Synergy between Membrane Currents Prevents Severe Bradycardia in Mouse Sinoatrial Node Tissue
Abstract
:1. Introduction
2. Results
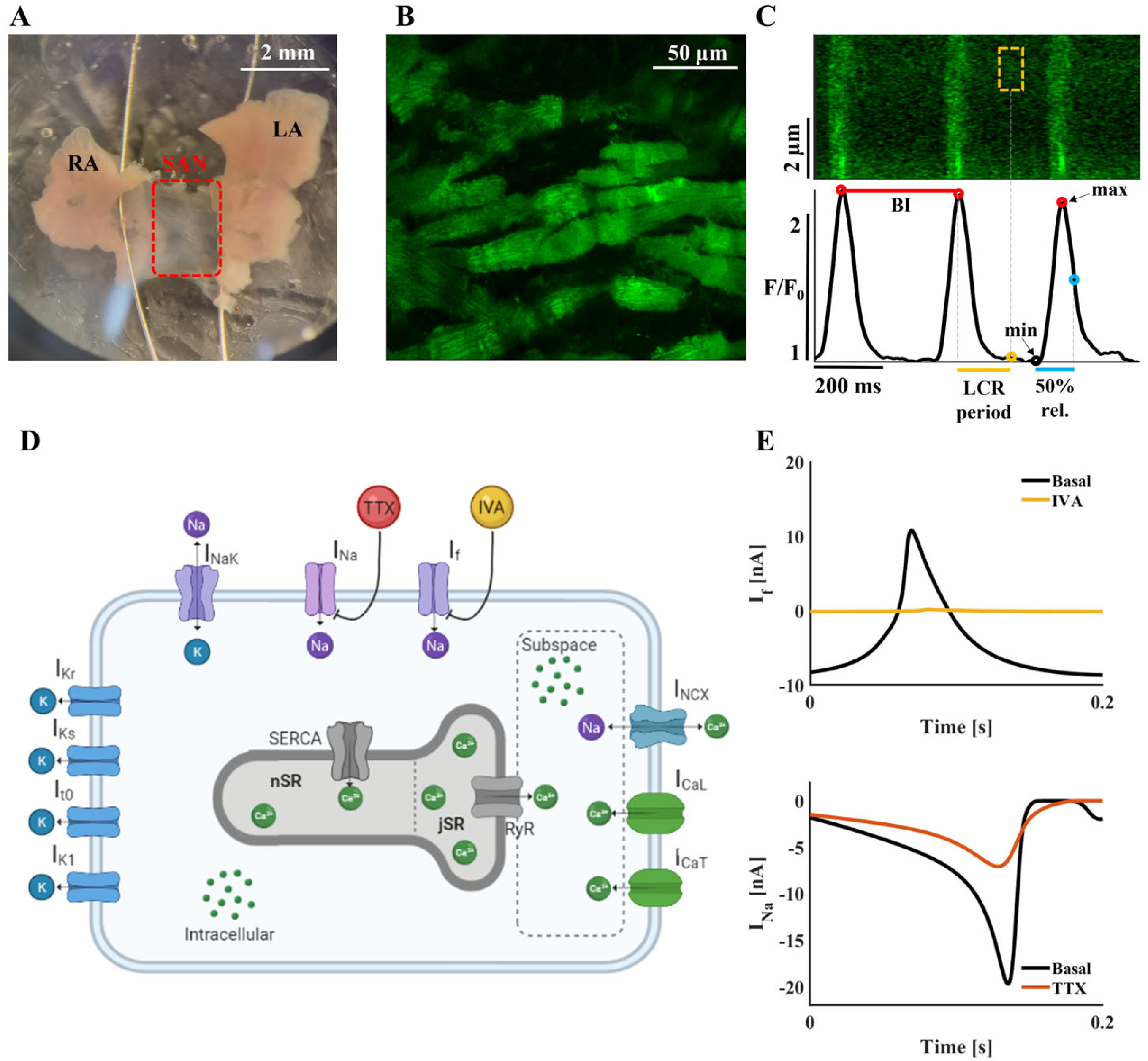
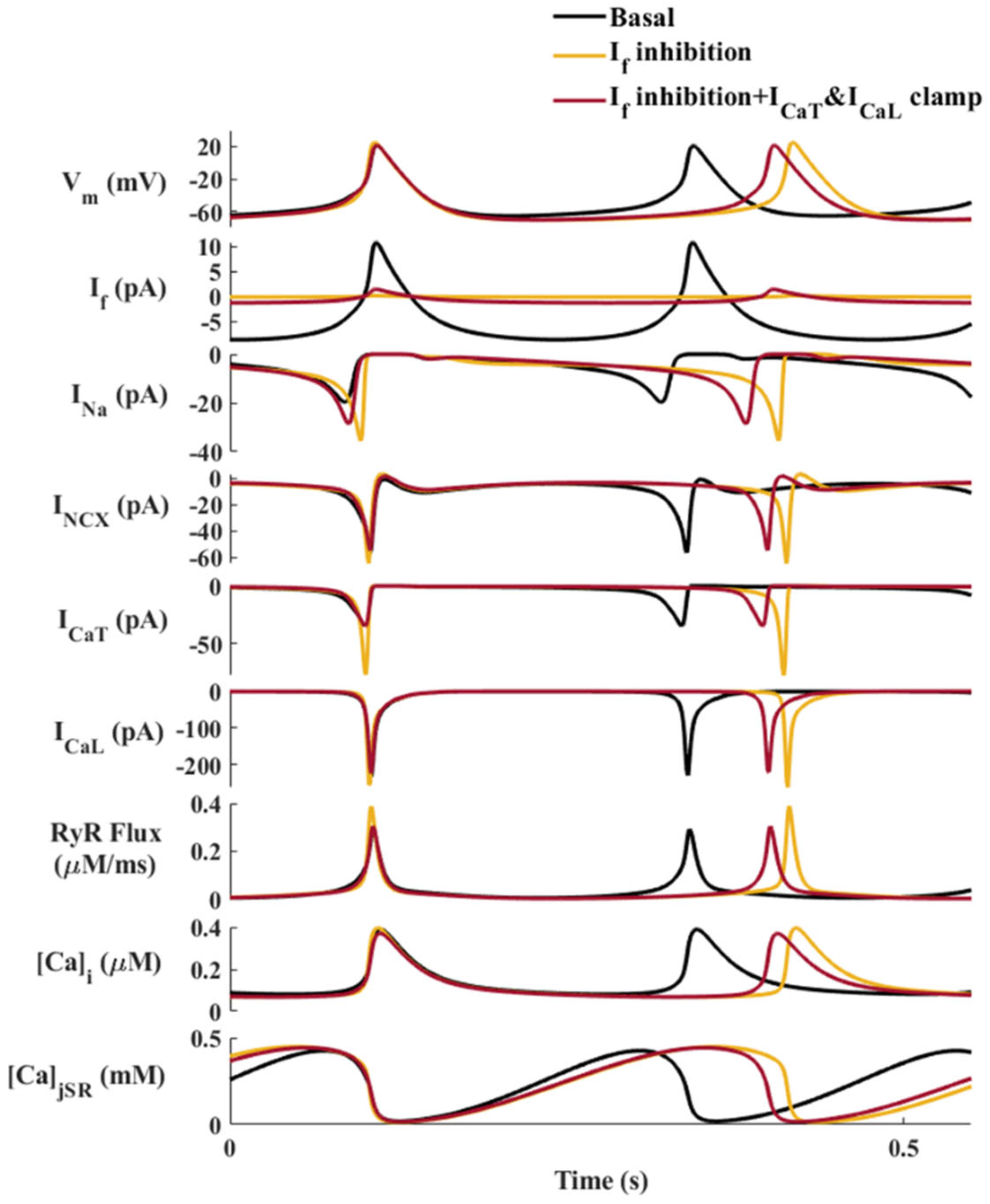
2.1. ICaT and INa Are Upregulated When If Is Inhibited: Computational Evidence
2.2. There Is No Synergy between If and ICaT
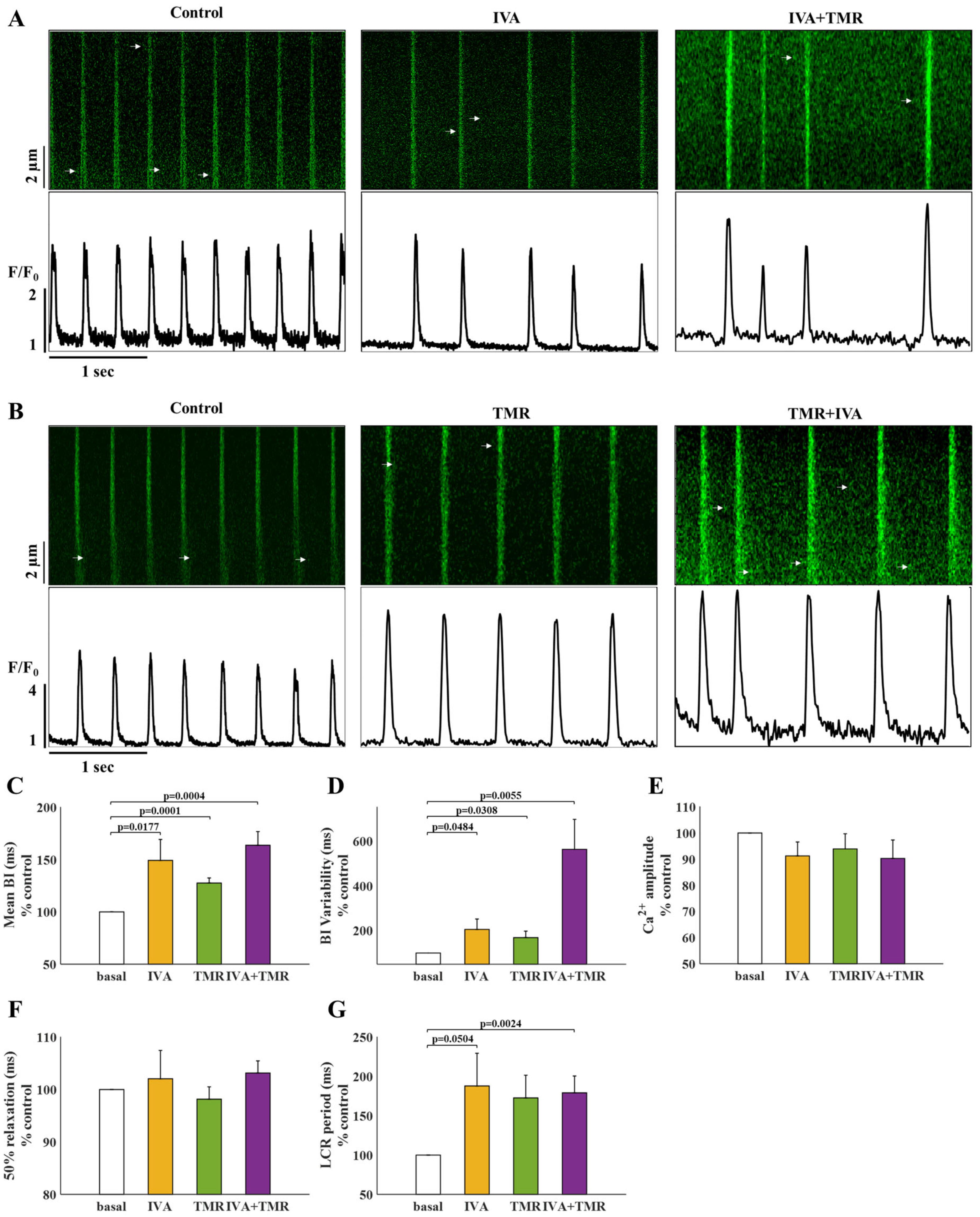
2.3. If and INa Blockers Have a Synergistic Effect on BI
2.4. The Molecular Mechanisms That Mediate the Synergy between If and INa: Computational Evidence
3. Discussion
Limitations
4. Materials and Methods
4.1. Mouse SAN Isolation
4.2. SAN Confocal Ca2+ Imaging
4.3. Ca2+ Analysis
4.4. Drugs
4.5. Statistics
4.6. Computational Model
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dharod, A.; Soliman, E.Z.; Dawood, F.; Chen, H.; Shea, S.; Nazarian, S.; Bertoni, A.G. Association of asymptomatic bradycardia with incident cardiovascular disease and mortality: The Multi-Ethnic Study of Atherosclerosis (MESA). JAMA Intern. Med. 2016, 176, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wung, S.F. Bradyarrhythmias: Clinical presentation, diagnosis, and management. Crit. Care Nurs. Clin. N. Am. 2016, 28, 297–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yaniv, Y.; Lakatta, E.G.; Maltsev, V.A. From two competing oscillators to one coupled-clock pacemaker cell system. Front. Physiol. 2015, 6, 28. [Google Scholar] [CrossRef] [Green Version]
- Yaniv, Y.; Sirenko, S.; Ziman, B.D.; Spurgeon, H.A.; Maltsev, V.A.; Lakatta, E.G. New evidence for coupled clock regulation of the normal automaticity of sinoatrial nodal pacemaker cells: Bradycardic effects of ivabradine are linked to suppression of intracellular Ca2+ cycling. J. Mol. Cell. Cardiol. 2013, 62, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.B.; Val-Blasco, A.; Davoodi, M.; Gómez, S.; Yaniv, Y.; Benitah, J.P.; Gómez, A.M. Heart failure in mice induces a dysfunction of the sinus node associated with reduced CaMKII signaling. J. Gen. Physiol. 2022, 154, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.O.; Wilders, R.; Coronel, R.; Ravesloot, J.H.; Verheijck, E.E. Ionic remodeling of sinoatrial node cells by heart failure. Circulation 2003, 108, 760–766. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Du, Y.; Zhang, J.; Ma, A.; Yang, P.; Huang, X.; Yang, P.; Du, Y.; Zhang, J.; Ma, A. Age-related down-regulation of HCN channels in rat sinoatrial node. Basic Res. Cardiol. 2007, 102, 429–435. [Google Scholar] [CrossRef]
- Yamanushi, T.T.; Yanni, J.; Dobrzynski, H.; Kabuto, H.; Boyett, M.R. Changes in ion channel expression in right-sided congestive heart failure. J. Mol. Cell. Cardiol. 2010, 48, 496–508. [Google Scholar]
- Zhang, Y.; Wang, Y.; Yanni, J.; Qureshi, M.A.; Logantha, S.J.R.J.; Kassab, S.; Boyett, M.R.; Gardiner, N.J.; Sun, H.; Howarth, F.C.; et al. Electrical conduction system remodeling in streptozotocin-induced diabetes mellitus rat heart. Front. Physiol. 2019, 10, 826. [Google Scholar] [CrossRef]
- Yung-Hsin, Y.; Burstein, B.; Qi, X.Y.; Sakabe, M.; Chartier, D.; Comtois, P.; Wang, Z.; Kuo, C.T.; Nattel, S. Funny current downregulation and sinus node dysfunction associated with atrial tachyarrhythmia. Circulation 2009, 119, 1576–1585. [Google Scholar] [CrossRef] [Green Version]
- Maltsev, V.A.; Lakatta, E.G. A novel quantitative explanation for the autonomic modulation of cardiac pacemaker cell automaticity via a dynamic system of sarcolemmal and intracellular proteins. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H2010–H2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyashkov, A.E.; Beahr, J.; Lakatta, E.G.; Yaniv, Y.; Maltsev, V.A. Positive feedback mechanisms among local Ca releases, NCX, and I CaL ignite pacemaker action potentials. Biophys. J. 2018, 114, 1176–1189. [Google Scholar] [CrossRef] [PubMed]
- Arbel-Ganon, L.; Behar, J.A.; Gómez, A.M.; Yaniv, Y. Distinct mechanisms mediate pacemaker dysfunction associated with catecholaminergic polymorphic ventricular tachycardia mutations: Insights from computational modeling. J. Mol. Cell. Cardiol. 2020, 143, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Monfredi, O.; Tsutsui, K.; Ziman, B.; Stern, M.D.; Lakatta, E.G.; Maltsev, V.A. Electrophysiological heterogeneity of pacemaker cells in the rabbit intercaval region, including the SA node: Insights from recording multiple ion currents in each cell. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H403–H414. [Google Scholar] [CrossRef]
- Yaniv, Y.; Maltsev, V.A.; Ziman, B.D.; Spurgeon, H.A.; Lakatta, E.G. The “Funny” Current (If) inhibition by Ivabradine at membrane potentials encompassing spontaneous depolarization in pacemaker cells. Molecules 2012, 17, 8241–8254. [Google Scholar] [CrossRef] [Green Version]
- Bois, P.; Bescond, J.; Renaudon, B.; Lenfant, J. Mode of action of bradycardic agent, S 16257, on ionic currents of rabbit sinoatrial node cells. Br. J. Pharmacol. 1996, 118, 1051–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nobuhisa Hagiwara, B.; Irisawa, H.; Kameyama, M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J. Physiol. 1988, 395, 233–253. [Google Scholar] [CrossRef]
- Haechl, N.; Ebner, J.; Hilber, K.; Todt, H.; Koenig, X. Pharmacological profile of the bradycardic agent ivabradine on human cardiac ion channels. Cell Physiol. Biochem. 2019, 53, 36–48. [Google Scholar] [CrossRef] [Green Version]
- Vinogradova, T.M.; Bogdanov, K.Y.; Lakatta, E.G. Beta-adrenergic stimulation modulates ryanodine receptor Ca2+ release during diastolic depolarization to accelerate pacemaker activity in rabbit sinoatrial nodal cells. Circ. Res. 2002, 90, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Haron-Khun, S.; Weisbrod, D.; Bueno, H.; Yadin, D.; Behar, J.; Peretz, A.; Binah, O.; Hochhauser, E.; Eldar, M.; Yaniv, Y.; et al. SK4 K+ channels are therapeutic targets for the treatment of cardiac arrhythmias. EMBO Mol. Med. 2017, 9, 415–429. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Maltsev, V.A.; Vinogradova, T.M. A coupled SYSTEM of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart’s pacemaker. Circ. Res. 2010, 106, 659–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Zhang, Q.; Timofeyev, V.; Zhang, Z.; Young, J.N.; Shin, H.S.; Knowlton, A.A.; Chiamvimonvat, N. Molecular coupling of a Ca2+-activated K+ channel to L-Type Ca2+ channels via Alpha-Actinin2. Circ. Res. 2007, 100, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Kunzelmann, K.; Kongsuphol, P.; Chootip, K.; Toledo, C.; Martins, J.R.; Almaca, J.; Tian, Y.; Witzgall, R.; Ousingsawat, J.; Schreiber, R. Role of the Ca2+-activated Cl− channels bestrophin and anoctamin in epithelial cells. Biol. Chem. 2011, 392, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Monfredi, O.; Lyashkov, A.E.; Johnsen, A.B.; Inada, S.; Schneider, H.; Wang, R.; Nirmalan, M.; Wisloff, U.; Maltsev, V.A.; Lakatta, E.G.; et al. Biophysical characterization of the underappreciated and important relationship between heart rate variability and heart rate. Hypertension 2014, 64, 1334–1343. [Google Scholar] [CrossRef] [Green Version]
- Rocchetti, M.; Malfatto, G.; Lombardi, F.; Zaza, A. Role of the input/output relation of sinoatrial myocytes in cholinergic modulation of heart rate variability. J. Cardiovasc. Electrophysiol. 2000, 11, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, Y.; Lyashkov, A.E.; Sirenko, S.; Okamoto, Y.; Guiriba, T.-R.R.; Ziman, B.D.; Morrell, C.H.; Lakatta, E.G. Stochasticity intrinsic to coupled-clock mechanisms underlies beat-to-beat variability of spontaneous action potential firing in sinoatrial node pacemaker cells. J. Mol. Cell. Cardiol. 2014, 77, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torrente, A.G.; Mesirca, P.; Bidaud, I.; Mangoni, M.E. Channelopathies of voltage-gated L-Type Cav1.3/A1D and T-Type Cav3.1/A1G Ca2+ channels in dysfunction of heart automaticity. Pflug. Arch. Eur. J. Physiol. 2020, 472, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Mesirca, P.; Torrente, A.G.; Mangoni, M.E. T-type channels in the sino-atrial and atrioventricular pacemaker mechanism. Pflug. Arch. Eur. J. Physiol. 2014, 466, 791–799. [Google Scholar] [CrossRef]
- Neher, E.; Sakmann, B.; Steinbach, J.H. The extracellular patch clamp: A method for resolving currents through individual open channels in biological membranes. Pflug. Arch. 1978, 375, 219–228. [Google Scholar] [CrossRef]
- Boyett, M.R.; Honjo, H.; Kodama, I. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc. Res. 2000, 47, 658–687. [Google Scholar] [CrossRef]
- Li, N.; Kalyanasundaram, A.; Hansen, B.J.; Artiga, E.J.; Sharma, R.; Abudulwahed, S.H.; Helfrich, K.M.; Rozenberg, G.; Wu, P.J.; Zakharkin, S.; et al. Impaired neuronal sodium channels cause intranodal conduction failure and reentrant arrhythmias in human sinoatrial node. Nat. Commun. 2020, 11, 512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verheijck, E.E.; Van Kempen, M.J.A.; Veereschild, M.; Lurvink, J.; Jongsma, H.J.; Bouman, L.N. Electrophysiological features of the mouse sinoatrial node in relation to connexin distribution. Cardiovasc. Res. 2001, 52, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dobrzynski, H.; Yanni, J.; Boyett, M.R.; Lei, M. Organisation of the mouse sinoatrial node: Structure and expression of HCN channels. Cardiovasc. Res. 2007, 73, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, B.; Komaromi, I.; Nanasi, P.P.; Szentandrassy, N. Selectivity problems with drugs acting on cardiac Na+ and Ca2+ channels. Curr. Med. Chem. 2013, 20, 2552–2571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Mesirca, P.; Marqués-Sulé, E.; Zahradnikova, A.; Villejoubert, O.; D’Ocon, P.; Ruiz, C.; Domingo, D.; Zorio, E.; Mangoni, M.E.; et al. RyR2R420Q catecholaminergic polymorphic ventricular tachycardia mutation induces bradycardia by disturbing the coupled clock pacemaker mechanism. JCI Insight 2017, 2, e91872. [Google Scholar] [CrossRef] [Green Version]
- Lyashkov, A.E.; Juhaszova, M.; Dobrzynski, H.; Vinogradova, T.M.; Maltsev, V.A.; Juhasz, O.; Spurgeon, H.A.; Sollott, S.J.; Lakatta, E.G. Calcium cycling protein density and functional importance to automaticity of isolated sinoatrial nodal cells are independent of cell size. Circ. Res. 2007, 100, 1723–1731. [Google Scholar] [CrossRef] [Green Version]
- Neco, P.; Torrente, A.G.; Mesirca, P.; Zorio, E.; Liu, N.; Priori, S.G.; Napolitano, C.; Richard, S.; Benitah, J.-P.P.; Mangoni, M.E.; et al. Paradoxical effect of increased diastolic Ca2+ release and decreased sinoatrial node activity in a mouse model of catecholaminergic polymorphic ventricular tachycardia. Circulation 2012, 126, 392–401. [Google Scholar] [CrossRef] [Green Version]
- Bychkov, R.; Juhaszova, M.; Tsutsui, K.; Coletta, C.; Stern, M.D.; Maltsev, V.A.; Lakatta, E.G. Synchronized cardiac impulses emerge from heterogeneous local calcium signals within and among cells of pacemaker tissue. JACC Clin. Electrophysiol. 2020, 6, 907–931. [Google Scholar] [CrossRef]
- Bychkov, R.; Juhaszova, M.; Calvo-Rubio Barrera, M.; Donald, L.A.H.; Coletta, C.; Shumaker, C.; Moorman, K.; Sirenko, S.T.; Maltsev, A.V.; Sollott, S.J.; et al. The heart’s pacemaker mimics brain cytoarchitecture and function: Novel interstitial cells expose complexity of the SAN. JACC Clin. Electrophysiol. 2022, 8, 1191–1215. [Google Scholar] [CrossRef]
- MacDonald, E.A.; Madl, J.; Greiner, J.; Ramadan, A.F.; Wells, S.M.; Torrente, A.G.; Kohl, P.; Rog-Zielinska, E.A.; Quinn, T.A. Sinoatrial node structure, mechanics, electrophysiology and the chronotropic response to stretch in rabbit and mouse. Front. Physiol. 2020, 11, 809. [Google Scholar] [CrossRef]
- Chong, H.L.; Ruben, P.C. Interaction between voltage-gated sodium channels and the neurotoxin, tetrodotoxin. Channels 2008, 2, 407–412. [Google Scholar] [CrossRef]
- Brennan, J.A.; Chen, Q.; Gams, A.; Dyavanapalli, J.; Mendelowitz, D.; Peng, W.; Efimov, I.R. Evidence of superior and inferior sinoatrial nodes in the mammalian heart. JACC Clin. Electrophysiol. 2020, 6, 1827–1840. [Google Scholar] [CrossRef]
- Davoodi, M.; Segal, S.; Kirschner Peretz, N.; Kamoun, D.; Yaniv, Y. Semi-automated program for analysis of local Ca2+ spark release with application for classification of heart cell type. Cell Calcium 2017, 64, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kharche, S.; Yu, J.; Lei, M.; Zhang, H. A Mathematical Model of Action Potentials of Mouse Sinoatrial Node Cells with Molecular Bases. AJP Hear. Circ. Physiol. 2011, 301, H945–H963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurata, Y.; Hisatome, I.; Imanishi, S.; Shibamoto, T. Dynamical Description of Sinoatrial Node Pacemaking: Improved Mathematical Model for Primary Pacemaker Cell. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H2074–H2101. [Google Scholar] [CrossRef] [PubMed]
- Saucerman, J.J.; Brunton, L.L.; Michailova, A.P.; McCulloch, A.D. Modeling β-Adrenergic Control of Cardiac Myocyte Contractility in Silico. J. Biol. Chem. 2003, 278, 47997–48003. [Google Scholar] [CrossRef] [Green Version]
- Demir, S.S.; Clark, J.W.; Giles, W.R. Parasympathetic Modulation of Sinoatrial Node Pacemaker Activity in Rabbit Heart: A Unifying Model. Am. J. Physiol. Heart Circ. Physiol. 1999, 276, H2221–H2244. [Google Scholar] [CrossRef]
- Luo, C.; Rudy, Y. A Dynamic Model of the Cardiac Ventricular Action Potential. I. Simulations of Ionic Currents and Concentration Changes. Circ. Res. 1994, 74, 1071–1096. [Google Scholar] [CrossRef] [Green Version]
- Yaniv, Y.; Sivan, R.; Landesberg, A. Analysis of Hystereses in Force Length and Force Calcium Relations. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H389–H399. [Google Scholar] [CrossRef] [Green Version]
- Catanzaro, J.N.; Nett, M.P.; Rota, M.; Vassalle, M. On the Mechanisms Underlying Diastolic Voltage Oscillations in the Sinoatrial Node. J. Electrocardiol. 2006, 39, 342. [Google Scholar] [CrossRef]
- Yaniv, Y.; Ganesan, A.; Yang, D.; Ziman, B.D.; Lyashkov, A.E.; Levchenko, A.; Zhang, J.; Lakatta, E.G. Real-Time Relationship between PKA Biochemical Signal Network Dynamics and Increased Action Potential Firing Rate in Heart Pacemaker Cells: Kinetics of PKA Activation in Heart Pacemaker Cells. J. Mol. Cell. Cardiol. 2015, 86, 168–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behar, J.; Yaniv, Y. Age-Related Pacemaker Deterioration Is Due to Impaired Intracellular and Membrane Mechanisms: Insights from Numerical Modeling. J. Gen. Physiol. 2017, 149, 935–949. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arbel Ganon, L.; Davoodi, M.; Alexandrovich, A.; Yaniv, Y. Synergy between Membrane Currents Prevents Severe Bradycardia in Mouse Sinoatrial Node Tissue. Int. J. Mol. Sci. 2023, 24, 5786. https://doi.org/10.3390/ijms24065786
Arbel Ganon L, Davoodi M, Alexandrovich A, Yaniv Y. Synergy between Membrane Currents Prevents Severe Bradycardia in Mouse Sinoatrial Node Tissue. International Journal of Molecular Sciences. 2023; 24(6):5786. https://doi.org/10.3390/ijms24065786
Chicago/Turabian StyleArbel Ganon, Limor, Moran Davoodi, Alexandra Alexandrovich, and Yael Yaniv. 2023. "Synergy between Membrane Currents Prevents Severe Bradycardia in Mouse Sinoatrial Node Tissue" International Journal of Molecular Sciences 24, no. 6: 5786. https://doi.org/10.3390/ijms24065786




