Zearalenone Induces Blood-Testis Barrier Damage through Endoplasmic Reticulum Stress-Mediated Paraptosis of Sertoli Cells in Goats
Abstract
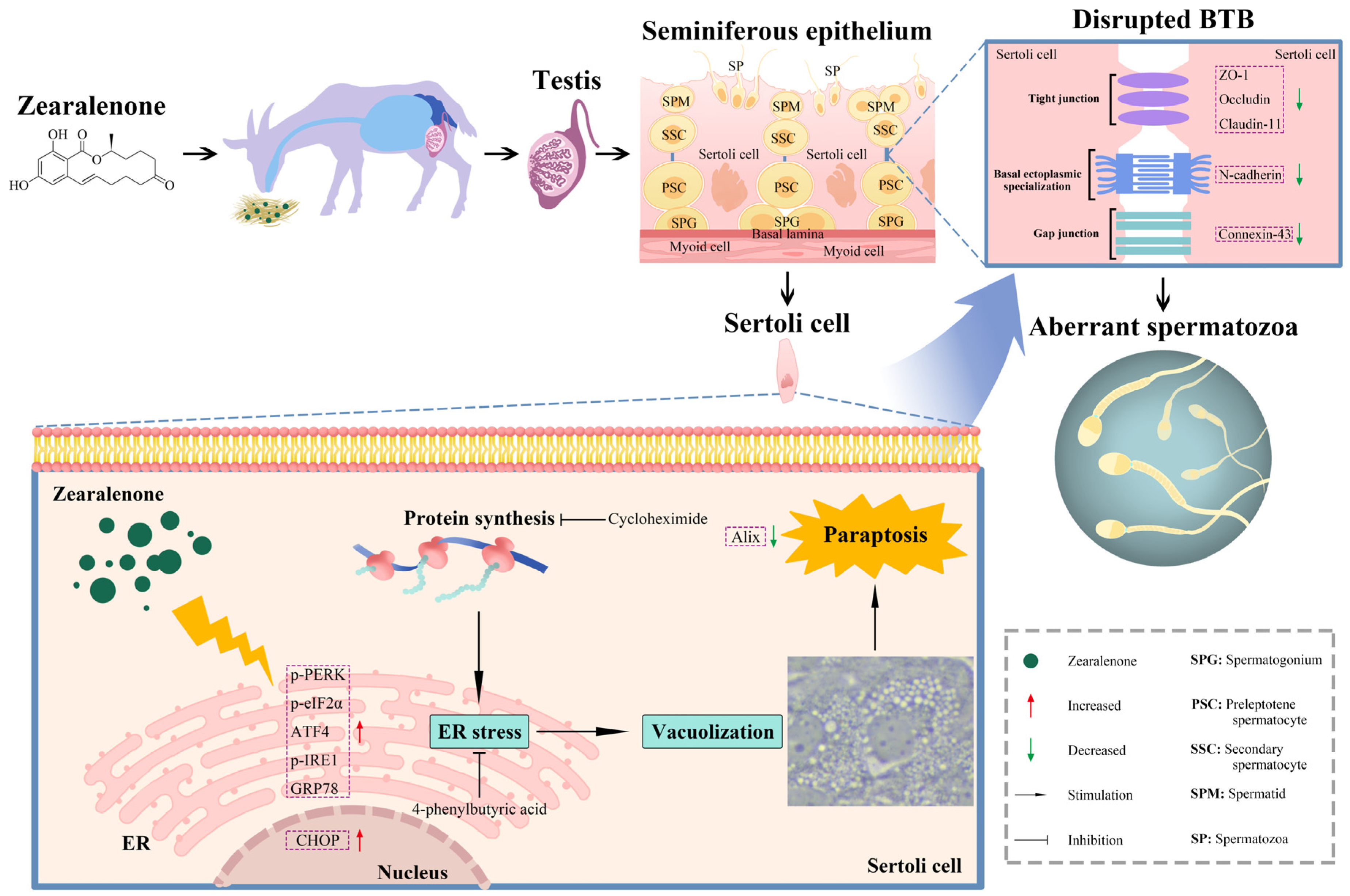
:1. Introduction
2. Results
2.1. ZEA Exposure Induced Testicular Dysfunction in Goats
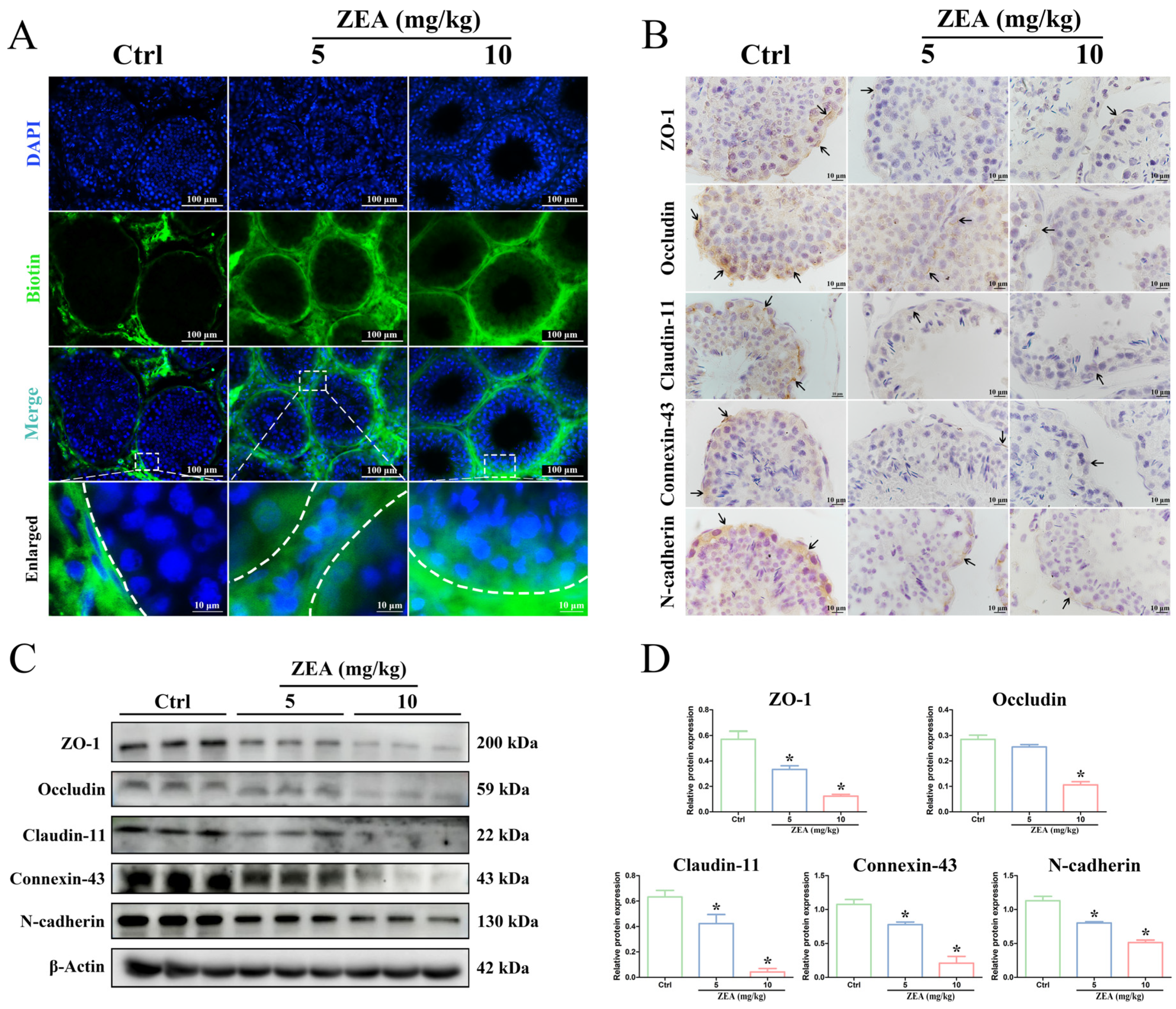
2.2. ZEA Exposure Disrupted BTB Integrity in Goat Testes
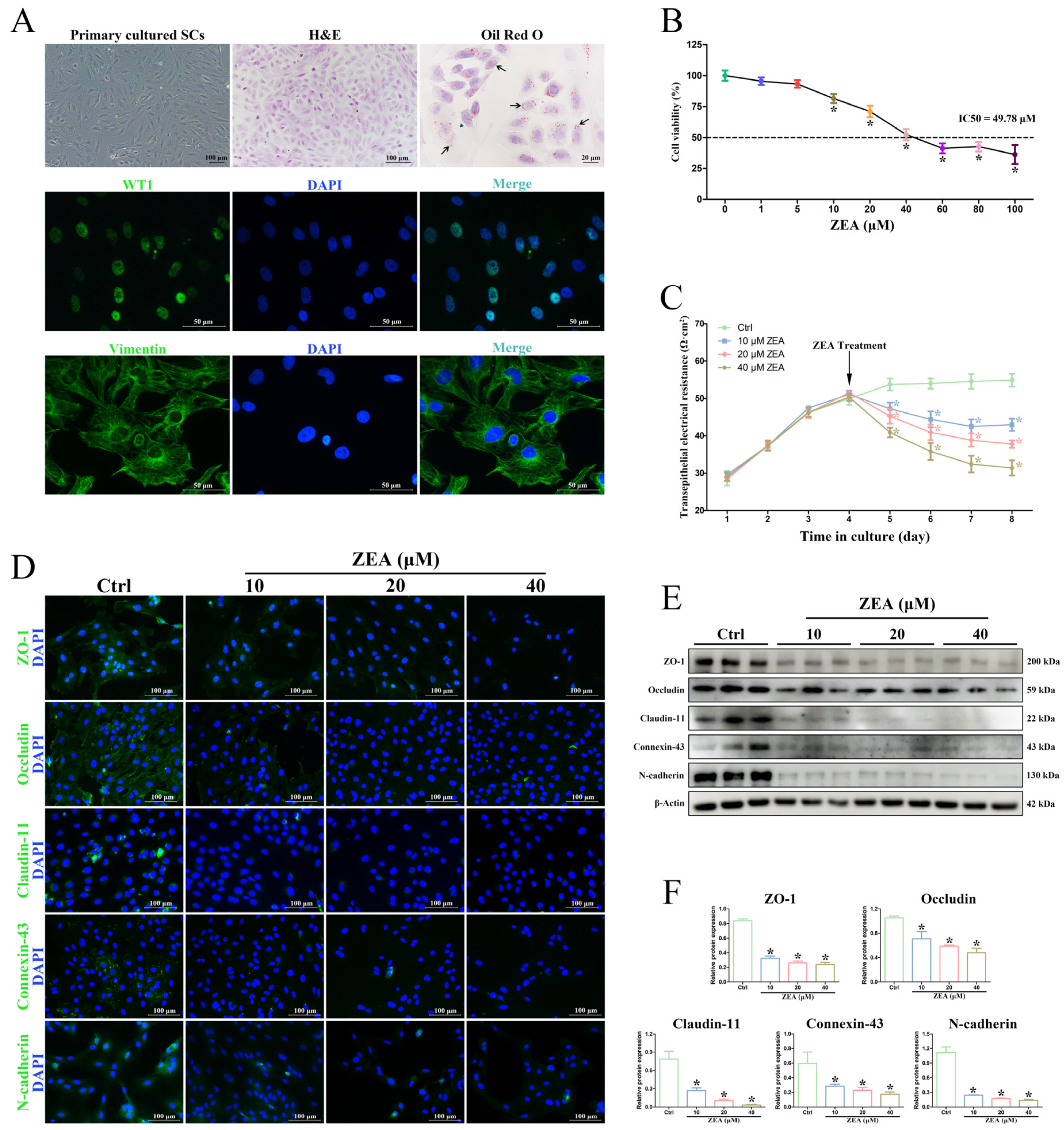
2.3. ZEA Decreased SC Viability and Induced BTB Damage In Vitro
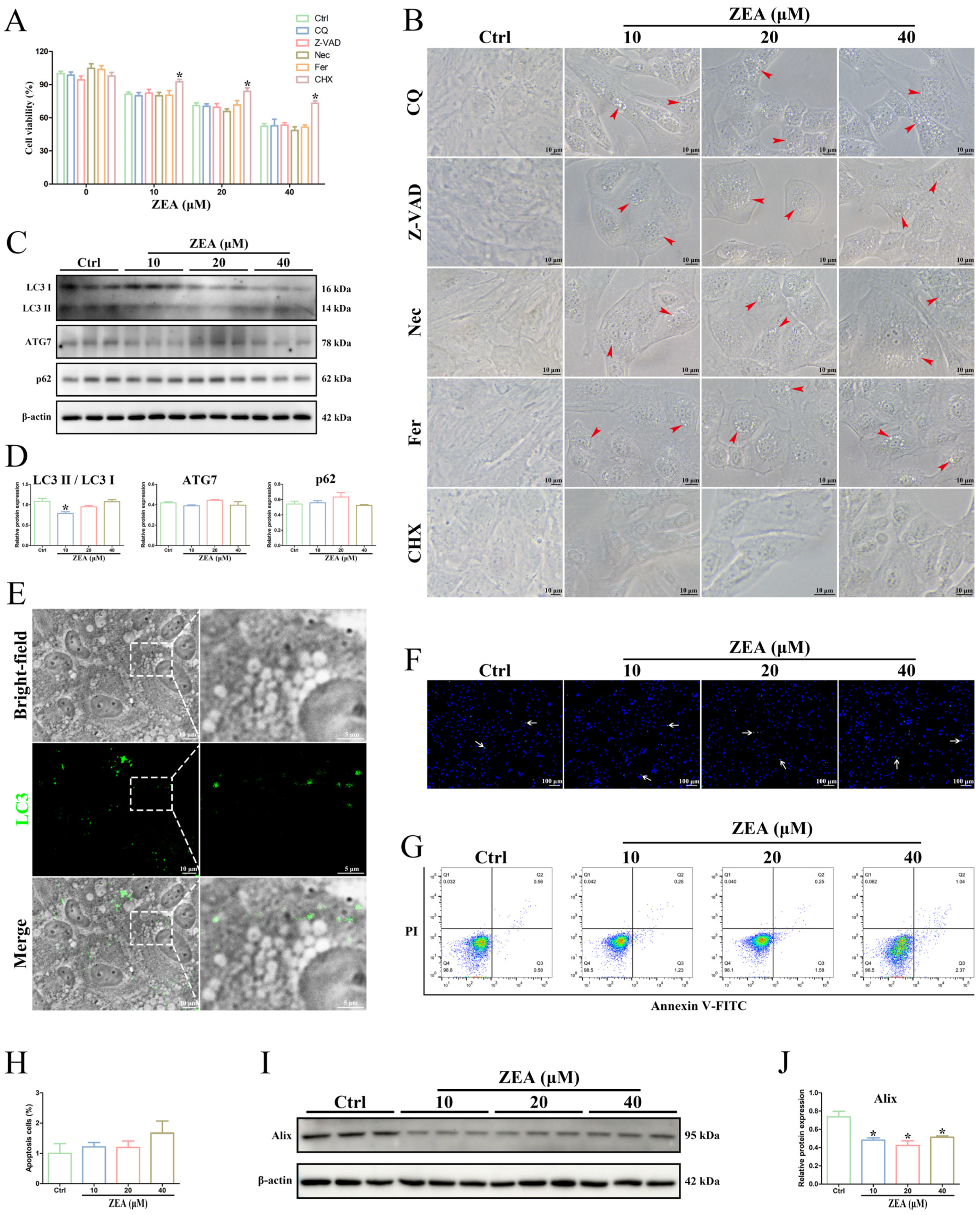
2.4. ZEA Treatment Induced Cytoplasmic Vacuolation in SCs
2.5. ZEA Exposure Triggered SC Paraptosis
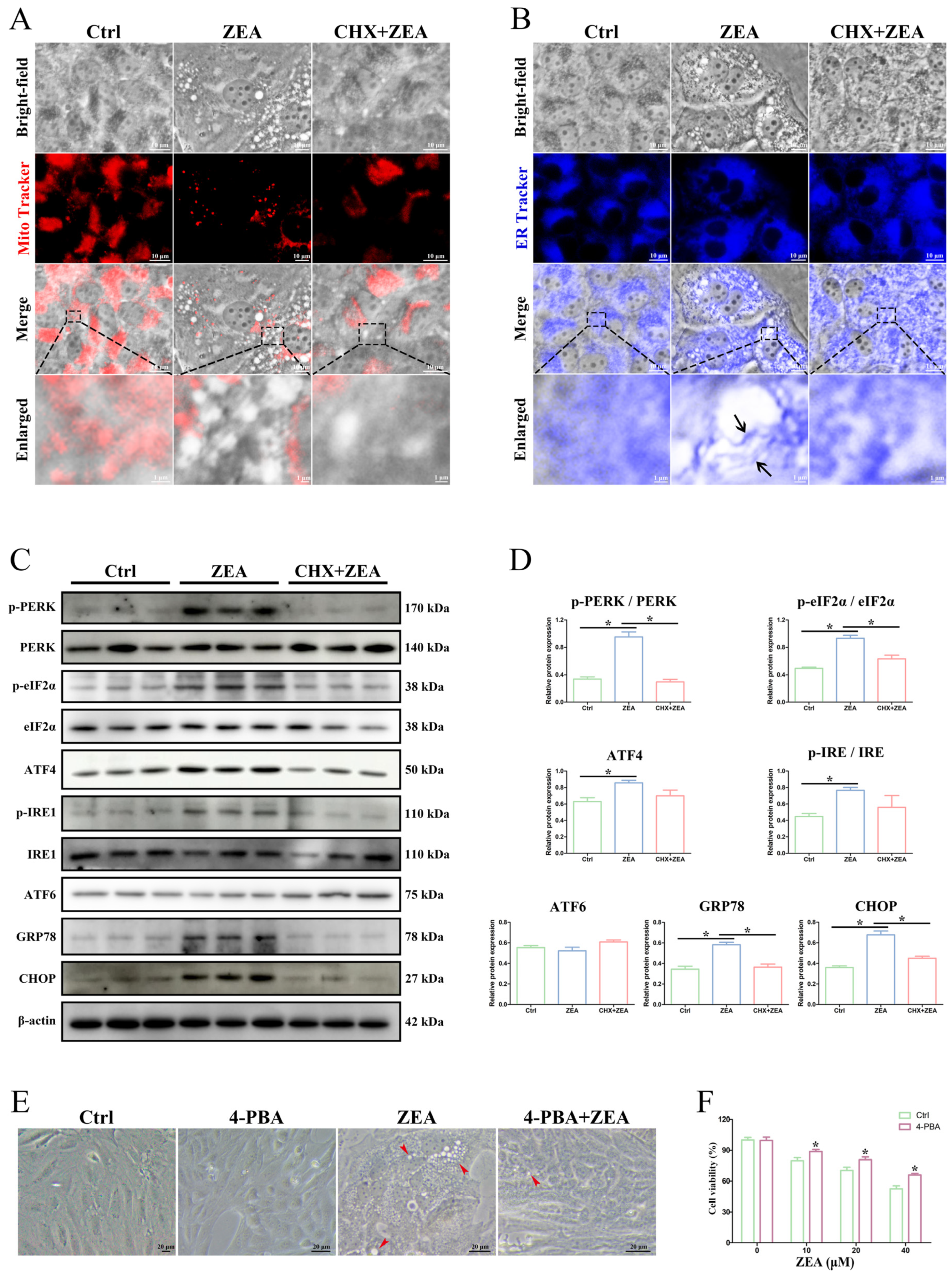
2.6. ZEA-Induced Paraptosis Was Accompanied by ER Stress in SCs
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Evaluation of Testicular Organ Coefficients
4.3. Spermatozoon Quality Analysis
4.4. Hematoxylin–Eosin (H&E) Staining
4.5. Transmission Electron Microscopy (TEM) Analysis
4.6. BTB Integrity Assay
4.7. Immunohistochemistry (IHC) Staining
4.8. Western Blot Analysis
4.9. Isolation, Culture, and Identification of Goat SCs
4.10. Cell Treatment
4.11. Cell Viability Assay
4.12. Transepithelial Electrical Resistance (TER) Measurement
4.13. Immunofluorescence (IF) Staining
4.14. Hoechst 33342 Staining
4.15. Flow Cytometry
4.16. Fluorescent Labeling of Mitochondrial and ER
4.17. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, H.; Wang, L.; Sun, J.; Wang, L.; Guo, H.; Ye, Y.; Sun, X. Microbial detoxification of mycotoxins in food and feed. Crit. Rev. Food Sci. Nutr. 2022, 62, 4951–4969. [Google Scholar] [CrossRef] [PubMed]
- Nešić, K.; Habschied, K.; Mastanjević, K. Modified Mycotoxins and Multitoxin Contamination of Food and Feed as Major Analytical Challenges. Toxins 2023, 15, 511. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef]
- Balló, A.; Busznyákné Székvári, K.; Czétány, P.; Márk, L.; Török, A.; Szántó, Á.; Máté, G. Estrogenic and Non-Estrogenic Disruptor Effect of Zearalenone on Male Reproduction: A Review. Int. J. Mol. Sci. 2023, 24, 1578. [Google Scholar] [CrossRef]
- Han, X.; Huangfu, B.; Xu, T.; Xu, W.; Asakiya, C.; Huang, K.; He, X. Research Progress of Safety of Zearalenone: A Review. Toxins 2022, 14, 386. [Google Scholar] [CrossRef]
- Zheng, W.; Feng, N.; Wang, Y.; Noll, L.; Xu, S.; Liu, X.; Lu, N.; Zou, H.; Gu, J.; Yuan, Y.; et al. Effects of zearalenone and its derivatives on the synthesis and secretion of mammalian sex steroid hormones: A review. Food Chem. Toxicol. 2019, 126, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, T.; Ren, X.; Li, B.; Wang, S. Male reproductive toxicity of zearalenone-meta-analysis with mechanism review. Ecotoxicol. Environ. Saf. 2021, 221, 112457. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, L.; Jesse, C.R.; de Gomes, M.G.; Borges Filho, C.; Donato, F.; Souza, L.C.; Goes, A.R.; Furian, A.F.; Boeira, S.P. The flavonoid chrysin protects against zearalenone induced reproductive toxicity in male mice. Toxicon 2019, 165, 13–21. [Google Scholar] [CrossRef]
- Cai, P.; Feng, N.; Zou, H.; Gu, J.; Liu, X.; Liu, Z.; Yuan, Y.; Bian, J. Zearalenone damages the male reproductive system of rats by destroying testicular focal adhesion. Environ. Toxicol. 2023, 38, 278–288. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef]
- Liu, J.; Applegate, T. Zearalenone (ZEN) in Livestock and Poultry: Dose, Toxicokinetics, Toxicity and Estrogenicity. Toxins 2020, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Ren, C.; Gong, Y.; Gao, X.; Rajput, S.A.; Qi, D.; Wang, S. The insensitive mechanism of poultry to zearalenone: A review. Anim. Nutr. 2021, 7, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xi, H.; Han, S.; Zhang, H.; Hu, J. Zearalenone induces oxidative stress and autophagy in goat Sertoli cells. Ecotoxicol. Environ. Saf. 2023, 252, 114571. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Jiang, T.; Lin, P.; Chen, H.; Wang, L.; Wang, N.; Zhao, F.; Tang, K.; Zhou, D.; Wang, A.; et al. Apoptosis inducing factor gene depletion inhibits zearalenone-induced cell death in a goat Leydig cell line. Reprod. Toxicol. 2017, 67, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Mruk, D.D.; Cheng, C.Y. The Mammalian Blood-Testis Barrier: Its Biology and Regulation. Endocr. Rev. 2015, 36, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Luaces, J.P.; Toro-Urrego, N.; Otero-Losada, M.; Capani, F. What do we know about blood-testis barrier? current understanding of its structure and physiology. Front. Cell Dev. Biol. 2023, 11, 1114769. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Jiang, X.; Sun, J.; Li, X.; Li, X.; Jiao, R.; Peng, Z.; Li, Y.; Bai, W. Toxic effects of zearalenone on gametogenesis and embryonic development: A molecular point of review. Food Chem. Toxicol. 2018, 119, 24–30. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, D.; Fayjaloun, S.; Nassar, M.; Sahakian, J.; Aad, P.Y. Updates on the Effect of Mycotoxins on Male Reproductive Efficiency in Mammals. Toxins 2019, 11, 515. [Google Scholar] [CrossRef]
- Cao, Z.; Huang, W.; Sun, Y.; Li, Y. Deoxynivalenol induced spermatogenesis disorder by blood-testis barrier disruption associated with testosterone deficiency and inflammation in mice. Environ. Pollut. 2020, 264, 114748. [Google Scholar] [CrossRef]
- Huang, W.; Liu, M.; Xiao, B.; Zhang, J.; Song, M.; Li, Y.; Cao, Z. Aflatoxin B1 disrupts blood-testis barrier integrity by reducing junction protein and promoting apoptosis in mice testes. Food Chem. Toxicol. 2021, 148, 111972. [Google Scholar] [CrossRef]
- Karacaoğlu, E.; Selmanoğlu, G. T-2 toxin induces cytotoxicity and disrupts tight junction barrier in SerW3 cells. Environ. Toxicol. Pharmacol. 2017, 56, 259–267. [Google Scholar] [CrossRef]
- She, J.; Feng, N.; Zheng, W.; Zheng, H.; Cai, P.; Zou, H.; Yuan, Y.; Gu, J.; Liu, Z.; Bian, J. Zearalenone Exposure Disrupts Blood-Testis Barrier Integrity through Excessive Ca2+-Mediated Autophagy. Toxins 2021, 13, 875. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Yang, S.; Dong, S.; Chen, X.; Zhang, Y.; He, J. Characterization of semen quality, testicular marker enzyme activities and gene expression changes in the blood testis barrier of Kunming mice following acute exposure to zearalenone. Environ. Sci. Pollut. Res. Int. 2017, 24, 27235–27243. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.H.; Wang, L.; Ma, H.H.; Zhao, A.H.; Xiao, H.W.; Zhang, X.F. Identification of apoptotic pathways in zearalenone-treated mouse sertoli cells. J. Toxicol. Sci. 2022, 47, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.C.; Lin, Y.F.; Huang, M.Y.; Zhang, X.L.; Wang, P.; Huang, M.Q.; Lu, J.J. Paraptosis: A non-classical paradigm of cell death for cancer therapy. Acta Pharmacol. Sin. 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Di Domizio, A.; Limonta, P. The emerging role of paraptosis in tumor cell biology: Perspectives for cancer prevention and therapy with natural compounds. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188338. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.; Dharan, A.; PV, J.; Pal, S.; Nair, B.G.; Kar, R.; Mishra, N. Paraptosis: A unique cell death mode for targeting cancer. Front. Pharmacol. 2023, 14, 1159409. [Google Scholar] [CrossRef]
- Schwarz, D.S.; Blower, M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef]
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic reticulum stress signals in the tumour and its microenvironment. Nat. Rev. Cancer 2021, 21, 71–88. [Google Scholar] [CrossRef]
- Cubillos-Ruiz, J.R.; Bettigole, S.E.; Glimcher, L.H. Tumorigenic and Immunosuppressive Effects of Endoplasmic Reticulum Stress in Cancer. Cell 2017, 168, 692–706. [Google Scholar] [CrossRef]
- Mandula, J.K.; Chang, S.; Mohamed, E.; Jimenez, R.; Sierra-Mondragon, R.A.; Chang, D.C.; Obermayer, A.N.; Moran-Segura, C.M.; Das, S.; Vazquez-Martinez, J.A.; et al. Ablation of the endoplasmic reticulum stress kinase PERK induces paraptosis and type I interferon to promote anti-tumor T cell responses. Cancer Cell 2022, 40, 1145–1160.e9. [Google Scholar] [CrossRef] [PubMed]
- Li, G.N.; Zhao, X.J.; Wang, Z.; Luo, M.S.; Shi, S.N.; Yan, D.M.; Li, H.Y.; Liu, J.H.; Yang, Y.; Tan, J.H.; et al. Elaiophylin triggers paraptosis and preferentially kills ovarian cancer drug-resistant cells by inducing MAPK hyperactivation. Signal Transduct. Target. Ther. 2022, 7, 317. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Lee, D.M.; Seo, M.J.; Lee, H.J.; Choi, K.S. Intracellular Ca2+ Imbalance Critically Contributes to Paraptosis. Front. Cell Dev. Biol. 2021, 8, 607844. [Google Scholar] [CrossRef] [PubMed]
- Korsnes, M.S.; Espenes, A.; Hetland, D.L.; Hermansen, L.C. Paraptosis-like cell death induced by yessotoxin. Toxicol. Vitro 2011, 25, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guo, Y.; Yang, C.; Huang, R.; Wen, Y.; Zhang, C.; Wu, C.; Zhao, B. Swainsonine Triggers Paraptosis via ER Stress and MAPK Signaling Pathway in Rat Primary Renal Tubular Epithelial Cells. Front. Pharmacol. 2021, 12, 715285. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gu, Y.; Bian, Y.; Cai, D.; Li, Y.; Zhao, Y.; Zhang, Z.; Xue, M.; Zhang, L. Honokiol induces paraptosis-like cell death of acute promyelocytic leukemia via mTOR & MAPK signaling pathways activation. Apoptosis 2021, 26, 195–208. [Google Scholar] [CrossRef]
- Xu, M.L.; Hu, J.; Guo, B.P.; Niu, Y.R.; Xiao, C.; Xu, Y.X. Exploration of intrinsic and extrinsic apoptotic pathways in zearalenone-treated rat sertoli cells. Environ. Toxicol. 2016, 31, 1731–1739. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Z.; Cui, H.; Ding, K.; Zhao, Y.; Ma, X.; Adetunji, A.O.; Min, L. Effect of Zearalenone-Induced Ferroptosis on Mice Spermatogenesis. Animals 2022, 12, 3026. [Google Scholar] [CrossRef]
- Vargas, J.N.S.; Hamasaki, M.; Kawabata, T.; Youle, R.J.; Yoshimori, T. The mechanisms and roles of selective autophagy in mammals. Nat. Rev. Mol. Cell Biol. 2023, 24, 167–185. [Google Scholar] [CrossRef]
- Ketelut-Carneiro, N.; Fitzgerald, K.A. Apoptosis, Pyroptosis, and Necroptosis-Oh My! The Many Ways a Cell Can Die. J. Mol. Biol. 2022, 434, 167378. [Google Scholar] [CrossRef]
- Sperandio, S.; Poksay, K.; de Belle, I.; Lafuente, M.J.; Liu, B.; Nasir, J.; Bredesen, D.E. Paraptosis: Mediation by MAP kinases and inhibition by AIP-1/Alix. Cell Death Differ. 2004, 11, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Zhao, Y.; Zhang, P.; Zhang, H.; Gao, Y.; Liu, J.; Feng, Y.; Li, L.; Shen, W.; Sun, Z.; et al. Gestational exposure to low-dose zearalenone disrupting offspring spermatogenesis might be through epigenetic modifications. Basic Clin. Pharmacol. Toxicol. 2019, 125, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhao, Y.; Zhang, H.; Zhang, P.; Liu, J.; Feng, Y.; Men, Y.; Li, L.; Shen, W.; Sun, Z.; et al. Pubertal exposure to low doses of zearalenone disrupting spermatogenesis through ERα related genetic and epigenetic pathways. Toxicol. Lett. 2019, 315, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Fijak, M.; Meinhardt, A. The testis in immune privilege. Immunol. Rev. 2006, 213, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Yang, S.H.; Shi, W.; Li, P.; Guo, Y.; Guo, J.; He, J.B.; Zhang, Y. Protective effect of proanthocyanidin on mice Sertoli cell apoptosis induced by zearalenone via the Nrf2/ARE signalling pathway. Environ. Sci. Pollut. Res. Int. 2017, 24, 26724–26733. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Chen, C.; Hai, S.; Wang, C.; Rahman, S.U.; Huang, W.; Zhao, C.; Feng, S.; Wang, X. Inhibition of Mitochondrial Fission Alleviates Zearalenone-Induced Mitochondria-Associated Endoplasmic Reticulum Membrane Dysfunction in Piglet Sertoli Cells. Toxins 2023, 15, 253. [Google Scholar] [CrossRef]
- Sekido, R.; Lovell-Badge, R. Genetic control of testis development. Sex. Dev. 2013, 7, 21–32. [Google Scholar] [CrossRef]
- O’Donnell, L.; Smith, L.B.; Rebourcet, D. Sertoli cells as key drivers of testis function. Semin. Cell Dev. Biol. 2022, 121, 2–9. [Google Scholar] [CrossRef]
- Cao, L.; Zhao, J.; Xu, J.; Zhu, L.; Rahman, S.U.; Feng, S.; Li, Y.; Wu, J.; Wang, X. N-acetylcysteine ameliorate cytotoxic injury in piglets sertoli cells induced by zearalenone and deoxynivalenol. Environ. Sci. Pollut. Res. Int. 2021, 28, 60276–60289. [Google Scholar] [CrossRef]
- Ma, L.; Xuan, X.; Fan, M.; Zhang, Y.; Yuan, G.; Huang, G.; Liu, Z. A novel 8-hydroxyquinoline derivative induces breast cancer cell death through paraptosis and apoptosis. Apoptosis 2022, 27, 577–589. [Google Scholar] [CrossRef]
- Zhao, L.; Zhong, B.; Zhu, Y.; Zheng, H.; Wang, X.; Hou, Y.; Lu, J.J.; Ai, N.; Guo, X.; Ge, W.; et al. Nitrovin (difurazone), an antibacterial growth promoter, induces ROS-mediated paraptosis-like cell death by targeting thioredoxin reductase 1 (TrxR1). Biochem. Pharmacol. 2023, 210, 115487. [Google Scholar] [CrossRef] [PubMed]
- Nedungadi, D.; Binoy, A.; Vinod, V.; Vanuopadath, M.; Nair, S.S.; Nair, B.G.; Mishra, N. Ginger extract activates caspase independent paraptosis in cancer cells via ER stress, mitochondrial dysfunction, AIF translocation and DNA damage. Nutr. Cancer 2021, 73, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Schröder, M.; Kaufman, R.J. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005, 74, 739–789. [Google Scholar] [CrossRef] [PubMed]
- Urra, H.; Dufey, E.; Avril, T.; Chevet, E.; Hetz, C. Endoplasmic Reticulum Stress and the Hallmarks of Cancer. Trends Cancer 2016, 2, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.; Teodoro, T.; Volchuk, A. Endoplasmic reticulum stress: Signaling the unfolded protein response. Physiology 2007, 22, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Chevet, E.; Hetz, C.; Samali, A. Endoplasmic reticulum stress-activated cell reprogramming in oncogenesis. Cancer Discov. 2015, 5, 586–597. [Google Scholar] [CrossRef] [PubMed]
- Binoy, A.; Nedungadi, D.; Katiyar, N.; Bose, C.; Shankarappa, S.A.; Nair, B.G.; Mishra, N. Plumbagin induces paraptosis in cancer cells by disrupting the sulfhydryl homeostasis and proteasomal function. Chem. Biol. Interact. 2019, 310, 108733. [Google Scholar] [CrossRef]
- Torres-Ramírez, N.; Escobar, M.L.; Vázquez-Nin, G.H.; Ortiz, R.; Echeverría, O.M. Paraptosis-like cell death in Wistar rat granulosa cells. Dev. Growth Differ. 2016, 58, 651–663. [Google Scholar] [CrossRef]
- Hu, L.; Shi, J.; Shen, D.; Zhai, X.; Liang, D.; Wang, J.; Xie, C.; Xia, Z.; Cui, J.; Liu, F.; et al. Osimertinib induces paraptosis and TRIP13 confers resistance in glioblastoma cells. Cell Death Discov. 2023, 9, 333. [Google Scholar] [CrossRef]
- Caamaño, J.N.; Santiago-Moreno, J.; Martínez-Pastor, F.; Tamargo, C.; Salman, A.; Fernández, Á.; Merino, M.J.; Lacalle, E.; Toledano-Díaz, A.; Hidalgo, C.O. Use of the flavonoid taxifolin for sperm cryopreservation from the threatened Bermeya goat breed. Theriogenology 2023, 206, 18–27. [Google Scholar] [CrossRef]
- Zhou, G.X.; Liu, W.B.; Dai, L.M.; Zhu, H.L.; Xiong, Y.W.; Li, D.X.; Xu, D.X.; Wang, H. Environmental cadmium impairs blood-testis barrier via activating HRI-responsive mitochondrial stress in mice. Sci. Total Environ. 2022, 810, 152247. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Lu, X.; Bao, X.; Zhang, C.; Li, J.; Ren, C.; Zhu, Z.; Ma, B.; Zhang, N.; Jin, X.; et al. Aucubin supplementation alleviate diabetes induced-disruption of blood-testis barrier and testicular damage via stabilizing cell junction integrity. Eur. J. Pharmacol. 2023, 938, 175430. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yang, Q.; Ouyang, Y.; Lou, Y.; Cui, H.; Deng, H.; Zhu, Y.; Geng, Y.; Ouyang, P.; Chen, L.; et al. Nickel induces blood-testis barrier damage through ROS-mediated p38 MAPK pathways in mice. Redox Biol. 2023, 67, 102886. [Google Scholar] [CrossRef]
- Xi, H.; Ren, F.; Li, Y.; Xian, M.; Wang, L.; Hu, J. FSH inhibits autophagy and lysosomal biogenesis to regulate protein degradation in cultured goat Sertoli cells. Mol. Cell. Endocrinol. 2022, 540, 111505. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, M.S.; Wang, J.X.; Cui, J.G.; Zhang, H.; Li, X.N.; Li, J.L. Connexin-43 is a promising target for lycopene preventing phthalate-induced spermatogenic disorders. J. Adv. Res. 2023, 49, 115–126. [Google Scholar] [CrossRef]
- Zhang, S.R.; Zhang, X.C.; Liang, J.F.; Fang, H.M.; Huang, H.X.; Zhao, Y.Y.; Chen, X.Q.; Ma, S.L. Chalcomoracin inhibits cell proliferation and increases sensitivity to radiotherapy in human non-small cell lung cancer cells via inducing endoplasmic reticulum stress-mediated paraptosis. Acta Pharmacol. Sin. 2020, 41, 825–834. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Liu, G.; Xu, Y.; Huang, Y.; Zhang, Y.; Wu, Y.; Xu, Y. Zearalenone Induces Blood-Testis Barrier Damage through Endoplasmic Reticulum Stress-Mediated Paraptosis of Sertoli Cells in Goats. Int. J. Mol. Sci. 2024, 25, 553. https://doi.org/10.3390/ijms25010553
Liu T, Liu G, Xu Y, Huang Y, Zhang Y, Wu Y, Xu Y. Zearalenone Induces Blood-Testis Barrier Damage through Endoplasmic Reticulum Stress-Mediated Paraptosis of Sertoli Cells in Goats. International Journal of Molecular Sciences. 2024; 25(1):553. https://doi.org/10.3390/ijms25010553
Chicago/Turabian StyleLiu, Tengfei, Gengchen Liu, Yinghuan Xu, Yuqi Huang, Yunxuan Zhang, Yongjie Wu, and Yongping Xu. 2024. "Zearalenone Induces Blood-Testis Barrier Damage through Endoplasmic Reticulum Stress-Mediated Paraptosis of Sertoli Cells in Goats" International Journal of Molecular Sciences 25, no. 1: 553. https://doi.org/10.3390/ijms25010553



