Voltammetric Behavior of o-Nitrophenol and Damage to DNA
Abstract
:1. Introduction
2. Results and discussion
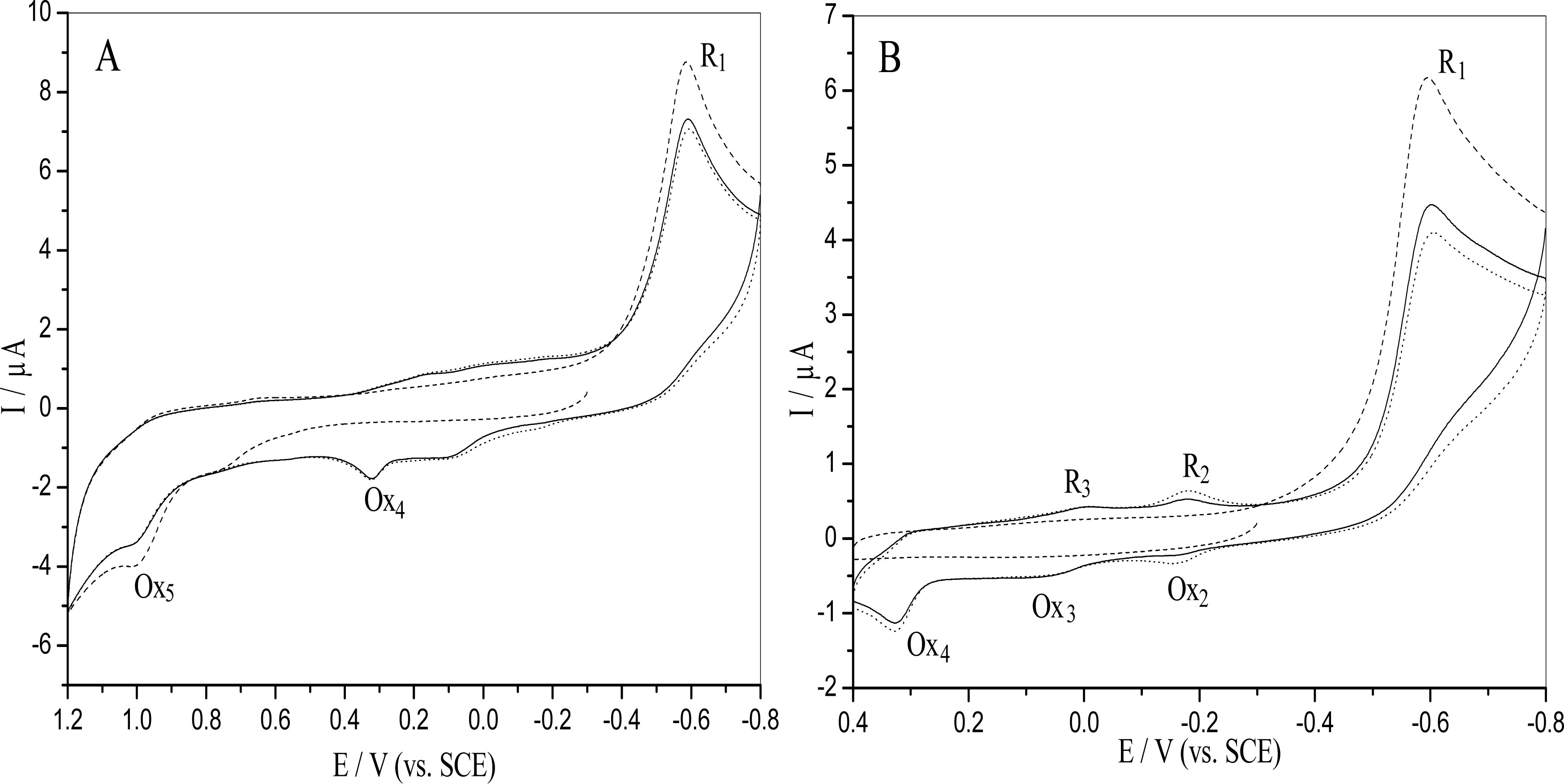
2.1. Electrochemical behavior of o-nitrophenol
2.2. o-Nitrophenol-DNA interaction
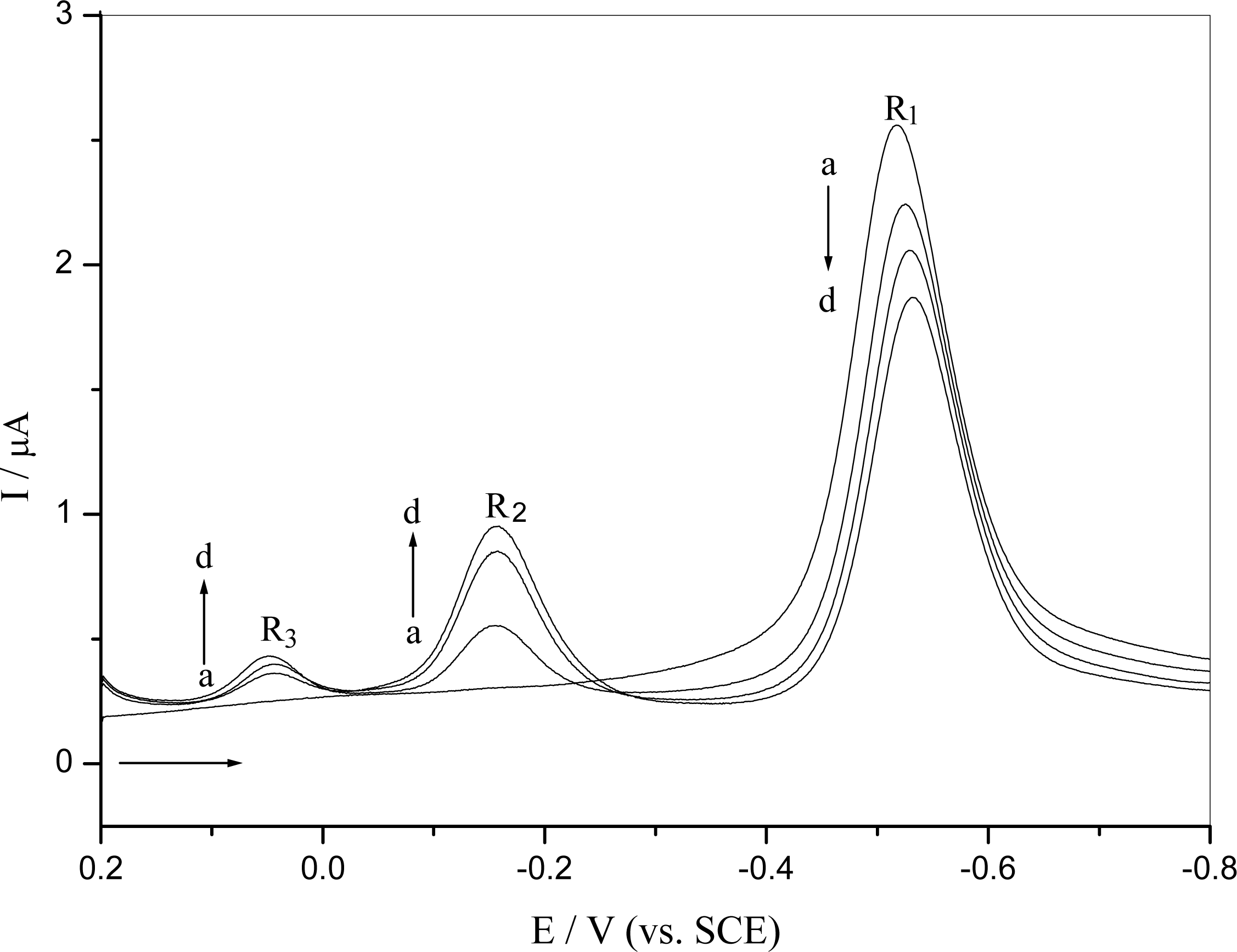
2.2.1. Interaction of o-nitrophenol with DNA in solution
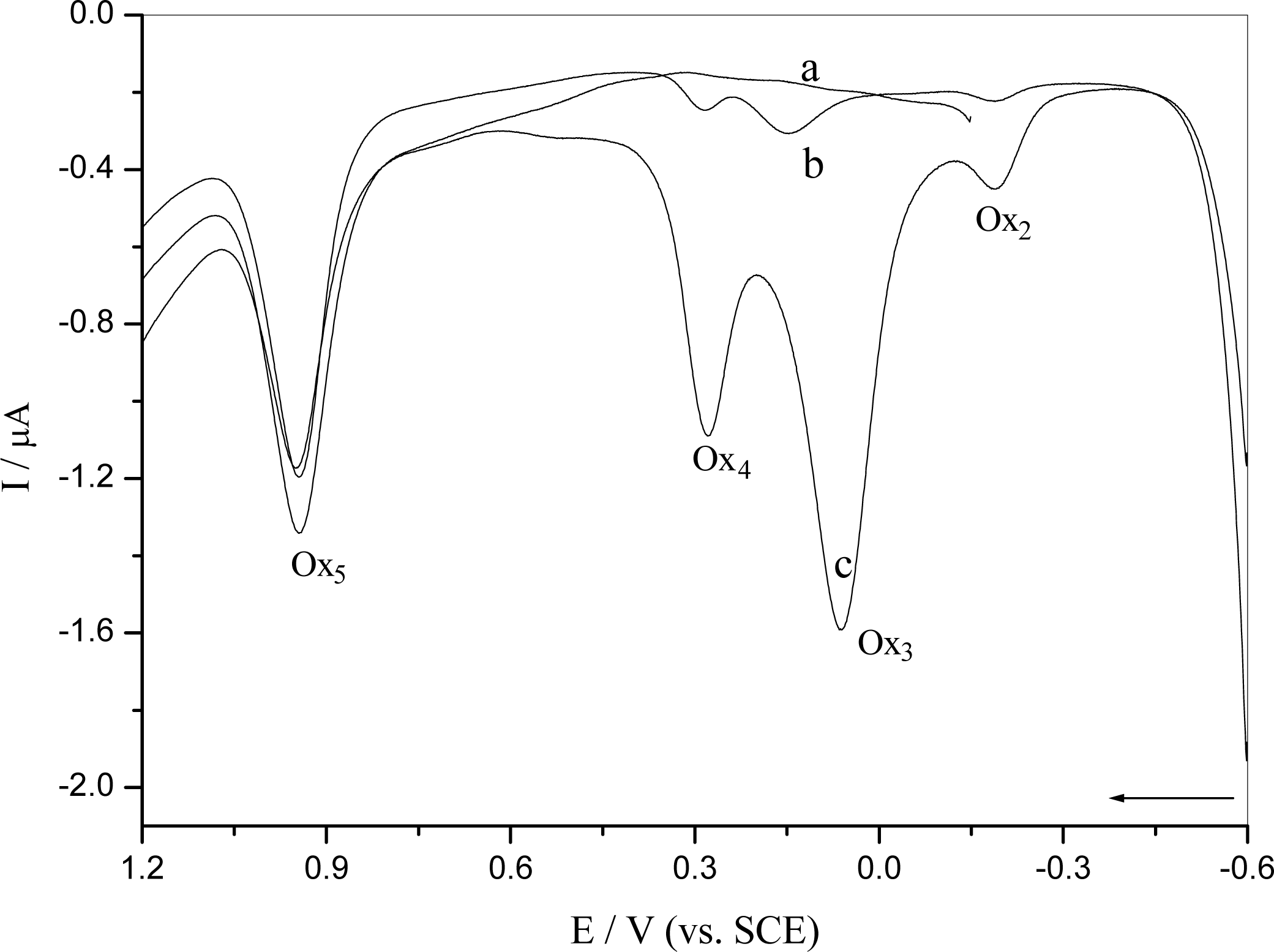
2.2.2 Interaction of o-nitrophenol with DNA immobilized at GCE surface
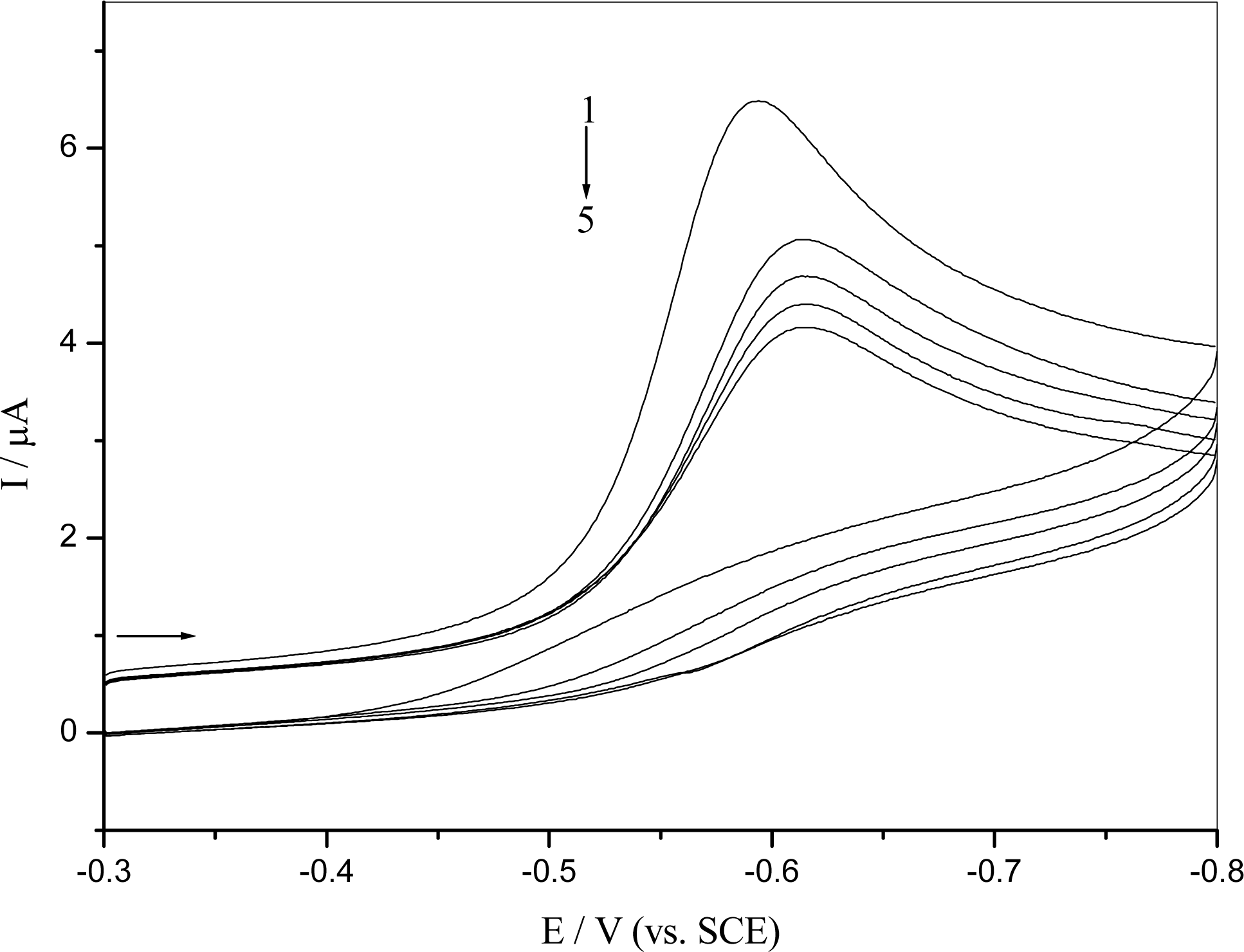
2.3 DNA damage
2.4 Mechanism of the o-nitrophenol redox and damage to dsDNA
3. Experimental
3.1 Chemicals and Apparatus
3.2 The process of preparing DNA-modified glassy carbon electrode
4. Conclusions
Acknowledgments
References
- Uberoi, V; Bhattacharya, SK. Toxicity and degradability of nitrophenols in anaerobic systems. Water. Environ. Res. 1997, 69, 146–156. [Google Scholar]
- Sethunathan, N. Degradation of parathion in flooded acid soil. J. Agr. Food. Chem. 1973, 21, 602–604. [Google Scholar]
- Nelson, LM. Biologically induced hydrolysis of parathion in soil: isolation of hydrolyzing bacteria. Soil. Biol. Biochem. 1982, 14, 219–222. [Google Scholar]
- Haghighi-Podeh, MR; Bhattacharya, SK; Qu, M. Effects of nitrophenols on acetate utilizing methanorgenic system. Water. Res. 1995, 29, 391–399. [Google Scholar]
- Environmental Protection Agency, Ambient water quality criteria for nitrophenols. 1980; EPA-440/s-80-063.
- Blasco, R; Castillo, F. Light-dependent degradation of nitrophenols by the phototrophic bacterium Rhodabacter capsulatus EIFI. Appl. Environ. Microb. 1992, 58, 690–695. [Google Scholar]
- Eckenfelder, WWJ. Industrial water pollution control, 2nd Ed. ed; McGraw-Hill: New York, NY, 1989; pp. 173–284. [Google Scholar]
- Karim, K; Gupta, SK. Effects of alteration carbon sources on biological transformation of nitrophenols. Biodegradation 2002, 13, 353–360. [Google Scholar]
- Karim, K; Gupta, SK. Biotransformation of nitrophenols in up flow anaerobic sludge blanket reactors. Bioresource Technol. 2001, 80, 179–186. [Google Scholar]
- Workman, P; Walton, ML; Lee, FY. Benznidazole: nitroreduction and inhibition of cytochrome P-450 in chemosensitization of tumor response to cytoxic drugs. Biochem. Pharmacol. 1986, 35, 117–119. [Google Scholar]
- Guissani, A; Henry, Y; Lougmani, N; Hickel, B. Kinetic studies of four types of nitroheterocyclic radicals by pulse radiolysis: Correlation of pharmacological properties to decay rats. Free. Radical. Bio. Med. 1990, 8, 173–189. [Google Scholar]
- Declerck, PJ; Ranter, CJD. Polarographic evidence for the interaction of reduced nitroimidazole derivatives with DNA bases. J. Chem. Soc. Faraday Trans l. 1987, 83, 257–265. [Google Scholar]
- Edwards, DI. Nitroimidazole drugs action and resistance mechanism I: Mechanism of action. J. Antimicrob. Chemother. 1993, 31, 9–20. [Google Scholar]
- Edwards, DI. Nitroimidazole drugs action and resistance mechanism II: Mechanism of resistance. J. Antimicrob. Chemother. 1993, 31, 201–210. [Google Scholar]
- Chien, YW; Mizuba, SS. Activity electroreduction relationship of antimicrobial metronidazole. J. Med. Chem. 1978, 21, 374–380. [Google Scholar]
- Oliveira-Brett, AM; Serrano, SHP; Guta, I; La-scalea, MA. Electrochemical reduction of metronidazole at a DNA-modified glassy carbon electrode. Bioelectrochem. Bioenerg. 1997, 42, 175–178. [Google Scholar]
- La-Scale, MA; Serrano, SHP; Ferreira, EI; Olveira-Brett, AM. Voltammetric behavior of benznidazole at a DNA-electrochemical biosensor. J. Pharmaceut. Biomed. 2002, 29, 561–568. [Google Scholar]
- Abreu, FC; Goulart, MOF; Oliveira-Brett, AM. Detection of the damage caused to DNA by niclosamide using an electrochemical DNA-biosensor. Biosens. Bioelectron. 2002, 17, 913–919. [Google Scholar]
- Chen, G; Zhao, J; Lin, X; Gao, G; Huang, J; Li, G. Electrochemical sensing DNA-damage with nano-titanium dioxide and repair with a medicinal herb species resveratrol. J. Biotechnol. 2007, 127, 653–656. [Google Scholar]
- Oliveira-Brett, AM; Diculescu, VC. Electrochemical study of quercetin-DNA interactions Part II. In situ sensing with DNA biosensors. Bioelectrochemistry 2004, 64, 143–150. [Google Scholar]
- Karakus, C; Zuman, P. Polarographic and electrochemical studies of some aromatic and heterocyclic nitro compounds part 9. Substituent effects on protonation of the radical ArNO2H· and its reaction with hydroxylamino and nitrosocompounds in buffered mixtures of water and DMF1–4. J. Electroanal. Chem. 1995, 396, 499–505. [Google Scholar]
- Nunez-Vergara, LJ; Aldunate, J; Letelier, ME; Bollo, S; Repetto, Y; Morello, A; Spencer, PL; Squella, JA. Nitro radical anion formation from nifurtimox II: electrochemical evidence. Bioelectrochem. Bioenerg 1995, 38, 355–358. [Google Scholar]
- Fijalek, Z; Pugia, M; Zuman, P. Polarographic and electrochemical studies of some aromatic nitro group compound: part VII. Polarographic, voltammetic and controlled potential electrolytic reduction of meta- and para-nitrobenzaldehyde. Electroanal. 1993, 5, 65–78. [Google Scholar]
- Lund, H. Cathodic reduction of nitro and related compound. In Organic Electrochemistry, 4th Ed.; Lund, H, Hammerich, O, Eds.; Marcel Dekker: New York, NY, 2001; pp. 389–390. [Google Scholar]
- Sun, Z; Ma, Z; Zhang, W; Wang, X; Fan, C; Li, G. Electrochemical investigations of baicalin and DNA-baicalin interactions. Anal. Bioanal. Chem. 2004, 379, 283–286. [Google Scholar]
- Zhou, H; Sun, Z; Hoshi, T; Kashiwagi, Y; Anzai, J; Li, G. Electrochemical studies of danthron and the DNA-danthron interaction. Biophys. Chem. 2005, 114, 21–26. [Google Scholar]
- Zheng, X; Chen, A; Hoshi, T; Anzai, J; Li, G. Electrochemical studies of (-)-epigallocatechin gallate and its interaction with DNA. Anal. Bioanal. Chem. 2006, 386, 1913–1919. [Google Scholar]
- De-los-Santos-Alvarez, P; Lobo-Castanon, MJ; Miranda-Orderes, AJ; Tunon-Blanco, P. Electrochemistry of Nucleic Acid at Solid Electrodes and Its Applications. Electroanal. 2004, 16, 1193–1204. [Google Scholar]
- Oliveira-Brett, AM; Serrano, SHP. The electrochemical oxidation of DNA. J. Brazil. Chem. Soc. 1995, 6, 97–100. [Google Scholar]
- Nunez-Vergara, LJ; Bonta, M; Nauarrete-Encina, PA; Squella, JA. Electrochemical characterization of ortho- and meta-nitrotoluene derivatives in different electrolytic media. Free radical formation. Electrochim. Acta. 2001, 46, 4289–4300. [Google Scholar]
- Tocher, JH. Reductive Activation of Nitroheterocyclic Compounds. Gen. Pharmac. 1997, 28, 485–487. [Google Scholar]



Share and Cite
Zhang, D.-P.; Wu, W.-L.; Long, H.-Y.; Liu, Y.-C.; Yang, Z.-S. Voltammetric Behavior of o-Nitrophenol and Damage to DNA. Int. J. Mol. Sci. 2008, 9, 316-326. https://doi.org/10.3390/ijms9030316
Zhang D-P, Wu W-L, Long H-Y, Liu Y-C, Yang Z-S. Voltammetric Behavior of o-Nitrophenol and Damage to DNA. International Journal of Molecular Sciences. 2008; 9(3):316-326. https://doi.org/10.3390/ijms9030316
Chicago/Turabian StyleZhang, Da-Peng, Wei-Li Wu, Hai-Yan Long, Yun-Chun Liu, and Zhou-Sheng Yang. 2008. "Voltammetric Behavior of o-Nitrophenol and Damage to DNA" International Journal of Molecular Sciences 9, no. 3: 316-326. https://doi.org/10.3390/ijms9030316



