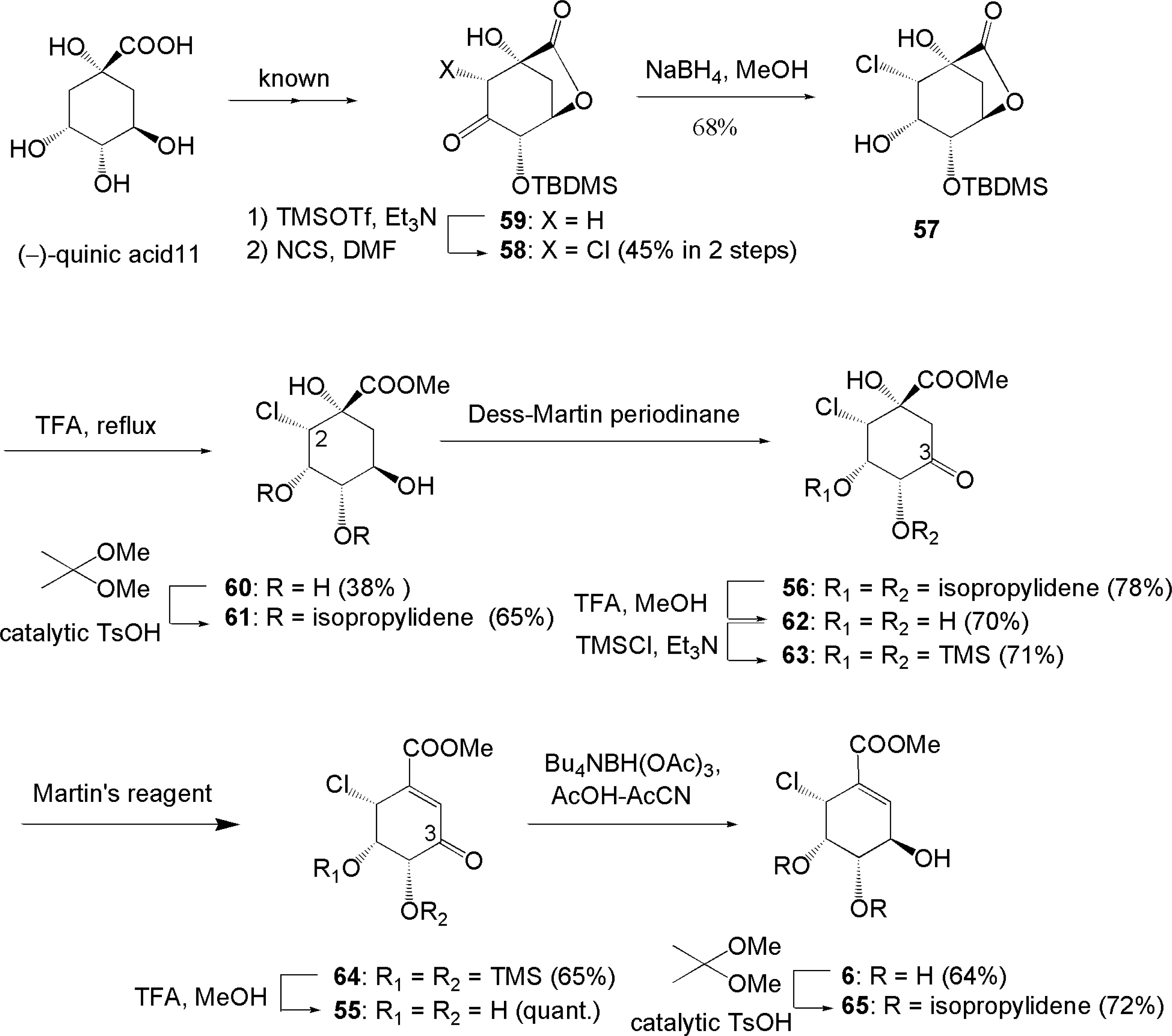
3.3. Total synthesis of initially assigned pericosines A and D [64, 65]
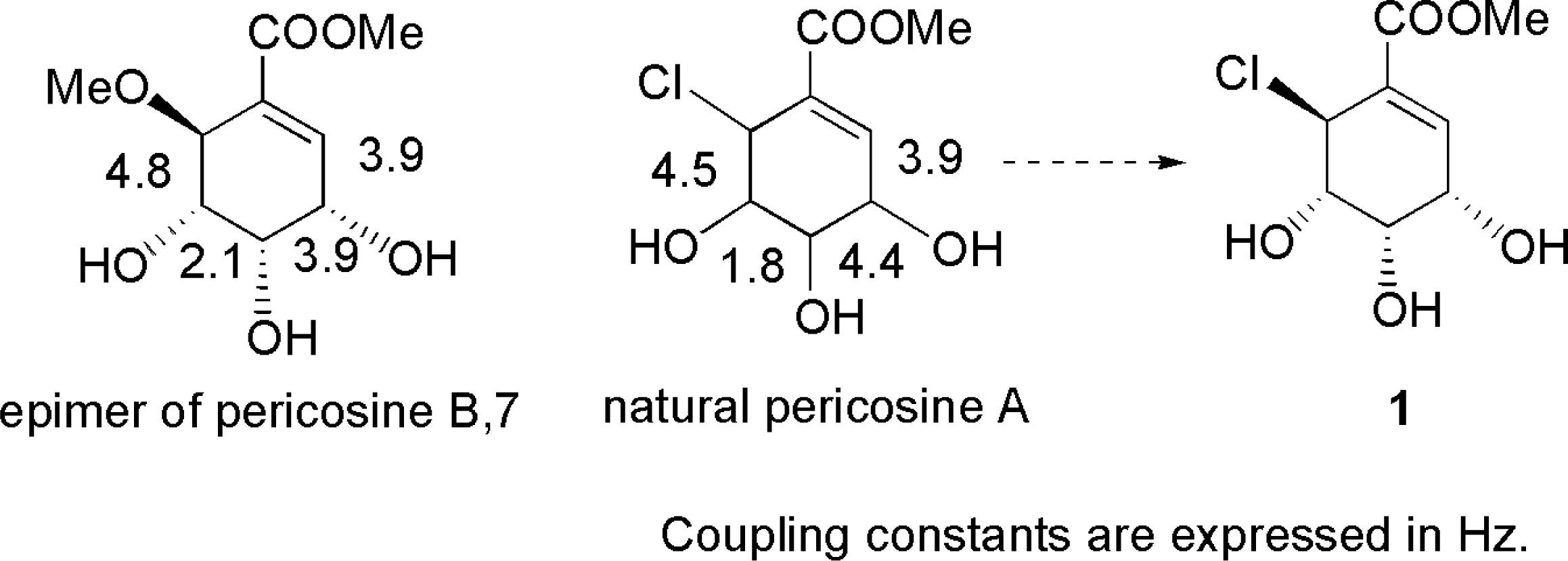
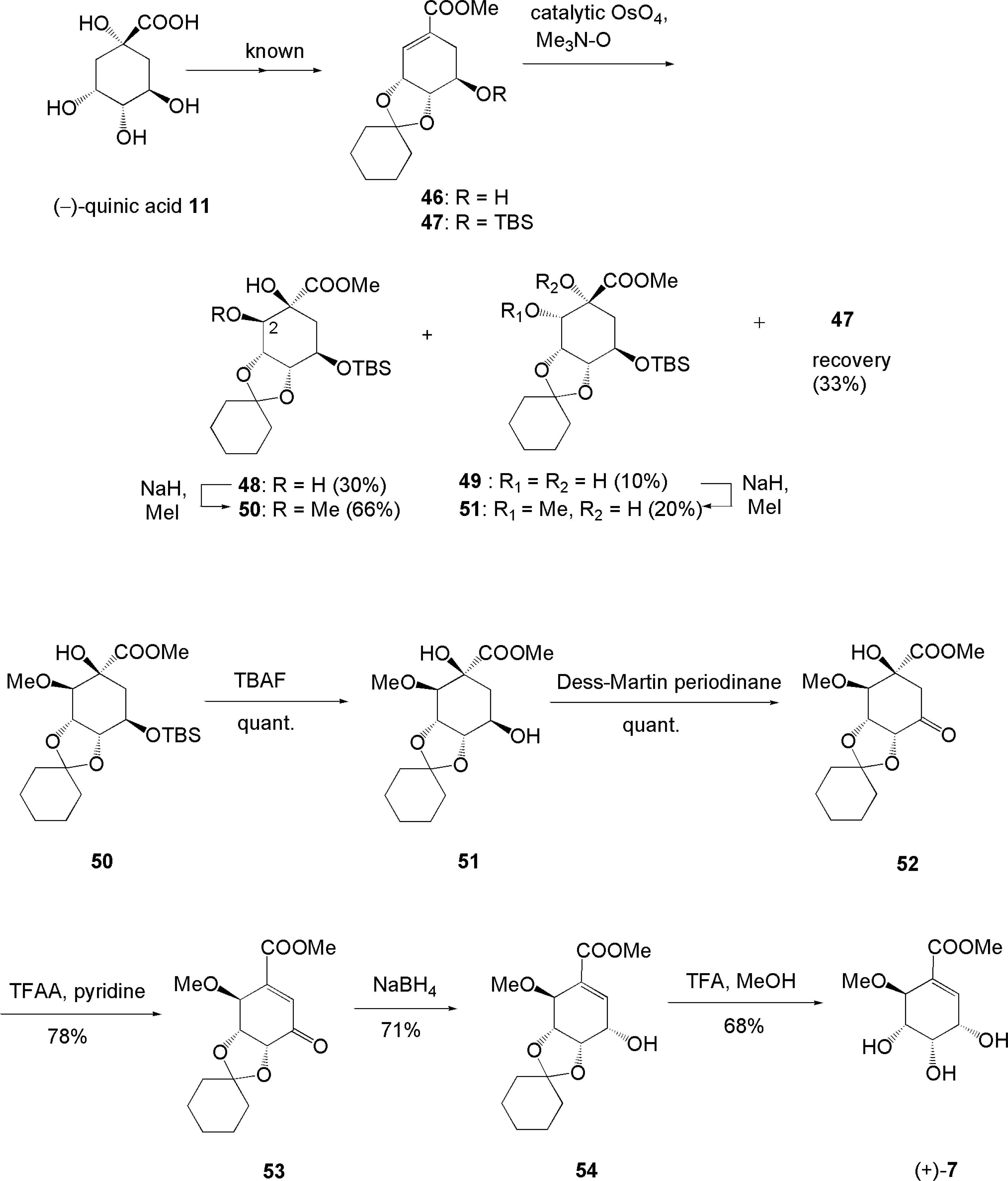
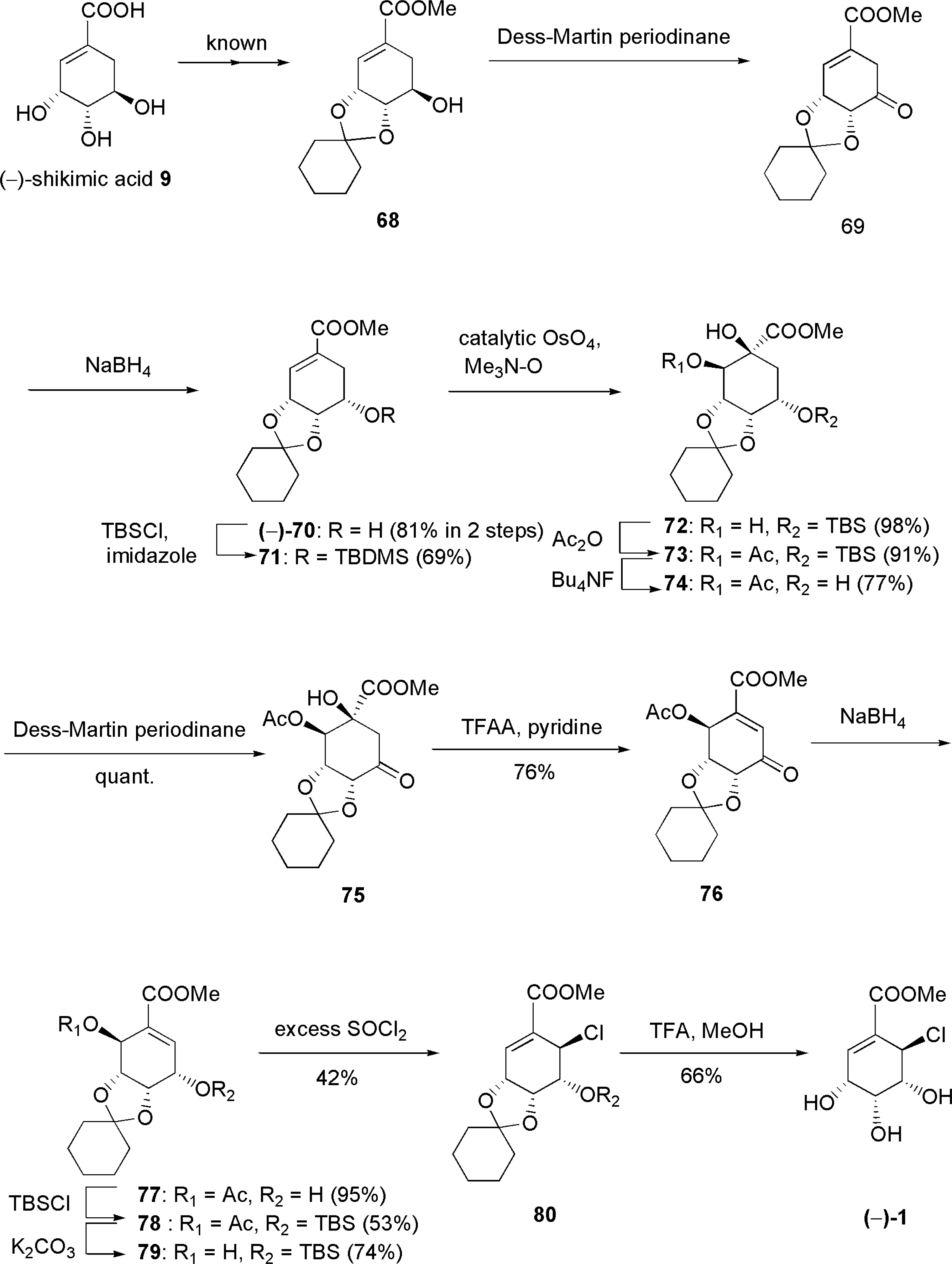
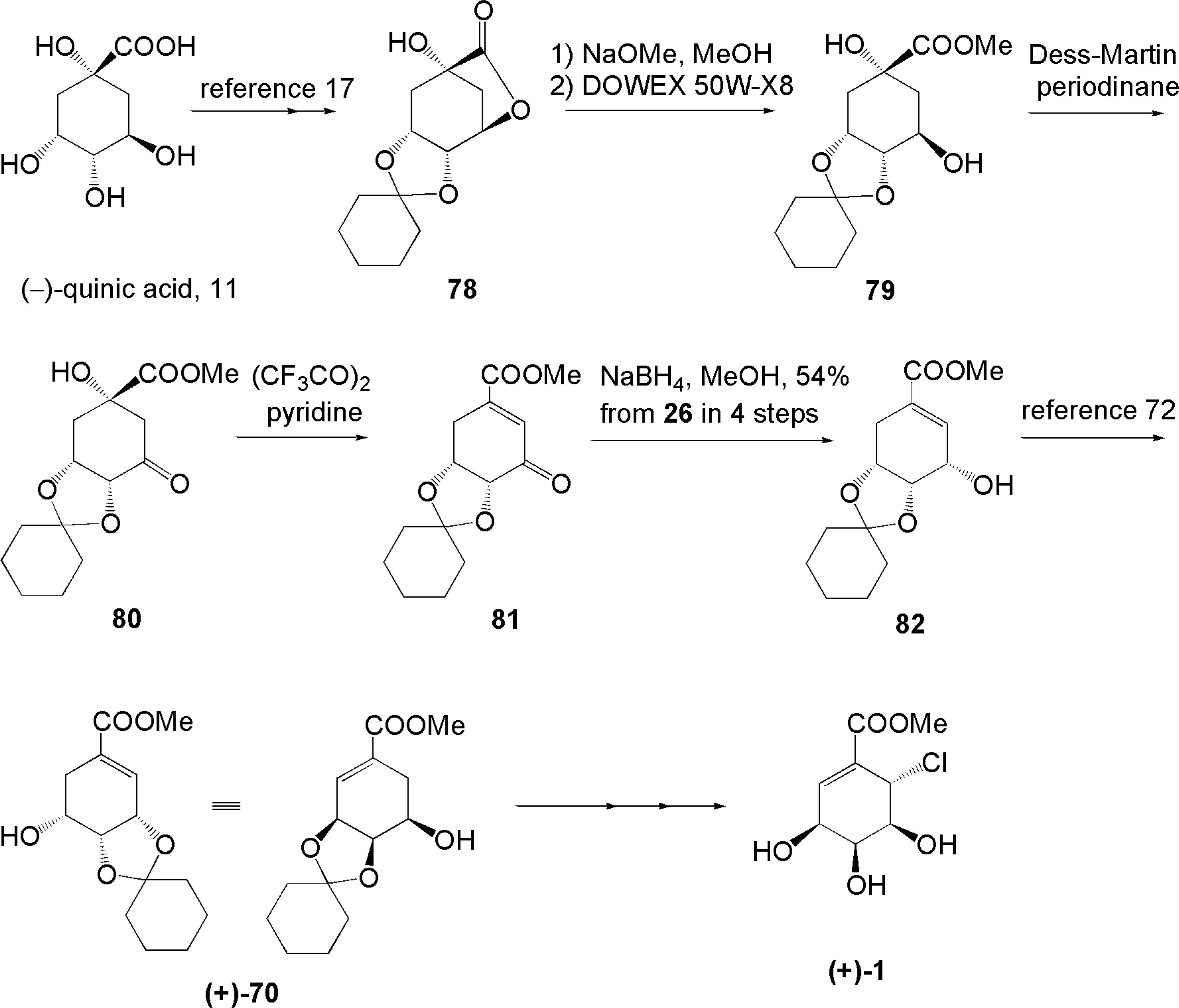
3.4. Total synthesis for structure revision and determination of absolute configuration of pericosine A [70, 71]
4. Further Discussion
Acknowledgements
References and Notes
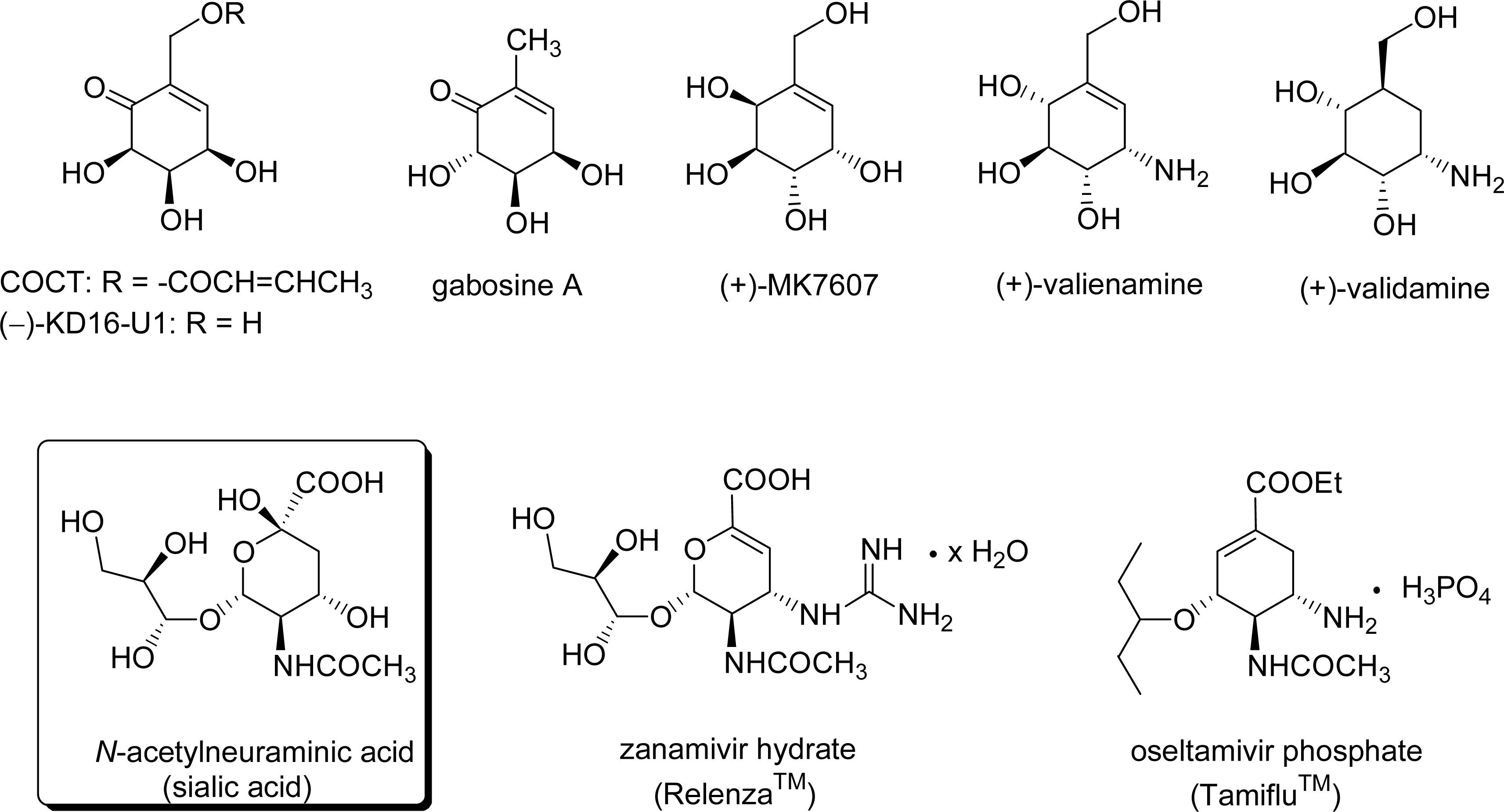
- Suami, T. Synthetic ventures in pseudo-sugar chemistry. Pure Appl Chem 1987, 59, 1509–1520, DOI 10.1351/pac198759111509. [Google Scholar]
- Berecibar, A; Grandjean, C; Sinwardena, A. Synthesis and Biological Activity of Natural Aminocyclopentitol Glycosidase Inhibitors: Mannostatins, Trehazolin, Allosamidins, and Their Analogs. Chem Rev 1999, 99, 779–844, DOI 10.1021/cr980033l; PubMed 11749432. [Google Scholar]
- Arjona, O; Gomez, AM; Lopez, JC; Plumet, J. Synthesis and conformational and biological aspects of carbasugars. Chem Rev 2007, 107, 1919–2036, DOI 10.1021/cr0203701; PubMed 17488060. [Google Scholar]
- Mirza, S; Molleyres, LP; Vasella, A. Synthesis of a glyoxalase I inhibitor from Streptomyces griseosporeus Niida et Ogasawara. Helvetica Chimica Acta 1985, 68, 988–996, DOI 10.1002/hlca.19850680425. [Google Scholar]
- Takayama, H; Hayashi, K; Koizumi, T. Enantioselective total synthesis of glyoxalase I inhibitor using asymmetric Diels-Alder reaction of a new chiral dienophile, (S)S-3-(3-trifluoromethylpyrid-2-ylsulfinyl)acrylate. Tetrahedron Lett 1986, 27, 5509–5512, DOI 10.1016/S0040-4039(00)85252-1. [Google Scholar]
- Yamakoshi, Y; Nakajima, Y; Ge, W-Y; Sugita, J; Okayama, K; Takahashi, T; Koizumi, T. High pressure mediated asymmetric Diels-Alder reaction of chiral sulfinylacrylate derivatives with furan and 2-methoxyfuran. Heterocycles 1996, 42, 129–133. [Google Scholar]
- Takahashi, T; Yamakoshi, Y; Okayama, K; Yamada, J; Ge, W-Y; Koizumi, T. High-pressure mediated asymmetric Diels-Alder reaction of chiral sulfinylacrylate derivatives and its application to chiral synthesis of (−)-COTC and (−)-gabosine C. Heterocycles 2002, 56, 209–220. [Google Scholar]
- Arthurs, CL; Wind, NS; Whitehead, RC; Stratford, IJ. Analogues of 2-crotonyloxymethyl-(4R,5R,6R)-4,5,6-trihydroxycyclohex-2-enone (COTC) with anti-tumor properties. Bioorg & Med Chem Lett 2007, 17, 553–557, DOI 10.1016/j.bmcl.2006.09.072. [Google Scholar]
- Arthurs, CL; Raftery, J; Whitby, HL; Whitehead, RC; Wind, NS; Stratford, IJ. Arene cis-dihydrodiols: Useful precursors for the preparation of analogs of the antitumor agent, 2-crotonyloxymethyl-(4R,5R,6R)-4,5,6-trihydroxycyclohex-2-enone (COTC). Bioorg & Med Chem Lett 2007, 17, 5974–5977, DOI 10.1016/j.bmcl.2007.07.070. [Google Scholar]
- Ramanaa, GV; Rao, BV. Stereoselective synthesis of (−)-gabosine C using a Nozaki-Hiyama-Kishi reaction and RCM. Tetrahedron Lett 2005, 46, 3049–3051, DOI: 10.1016/j.tetlet.2005.03.018. [Google Scholar]
- Huntley, CFM; Wood, HB; Ganem, B. A new synthesis of the glyoxalase-I inhibitor COTC. Tetrahedron Lett 2000, 41, 2031–2034, DOI 10.1016/S0040-4039(00)00103-9. [Google Scholar]
- Huntley, CF; Hamilton, DS; Creighton, DJ; Ganem, B. Reaction of COTC with glutathione: structure of the putative glyoxalase I inhibitor. Org Lett 2000, 2, 3143–3144, DOI 10.1021/ol006341z; PubMed 11009366. [Google Scholar]
- Shing, T; Tang, Y. Enantiospecific synthesis of 2-crotonyloxy-(4R,5R,6R)-4,5,6-trihydroxycyclohex-2-enone (COTC) from quinic acid. J Chem Soc, Chem Commun 1990, 312. [Google Scholar]
- Shing, T; Tang, Y. (−)-Quinic acid in organic synthesis. 1. A facile synthesis of 2-crotonyloxymethyl-(4R,5R,6R)-4,5,6-trihydroxycyclohex-2-enone. Tetrahedron 1990, 46, 6575–6584. [Google Scholar]
- Tatsuta, K; Yasuda, S; Araki, N; Takahashi, M; Kamiya, Y. Total synthesis of a glyoxalase I inhibitor and its precursor, (−)-KD16-U1. Tetrahedron Lett 1998, 39, 401–402, DOI 10.1016/S0040-4039(97)10559-7. [Google Scholar]
- Shinada, T; Fuji, T; Ohtani, Y; Yoshida, Y; Ohfune, Y. Syntheses of gabosine A, B, D, and E from allyl sulfide derived from (−)-quinic acid. Synlett 2002, 1341–1343. [Google Scholar]
- Lygo, B; Swiatyj, M; Trabsa, H; Voyle, M. Synthesis of (+)-Gabosines C and E from D-ribose Tetrahedron Lett. 1994, 35, 4197–4200, DOI (Either ISSN and Title must be supplied). [Google Scholar]
- Banwell, MG; Bray, AM; Wong, D. A concise and chemo-enzymatic synthesis of (−)-gabosine A, a carba-sugar enone from Streptomycetes. J New J Chem 2001, 25, 1351–1354. [Google Scholar]
- Lubineau, A; Billault, I. New Access to Unsaturated Keto Carba Sugars (Gabosines) Using an Intramolecular Nozaki-Kishi Reaction as the Key Step. J Org Chem 1998, 63, 5668–5671. [Google Scholar]
- Alibes, R; Bayon, P; De March, P; Figueredo, M; Font, J; Marjanet, G. Enantioselective synthesis and absolute configuration assignment of gabosine O. Synthesis of (+)- and (−)-gabosine N and (+)- and (−)-epigabosines N and O. Organic Lett 2006, 8, 1617–1620. [Google Scholar]
- Shing, TKM; Cheng, HM. Short Syntheses of Gabosine I and Gabosine G from δ-D-Gluconolactone. J Org Chem 2007, 72, 6610–6613, DOI 10.1021/jo0709697; PubMed 17637067. [Google Scholar]
- Mehta, G; Lakshminath, S. A norbornyl route to cyclohexitols: stereoselective synthesis of conduritol-E, allo-inositol, MK 7607 and gabosines. Tetrahedron Lett 2000, 41, 3509–3512, DOI 10.1016/S0040-4039(00)00409-3. [Google Scholar]
- Song, C; Jiang, S; Singh, G. Synthesis of (−)-MK7607 and Other Carbasugars from (−)-shikimic Acid. Synlett 2001, 1983–1985. [Google Scholar]
- Schmidt, RR; Koen, A. α-Glucosidase inhibitors. Part 4. Synthesis of valienamine. Angew Chem 1987, 99, 490–491. [Google Scholar]
- Park, TK; Danishefsky, SJ. A synthetic route to valienamine: an interesting observation concerning stereoelectronic preferences in the SN2’ reaction. Tetrahedron Lett 1994, 35, 2667–2670, DOI 10.1016/S0040-4039(00)77001-8. [Google Scholar]
- Fukase, H; Horii, S. Synthesis of a branched-chain inosose derivative, a versatile synthon of N-substituted valiolamine derivatives from D-glucose. J Org Chem 1992, 57, 3651–3658, DOI 10.1021/jo00039a026. [Google Scholar]
- Trost, BM; Chupak, S; Luebbers, T. Total Synthesis of (±)- and (+)-Valienamine via a Strategy Derived from New Palladium-Catalyzed Reactions. J Am Chem Soc 1998, 120, 1732–1740, DOI 10.1021/ja973081g. [Google Scholar]
- Shing, TKM; Li, TY; Kok, SH-L. Enantiospecific Syntheses of Valienamine and 2-epi-Valienamine. J Org Chem 1999, 64, 1941–1946, DOI 10.1021/jo982024i; PubMed 11674286. [Google Scholar]
- Yoshikawa, M; Cha, BC; Okaichi, Y; Takinami, Y; Yokokawa, Y; Kitagawa, I. Syntheses of validamine, epi-validamine, and valienamine, three optically active pseudo-amino-sugars, from D-glucose. Chem Pharm Bull 1988, 36, 4236–4239. [Google Scholar]
- Tatsuta, K; Mukai, H; Takahashi, M. Novel synthesis of natural pseudo-aminosugars, (+)-valienamine and (+)-validamine. J Antibiot 2000, 53, 430–435, PubMed 10866227. [Google Scholar]
- Chang, Y-K; Lee, B-Y; Lee, GS; Jeon, HB; Kim, KS. An Efficient Synthesis of Valienamine via Ring-Closing Metathesis. J Org Chem 2005, 70, 3299–3302, PubMed 15823000. [Google Scholar]
- von Itzstein, M; Wu, WY; Kok, GB; Pegg, MS; Dyason, JC; Jin, B; Van, PT; Smythe, ML; White, HF; Oliver, SW; Colman, PM; Varghese, JN; Ryan, DM; Woods, JM; Bethell, RC; Hotham, VJ; Cameron, JM; Penn, C. R Rational design of potent silalidase-based inhibitors of influenza virus replication. Nature 1993, 363, 418–423, PubMed 8502295. [Google Scholar]
- Kim, CU; Lew, W; Williams, MA; Zhang, L; Liu, H; Swaminathan, S; Bischofberger, N; Chen, MS; Tai, CY; Mendel, DB; Laver, WG; Stevens, RC. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J Am Chem Soc 1997, 119, 681–690, DOI 10.1021/ja963036t; PubMed 16526129. [Google Scholar]
- Rohloff, JC; Kent, KM; Postich, MJ; Becker, MW; Chapman, HH; Kelly, DE; Lew, W; Louie, MS; McGee, LR; Prisbe, EJ; Schultze, LM; Yu, RH; Zhang, L. Practical Total Synthesis of the Anti-Influenza Drug GS-4104. J Org Chem 1998, 63, 4545–4550, DOI 10.1021/jo980330q. [Google Scholar]
- Yeung, Y-Y; Hong, S; Corey, EJ. A Short Enantioselective Pathway for the Synthesis of the Anti-Influenza Neuramidase Inhibitor Oseltamivir from 1,3-Butadiene and Acrylic Acid. J Am Chem.Soc 2006, 128, 6310–6311, DOI 10.1021/ja0616433; PubMed 16683783. [Google Scholar]
- Fukuta, Y; Mita, T; Fukuda, N; Kanai, M; Shibasaki, M. Catalytic Asymmetric Total Synthesis of (+)-Lactacystin. J Am Chem Soc 2006, 128, 6312–6313, PubMed 16683784. [Google Scholar]
- Mita, T; Fukuda, N; Roca, FX; Kanai, M; Shibasaki, M. Second generation catalytic asymmetric synthesis of Tamiflu: allylic substitution route. Org Lett 2007, 9, 259–262, DOI 10.1021/ol062663c; PubMed 17217279. [Google Scholar]
- Satoh, N; Akiba, T; Yokoshima, S; Fukuyama, T. A practical synthesis of (−)-oseltamivir. Angewandte Chem, Int Ed 2007, 46, 5734–5736, DOI 10.1002/anie.200701754. [Google Scholar]
- Karchier, M; Michalak, K; Wicha, J. Anti-influenza drugs Synthesis of Tamiflu, a drug kept in stock to prevent a bird flu epidemic. Wiadomosci Chemiczne 2007, 61, 7–42. [Google Scholar]
- Shie, J-J; Fang, J-M; Wang, S-Y; Tsai, K-C; Cheng, Y-SE; Yang, A-S; Hsiao, S-C; Su, C-Y; Wong, C-H. Synthesis of Tamiflu and its Phosphonate Congeners Possessing Potent Anti-Influenza Activity. J Am Chem Soc 2007, 129, 11892–11893, DOI 10.1021/ja073992i; PubMed 17850083. [Google Scholar]
- Takeuchi, T; Chimura, H; Hamada, M; Umezawa, H; Yoshioka, O; Oguchi, N; Takahashi, Y; Matsuda, A. Glyoxalase I inhibitor of a new structural type produced by Streptomyces. J Antibiot 1975, 28, 737–742, PubMed 1102510. [Google Scholar]
- Tatsuta, K; Tsuchiya, N; Mikami, N; Umezawa, S; Umezawa, H; Naganawa, H. KD16-U1, a new metabolite of Streptomyces. Isolation and structural studies. J Antibiot 1974, 27, 579–586, PubMed 4436143. [Google Scholar]
- Bach, G; Breiding-Mack, S; Grabley, S; Hammann, P; Hutter, K; Thiericke, R; Uhr, H; Wink, J; Zeeck, A. Secondary metabolites by chemical screening. 22. Gabosines, new carba-sugars from Streptomyces. Liebigs Ann Chem 1993, 241–250. [Google Scholar]
- Yoshikawa, N; Chiba, N; Mikawa, T; Ueno, S; Harimaya, K; Iwata, M. Novel herbicidal MK7607 and its manufacture with Curvularia. Jpn Kokai Tokkyo Koho JP 1994. 06306000. [Google Scholar]
- Horii, S; Iwasa, T; Mizuta, E; Kameda, Y. Validamycins, new antibiotics. VI. Validamine, hydroxyvalidamine, and validatol, new cyclitols. J Antibiot 1971, 24, 59–63, PubMed 5541335. [Google Scholar]
- Kameda, Y; Horii, S. Structure of the antibiotic validamycin A. J Chem Soc, Chem Commun 1972, 746–747. [Google Scholar]
- Suami, T; Ogawa, S; Chida, N. The revised Structure of Validamycin A. J Antibiot 1980, 33, 98–99, PubMed 7372560. [Google Scholar]
- Laube, H; Fouladfar, M; Aubell, R; Schmitz, H. Effect of glucosidase inhibitor, Bay g 5421 (acarbose), on the blood glucose in obese diabetic patients type 2 (NIDDM). Arzneimittel-Forschung 1980, 30, 1154–1157, PubMed 7191299. [Google Scholar]
- Moore, RE; Corbett, TH; Patterson, GML; Valeriote, FA. The search for new antitumor drugs from blue-green algae. Current Pharmaceutical Design 1996, 2, 317–330. [Google Scholar]
- Newman, DJ; Cragg, GM. Natural Products as Sources of New Drugs over the Last 25 Years. J Nat Prod 2007, 70, 461–477, PubMed 17309302. [Google Scholar]
- Fenical, W; Jensen, PR. Developing a new resource for drug discovery: marine actinomycete bactereia. Nature Chemical Biology 2006, 2, 666–673, DOI 10.1038/nchembio841; PubMed 17108984. [Google Scholar]
- Newman, DJ; Cragg, GM. Natural products from marine invertebrates and microbes as modulators of antitumor targets. Current Drug Targets 2006, 7, 279–304, DOI 10.2174/138945006776054960; PubMed 16515528. [Google Scholar]
- Newman, DJ; Hill, RT. New drugs from marine microbes: the tide is turning. J Ind Microbiol Biotechnol 2006, 33, 539–544, DOI 10.1007/s10295-006-0115-2; PubMed 16598493. [Google Scholar]
- Numata, A; Iritani, M; Yamada, T; Minoura, K; Matsumura, E; Yamori, T; Tsuruo, T. Novel antitumor metabolites produced by a fungal strain from a sea hare. Tetrahedron Lett 1997, 38, 8215–8218, DOI 10.1016/S0040-4039(97)10198-8. [Google Scholar]
- Yamada, T; Iritani, M; Ohishi, H; Tanaka, K; Doi, M; Minoura, K; Numata, A. Pericosines, antitumor metabolites from the sea hare-derived fungus Periconia byssoides. Structures and biological activities. Org Bioorg Chem 2007, 5, 3979–3986. [Google Scholar]
- Yamada, T; Minoura, K; Numata, A. In. Proc. of the 119th Annual Meeting of the Pharmaceutical Society of Japan, Tokushima, Japan, 1999; Abstract 2. p. 159.
- Yamada, T. Ph. D. Thesis; Osaka University of Pharmaceutical Sciences: Japan, 2002.
- Donohoe, TJ; Blades, K; Helliwell, M; Waring, MJ; Newcombe, NJ. The Total Synthesis of (+)-Pericosine B. Tetrahedron Lett 1998, 39, 8755–8758, DOI 10.1016/S0040-4039(98)01989-3. [Google Scholar]
- Okamura, H; Nakamura, Y; Morishige, K; Ohura, R; Shimizu, H; Iwakawa, T; Nakatani, M. Development of Base Catalized Diels-Alder Reaction of 3-Hydroxy-2-pyrone and its Application to Synthesis of Biologically Active Compounds. 40th Tennen Yuki Kagobutsu Toronkai Koen Yoshisyu: Fukuoka, Japan, 1988; pp. 187–192. [Google Scholar]
- Usami, Y; Numata, A. Examination of the reactivities of hydroxy groups in multioxygenated cyclohexanoids: Synthetic study toward cytotoxic pericosine B. Chem Pharm Bull 2004, 52, 1125–1129, DOI 10.1248/cpb.52.1125; PubMed 15340203. [Google Scholar]
- Garcia Ruano, J; Alemparte, C; Lopez-Cantarero, J. (Z)-3-p-Tolylsulfinylacrylonitrile as a Chiral Dienophile: Diels-Alder Reactions with Furan and Acyclic Dienes. J Org Chem 2000, 65, 7938–7943, DOI 10.1021/jo000963g; PubMed 11073601. [Google Scholar]
- Garcia Ruano, J; Lopez-Cantarero, J; Martin Castro, AM; Adams, H; Rogriguez Ramos, JH. toward the Synthesis of (+)-Pericosine B. Phosphorus, Sulfur and Silicon and the Related Elements 2005, 180, 1493–1494, DOI 10.1080/10426500590913339. [Google Scholar]
- Usami, Y; Hatsuno, C; Yamamoto, H; Tanabe, M; Numata, A. Synthesis of the epimer of pericosine B from (−)-quinic acid. Chem Pharm Bull 2004, 52, 1130–1133, PubMed 15340204. [Google Scholar]
- Usami, Y; Ueda, Y. Synthetic Study toward Antitumour Natural Product Pericosine A. Chem Lett 2005, 34, 1062–1063, DOI 10.1246/cl.2005.1062. [Google Scholar]
- Usami, Y; Ueda, Y. Stereoselective Syntheses of Diastereomers of Antitumor Natural Product Pericosine A from (−)-Quinic Anid. Synthesis 2007, 3219–3225. [Google Scholar]
- Manthey, MK; Gonzalez-Bello, C; Abell, C. Synthesis of (2R)-2bromodehydroquinic acid and (2R)-2fluorodehydroquinic acid. J Chem Soc, Perkin Trans 1997, 625–628. [Google Scholar]
- Gonzalez-Bello, C; Manthey, MK; Harris, JH; Hawkins, AR; Coggins, JR; Abell, C. Synthesis of 2-Bromo- and 2-Fluoro-3-dehydroshikimic Acids and 2-Bromo- and 2-Fluoroshikimic Acids Using Synthetic and Enzymic Approaches. J Org Chem 1998, 63, 1591–1597. [Google Scholar]
- Alhalt, RJ; Martin, JC. Sulfuranes. VI. Reactions involving the alkoxy ligands of dialkoxydiarylsulfuranes. Formation of olefins and ethers. J Am Chem Soc 1972, 94, 5003–5010. [Google Scholar]
- Evans, DA; Chapman, KT. The directed reduction of β-hydroxy ketones employing Me4NHB(OAc)3. Tetrahedron Lett 1986, 27, 5939–5942, DOI 10.1016/S0040-4039(00)85367-8. [Google Scholar]
- Usami, Y; Horibe, Y; Takaoka, I; Ichikawa, H; Arimoto, M. First Total Synthesis of (−)-Pericosine A from (−)-Shikimic Acid: Structure Revision and Determination of the Absolute Configuration of Antitumor Natural Product Pericosine A. Synlett 2006, 1598–1600. [Google Scholar]
- Usami, Y; Takaoka, I; Ichikawa, H; Horibe, Y; Tomiyama, S; Ohtsuka, M; Imanishi, Y; Arimoto, M. First Total Synthesis of (+)- and (−)-Pericosine A: Determination of Absolute Stereo Structure. J Org Chem 2007, 72, 6127–6134, PubMed 17628106. [Google Scholar]
- Ulibarri, G; Nadler, W; Skrydstrup, T; Audrain, H; Chianori, A; Riche, C; Grierson, AA. Construction of the Bicyclic Core Structure of the Enediyne Antibiotic Esperamicin-A1 in Either Enantiomeric Form from (−)-Quinic Acid. J Org Chem 1995, 60, 2753–2761. [Google Scholar]
- Usami, Y; Mizuki, K; Ichikawa, H; Arimoto, M. In. Proc. of the 57th Annual Meeting of the Pharmaceutical Society of Japan Kinki-branch, Osaka, Japan, 2007; p. 36.







| Pericosine A | Pericosine B | Pericosine C | Pericosine D | Pericosine E | |
|---|---|---|---|---|---|
| ED50 (μg/mL) | 0.1 | 4.0 | 10.5 | 3.0 | 15.5 |
Share and Cite
Usami, Y.; Ichikawa, H.; Arimoto, M. Synthetic Efforts for Stereo Structure Determination of Cytotoxic Marine Natural Product Pericosines as Metabolites of Periconia sp. from Sea Hare. Int. J. Mol. Sci. 2008, 9, 401-421. https://doi.org/10.3390/ijms9030401
Usami Y, Ichikawa H, Arimoto M. Synthetic Efforts for Stereo Structure Determination of Cytotoxic Marine Natural Product Pericosines as Metabolites of Periconia sp. from Sea Hare. International Journal of Molecular Sciences. 2008; 9(3):401-421. https://doi.org/10.3390/ijms9030401
Chicago/Turabian StyleUsami, Yoshihide, Hayato Ichikawa, and Masao Arimoto. 2008. "Synthetic Efforts for Stereo Structure Determination of Cytotoxic Marine Natural Product Pericosines as Metabolites of Periconia sp. from Sea Hare" International Journal of Molecular Sciences 9, no. 3: 401-421. https://doi.org/10.3390/ijms9030401




