Hsp90 Maintains the Stability and Function of the Tau Phosphorylating Kinase GSK3β
Abstract
:1. Introduction
2. Materials and Methods
2.1 COS-7 Cell Cultures and Incubation with the Hsp90 Inhibitor
2.2 Primary Neuronal Cultures and Incubation with the Hsp90 Inhibitor
2.3 Cycloheximide Treatments
2.4 Immunoprecipitation
3. Results
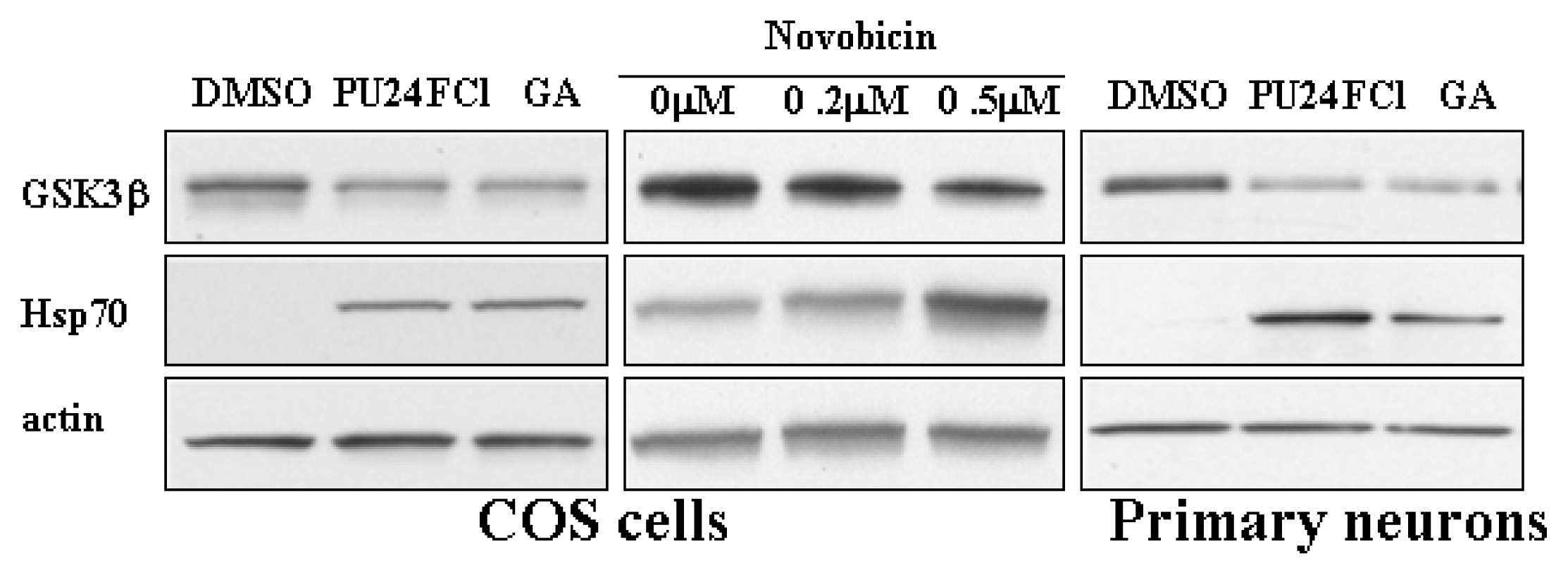
3.1 Inhibition of Hsp90 reduces tau phosphorylation level and protein level of GSK3β
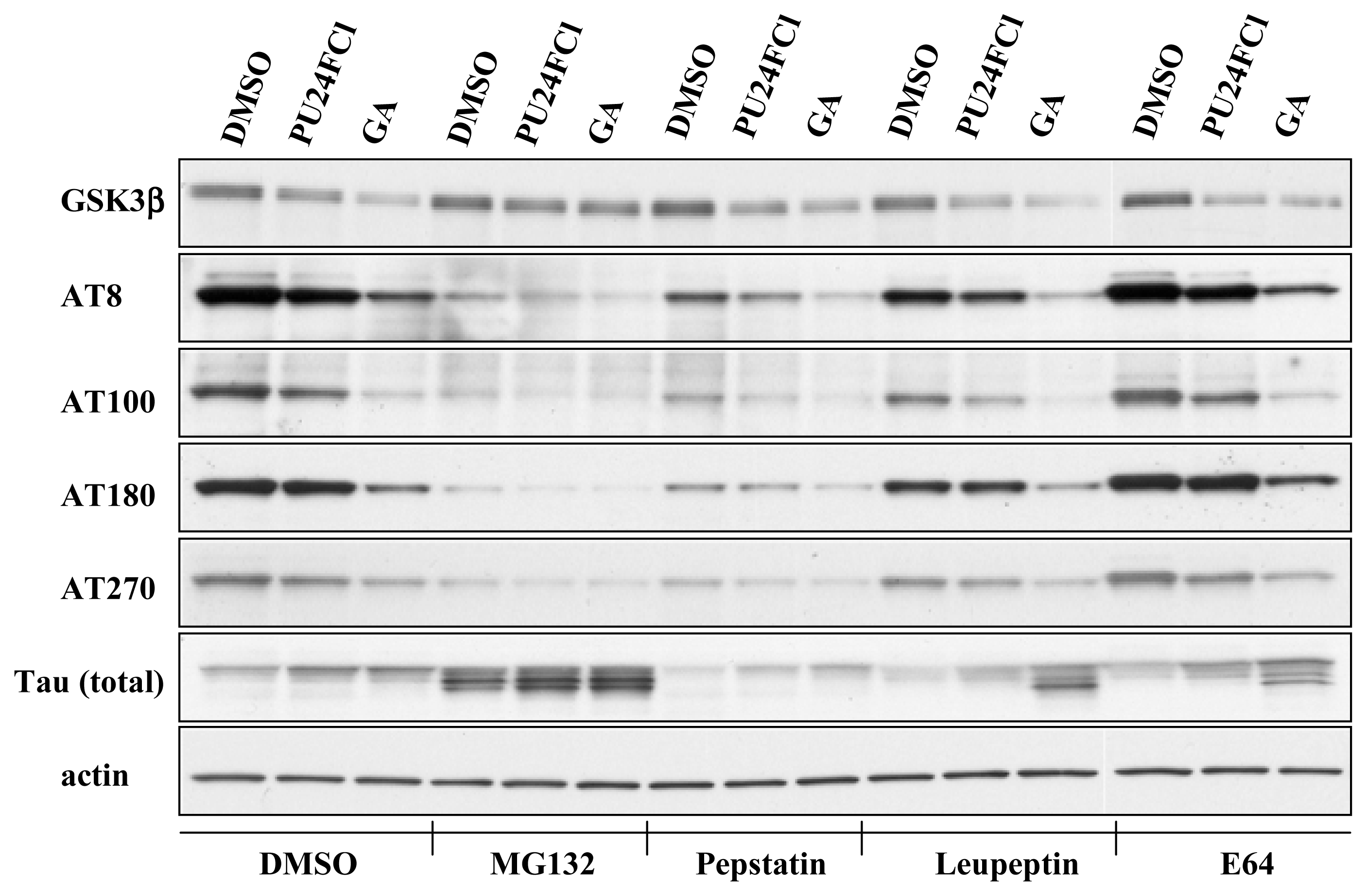
3.2 Degradation of GSK3β by Hsp90 inhibition is mediated by the proteasome
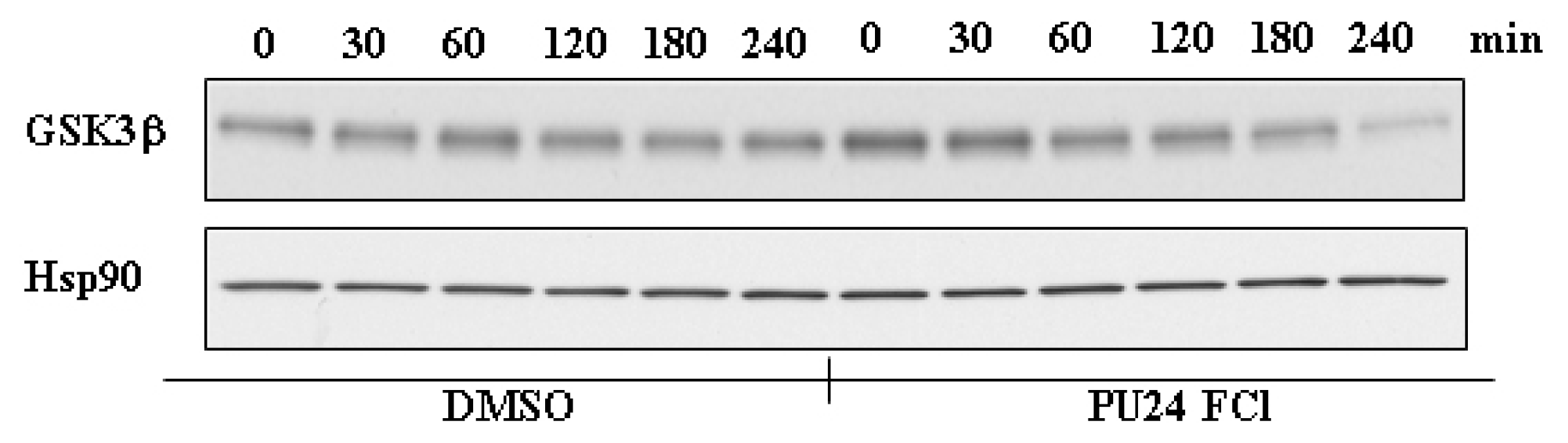
3.3 Hsp90 regulates the stability of GSK3β
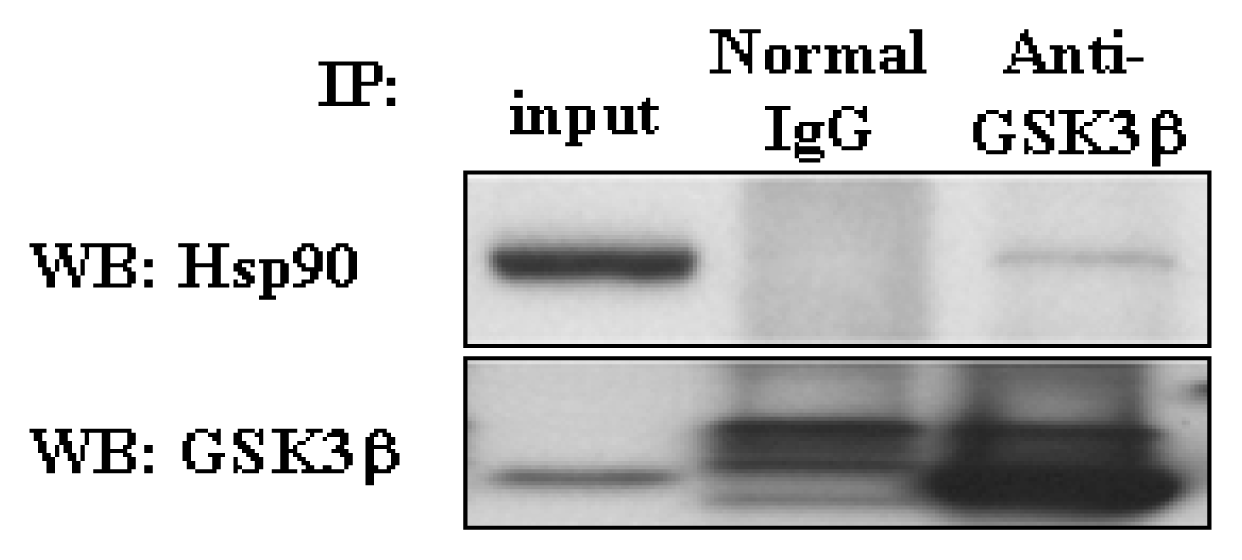
3. 4 GSK3β exist in a complex with Hsp90
Discussion
Acknowledgements
References and Notes
- Hartl, F.U.; Hayer-Hartl, M. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 2002, 295(5561), 1852–1858. [Google Scholar]
- Young, J.C.; et al. Pathways of chaperone-mediated protein folding in the cytosol. Nat Rev Mol Cell Biol 2004, 5(10), 781–791. [Google Scholar]
- Goldberg, A.L. Protein degradation and protection against misfolded or damaged proteins. Nature 2003, 426(6968), 895–899. [Google Scholar]
- Muchowski, P.J. Protein misfolding, amyloid formation, and neurodegeneration: a critical role for molecular chaperones? Neuron 2002, 35(1), 9–12. [Google Scholar]
- Sherman, M.Y.; Goldberg, A.L. Cellular defenses against unfolded proteins: a cell biologist thinks about neurodegenerative diseases. Neuron 2001, 29(1), 15–32. [Google Scholar]
- Muchowski, P.J.; Wacker, J.L. Modulation of neurodegeneration by molecular chaperones. Nat Rev Neurosci 2005, 6(1), 11–22. [Google Scholar]
- Slavotinek, AM; Biesecker, LG. Unfolding the role of chaperones and chaperonins in human disease. Trends Genet 2001, 17(9), 528–535. [Google Scholar]
- Carmichael, J; Chatellier, J; Woolfson, A; Milstein, C; Fersht, AR; Rubinsztein, DC. Bacterial and yeast chaperones reduce both aggregate formation and cell death in mammalian cell models of Huntington’s disease. Proc. Natl. Acad. Sci. USA 2000, 97(17), 9701–9705. [Google Scholar]
- Warrick, JM; Chan, HY; Gray-Board, GL; Chai, Y; Paulson, HL; Bonini, NM. Suppression of polyglutamine-mediated neurodegeneration in Drosophila by the molecular chaperone HSP70. Nat. Genet 1999, 23(4), 425–428. [Google Scholar]
- Sittler, A; Lurz, R; Lueder, G; Priller, J; Lehrach, H; Hayer-Hartl, MK; Hartl, FU; Wanker, EE. Geldanamycin activates a heat shock response and inhibits huntingtin aggregation in a cell culture model of Huntington’s disease. Hum. Mol. Genet 2001, 10(16), 1307–1315. [Google Scholar]
- Auluck, PK; Chan, HY; Trojanowski, JQ; Lee, VM; Bonini, NM. Chaperone suppression of alpha-synuclein toxicity in a Drosophila model for Parkinson’s disease. Science 2002, 295(5556), 865–868. [Google Scholar]
- Muchowski, PJ; Schaffar, G; Sittler, A; Wanker, EE; Hayer-Hartl, MK; Hartl, FU. Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc. Natl. Acad. Sci. USA 2000, 97(14), 7841–7846. [Google Scholar]
- Dou, F; Netzer, WJ; Tanemura, K; Li, F; Hartl, FU; Takashima, A; Gouras, GK; et al. Chaperones increase association of tau protein with microtubules. Proc. Natl. Acad. Sci. USA 2003, 100(2), 721–726. [Google Scholar]
- Xu, W; Mimnaugh, E; Rosser, MF; Nicchitta, C; Marcu, M; Yarden, Y; Neckers, L. Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J. Biol. Chem 2001, 276(5), 3702–3708. [Google Scholar]
- Sato, S; Fujita, N; Tsuruo, T. Modulation of Akt kinase activity by binding to Hsp90. Proc. Natl Acad. Sci. USA 2000, 97(20), 10832–10837. [Google Scholar]
- Schulte, TW; Blagosklonny, MV; Ingui, C; Neckers, L. Disruption of the Raf-1-Hsp90 molecular complex results in destabilization of Raf-1 and loss of Raf-1-Ras association. J. Biol. Chem 1995, 270(41), 24585–24588. [Google Scholar]
- de Carcer, G; do Carmo Avides, M; Lallena, MJ; Glover, DM; Gonzalez, C. Requirement of Hsp90 for centrosomal function reflects its regulation of Polo kinase stability. Eur. Mol. Biol. Org. J 2001, 20(11), 2878–2884. [Google Scholar]
- Dou, F.; Yuan, L.D.; Zhu, J.J. Heat shock protein 90 indirectly regulates ERK activity by affecting Raf protein metabolism. Acta Biochim Biophys Sin (Shanghai) 2005, 37(7), 501–505. [Google Scholar]
- Vilenchik, M.; et al. Targeting wide-range oncogenic transformation via PU24FCl, a specific inhibitor of tumor Hsp90. Chem. Biol 2004, 11, 787–797. [Google Scholar]
- Xu, H.; et al. Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nat Med 1998, 4(4), 447–51. [Google Scholar]
- Lee, V.M.; Goedert, M.; Trojanowski, J.Q. Neurodegenerative tauopathies, in. Annu Rev Neurosci 2001, 1121–1159. [Google Scholar]
- Billingsley, M.L.; Kincaid, R.L. Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem J 1997, 323 Pt 3, 577–91. [Google Scholar]
- Zou, J; Guo, Y; Guettouche, T; Smith, DF; Voellmy, R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell 1998, 94(4), 471–480. [Google Scholar]
- Singh, T. J.; Zaidi, T.; Grundke-Iqbal, I.; Iqbal, K. Modulation of GSK-3-catalyzed phosphorylation of microtubule-associated protein tau by non-prolinedependent protein kinases. FEBS Lett 1995, 358, 4–8. [Google Scholar]
- Mimnaugh, E.; Chavany, C.; Neckers, L. M. Polyubiquitination and proteasomal degradation of the p185c-erbB-2 receptor protein-tyrosine kinase induced by geldanamycin. J. Biol. Chem 1996, 271, 22796–22801. [Google Scholar]
- Goldbaum, O; Richter-Landsberg, C. Proteolytic stress causes heat shock protein induction, tau ubiquitination, and the recruitment of ubiquitin to tau-positive aggregates in oligodendrocytes in culture. J Neurosci 2004, 24(25), 5748–5757. [Google Scholar]
- Iqbal, K; Grundke-Iqbal, I. Inhibition of neurofibrillary degeneration: a promising approach to Alzheimer’s disease and other tauopathies. Curr Drug Targets 2004, 5(6), 495–502. [Google Scholar]
- Stoothoff, WH; Johnson, GV. Tau phosphorylation: physiological and pathological consequences. Biochim Biophys Acta 2005, 1739(2–3), 280–297. [Google Scholar]
- Monaco, EA, 3rd; Vallano, ML. Role of protein kinases in neurodegenerative disease: cyclin-dependent kinases in Alzheimer’s disease. Front Biosci 2005, 10, 143–159. [Google Scholar]
- Kosik, K. S.; Shimura, H. Phosphorylated tau and the neurodegenerative foldopathies. Biochim. Biophys. Acta 2005, 1739, 298–310. [Google Scholar]
- Sang, H.; et al. Phosphorylation of tau by glycogen synthase kinase 3β in intact mammalian cells influences the stability of microtubules. Neurosci. Lett 2001, 312, 141–144. [Google Scholar]
- Pei, J.J.; et al. Distribution of active glycogen synthase kinase 3β (GSK-3β) in brains staged for Alzheimer disease neurofibrillary changes. J. Neuropathol. Exp. Neurol 1999, 58, 1010–1019. [Google Scholar]
- Yamaguchi, H.; et al. Preferential labeling of Alzheimer neurofibrillary tangles with antisera for tau protein kinase (TPK) I/glycogen synthase kinase-3 β and cyclin-dependent kinase 5, a component of TPK II. Acta Neuropathol 1996, 92, 232–241. [Google Scholar]
- Neckers, L. Chaperoning oncogenes: Hsp90 as a target of geldanamycin. Handb. Exp. Pharmacol 2006, 172, 259–77. [Google Scholar]
- McDonald, E.; Workman, P.; Jones, K. Inhibitors of the HSP90 molecular chaperone: attacking the master regulator in cancer. Curr. Top. Med. Chem 2006, 6, 1091–1107. [Google Scholar]
- Chiosis, G. Targeting chaperones in transformed systems--a focus on Hsp90 and cancer. Expert Opin. Ther. Targets 2006, 10, 37–50. [Google Scholar]
- Noble, W; Planel, E; Zehr, C; Olm, V; Meyerson, J; Suleman, F; Gaynor, K; Wang, L; LaFrancois, J; Feinstein, B; Burns, M; Krishnamurthy, P; Wen, Y; Bhat, R; Lewis, J; Dickson, D; Duff, K. Inhibition of glycogen synthase kinase-3 by lithium correlates with reduced tauopathy and degeneration in vivo. Proc Natl Acad Sci U S A 2005, 102(19), 6990–6995. [Google Scholar]

© 2007 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Dou, F.; Chang, X.; Ma, D. Hsp90 Maintains the Stability and Function of the Tau Phosphorylating Kinase GSK3β. Int. J. Mol. Sci. 2007, 8, 51-60. https://doi.org/10.3390/i8010060
Dou F, Chang X, Ma D. Hsp90 Maintains the Stability and Function of the Tau Phosphorylating Kinase GSK3β. International Journal of Molecular Sciences. 2007; 8(1):51-60. https://doi.org/10.3390/i8010060
Chicago/Turabian StyleDou, Fei, Xingya Chang, and Da Ma. 2007. "Hsp90 Maintains the Stability and Function of the Tau Phosphorylating Kinase GSK3β" International Journal of Molecular Sciences 8, no. 1: 51-60. https://doi.org/10.3390/i8010060



