Extracellular Vesicle-microRNAs as Diagnostic Biomarkers in Preterm Neonates
Abstract
:1. Introduction
2. Definition of an Effective Diagnostic Biomarker
3. EVs as Effective Diagnostic Biomarkers
Methods of EV Characterization and miRNA Extraction
4. EV-miRNAs in Necrotizing Enterocolitis
5. EV-miRNAs in Bronchopulmonary Dysplasia
6. EV-miRNAs in Hypoxic-Ischemic Brain Damage
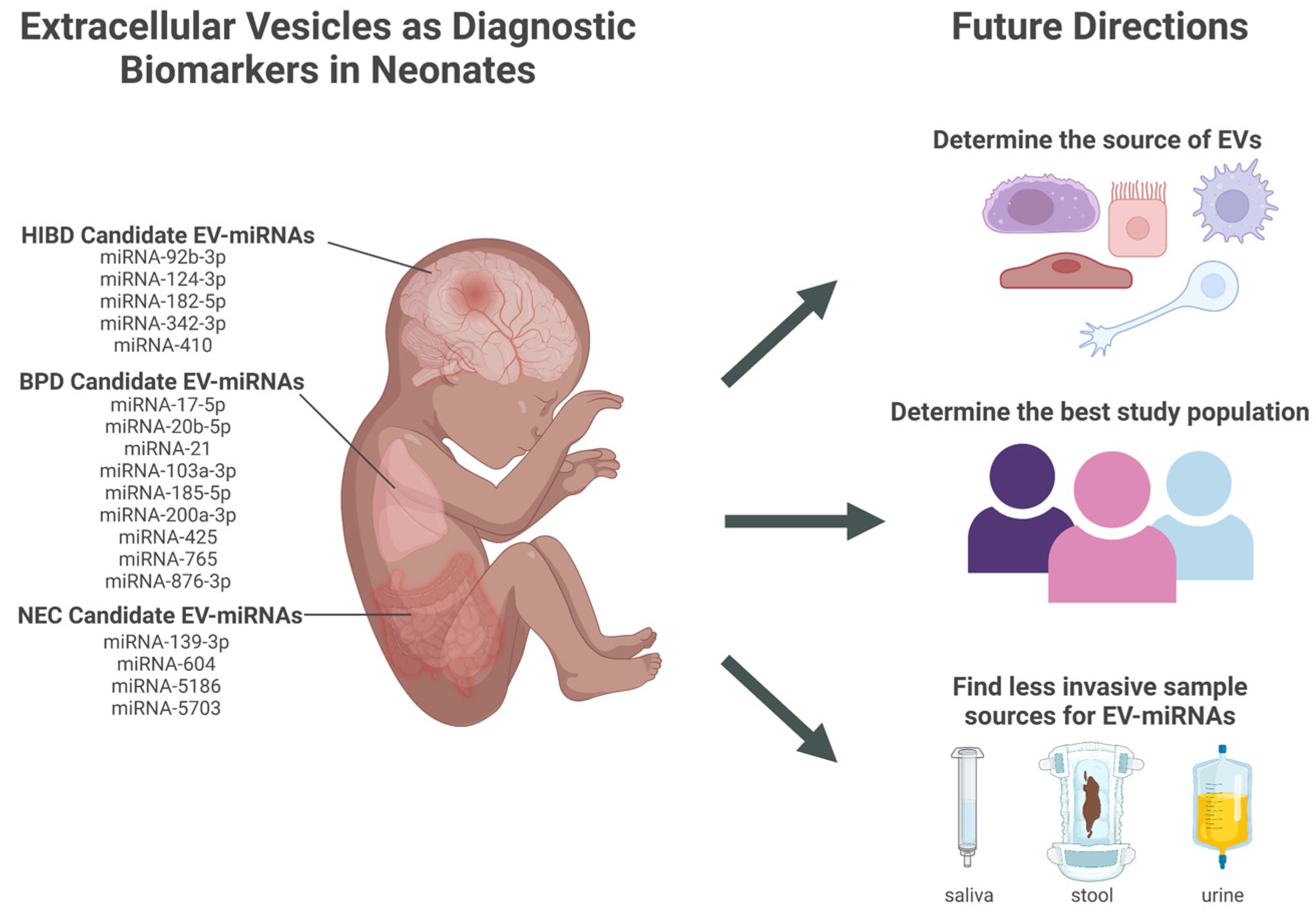
7. Next Steps in EV-miRNAs Biomarker Development in Premature Infants
7.1. Determine the Best Sample Source for Discovering Diagnostic Biomarkers from Neonatal EVs
7.2. Validation of EV-miRNAs as Reliable Diagnostic Biomarkers in the Premature Population
7.3. Determine the Feasibility of Implementing EV Diagnostic Testing: Testing Population and Role of EV Biomarkers in Clinical Decision Making
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheong, J.L.Y.; Doyle, L.W. An Update on Pulmonary and Neurodevelopmental Outcomes of Bronchopulmonary Dysplasia. Semin. Perinatol. 2018, 42, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Finder, M.; Boylan, G.B.; Twomey, D.; Ahearne, C.; Murray, D.M.; Hallberg, B. Two-Year Neurodevelopmental Outcomes After Mild Hypoxic Ischemic Encephalopathy in the Era of Therapeutic Hypothermia. Jama Pediatr. 2020, 174, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.H.; Hall, N.J. Contemporary Outcomes for Infants with Necrotizing Enterocolitis—A Systematic Review. J. Pediatr. 2020, 220, 86–92.e3. [Google Scholar] [CrossRef]
- Waitzman, N.J.; Jalali, A.; Grosse, S.D. Preterm Birth Lifetime Costs in the United States in 2016: An Update. Semin. Perinatol. 2021, 45, 151390. [Google Scholar] [CrossRef] [PubMed]
- Purdy, I.B.; Craig, J.W.; Zeanah, P. NICU Discharge Planning and beyond: Recommendations for Parent Psychosocial Support. J. Perinatol. 2015, 35, S24–S28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matei, A.C.; Antounians, L.; Zani, A. Extracellular Vesicles as a Potential Therapy for Neonatal Conditions: State of the Art and Challenges in Clinical Translation. Pharmaceutics 2019, 11, 404. [Google Scholar] [CrossRef] [Green Version]
- Murphy, C.A.; O’Reilly, D.P.; Neary, E.; EL-Khuffash, A.; NíAinle, F.; McCallion, N.; Maguire, P.B. A Review of the Role of Extracellular Vesicles in Neonatal Physiology and Pathology. Pediatr. Res. 2021, 90, 289–299. [Google Scholar] [CrossRef]
- FDA-NIH Biomarker Working Group. BEST (Biomarkers, EndpointS, and Other Tools) Resource. Available online: https://www.ncbi.nlm.nih.gov/books/NBK326791/ (accessed on 16 September 2022).
- Byrnes, S.A.; Weigl, B.H. Selecting Analytical Biomarkers for Diagnostic Applications: A First Principles Approach. Expert Rev. Mol. Diagn. 2017, 18, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Califf, R.M. Biomarker Definitions and Their Applications. Exp. Biol. Med. 2018, 243, 213–221. [Google Scholar] [CrossRef]
- Agakidou, E.; Agakidis, C.; Gika, H.; Sarafidis, K. Emerging Biomarkers for Prediction and Early Diagnosis of Necrotizing Enterocolitis in the Era of Metabolomics and Proteomics. Front. Pediatr. 2020, 8, 602255. [Google Scholar] [CrossRef]
- Yen, E.; Kaneko-Tarui, T.; Maron, J.L. Technical Considerations and Protocol Optimization for Neonatal Salivary Biomarker Discovery and Analysis. Front. Pediatr. 2021, 8, 618553. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Extracellular Vesicles Isolation and Their Biomarker Potential: Are We Ready for Testing? Ann. Transl. Med. 2017, 5, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyiadzis, M.; Whiteside, T.L. Information Transfer by Exosomes: A New Frontier in Hematologic Malignancies. Blood Rev. 2015, 29, 281–290. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA Delivery by Extracellular Vesicles in Mammalian Cells and Its Applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Raposo, G.; Stahl, P.D. Extracellular Vesicles: A New Communication Paradigm? Nat. Rev. Mol. Cell Biol. 2019, 20, 509–510. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Palmirotta, R.; Lovero, D.; Cafforio, P.; Felici, C.; Mannavola, F.; Pellè, E.; Quaresmini, D.; Tucci, M.; Silvestris, F. Liquid Biopsy of Cancer: A Multimodal Diagnostic Tool in Clinical Oncology. Ther. Adv. Med. Oncol. 2018, 10, 1758835918794630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurunathan, S.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on Factors Influences Biogenesis, Functions, Therapeutic and Clinical Implications of Exosomes. Int. J. Nanomed. 2021, 16, 1281–1312. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367. [Google Scholar] [CrossRef]
- Kamal, N.N.S.B.N.M.; Shahidan, W.N.S. Non-Exosomal and Exosomal Circulatory MicroRNAs: Which Are More Valid as Biomarkers? Front. Pharmacol. 2020, 10, 1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, S.; Bhowmik, S.; Majumdar, S.; Goswami, A.; Chakraborty, J.; Gupta, S.; Aggarwal, S.; Ray, S.; Chatterjee, R.; Bhattacharyya, S.; et al. The Exosome Encapsulated MicroRNAs as Circulating Diagnostic Marker for Hepatocellular Carcinoma with Low Alpha-fetoprotein. Int. J. Cancer 2020, 147, 2934–2947. [Google Scholar] [CrossRef] [PubMed]
- Admyre, C.; Johansson, S.M.; Qazi, K.R.; Filén, J.-J.; Lahesmaa, R.; Norman, M.; Neve, E.P.A.; Scheynius, A.; Gabrielsson, S. Exosomes with Immune Modulatory Features Are Present in Human Breast Milk. J. Immunol. 2007, 179, 1969–1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Sun, W.; Liu, C.; Na, Q. Umbilical Cord Blood-Derived Exosomes in Maternal–Fetal Disease: A Review. Reprod. Sci. 2022, 30, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.; Bajracharya, S.; Yuen, P.; Zhou, H.; Star, R.; Illei, G.; Alevizos, I. Exosomes from Human Saliva as a Source of MicroRNA Biomarkers. Oral. Dis. 2010, 16, 34–38. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.; Rupp, C.; Stoeck, A.; Runz, S.; Fogel, M.; Lugert, S.; Hager, H.-D.; Abdel-Bakky, M.S.; Gutwein, P.; Altevogt, P. CD24 Is a Marker of Exosomes Secreted into Urine and Amniotic Fluid. Kidney Int. 2007, 72, 1095–1102. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.; Patel, T.; Freedman, J.E. Circulating Extracellular Vesicles in Human Disease. New Engl. J. Med. 2018, 379, 2179–2181. [Google Scholar] [CrossRef]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef]
- Tkach, M.; Théry, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [Green Version]
- Linxweiler, J.; Junker, K. Extracellular Vesicles in Urological Malignancies: An Update. Nat. Rev. Urol. 2020, 17, 11–27. [Google Scholar] [CrossRef]
- Temoche-Diaz, M.M.; Shurtleff, M.J.; Nottingham, R.M.; Yao, J.; Fadadu, R.P.; Lambowitz, A.M.; Schekman, R. Distinct Mechanisms of MicroRNA Sorting into Cancer Cell-Derived Extracellular Vesicle Subtypes. Elife 2019, 8, e47544. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Li, C.; Zhang, Y.; Zhang, D.; Otterbein, L.E.; Jin, Y. Caveolin-1 Selectively Regulates MicroRNA Sorting into Microvesicles after Noxious Stimuli. J. Exp. Med. 2019, 216, 2202–2220. [Google Scholar] [CrossRef] [Green Version]
- Martins-Marques, T.; Costa, M.C.; Catarino, S.; Simoes, I.; Aasen, T.; Enguita, F.J.; Girao, H. Cx43-mediated Sorting of MiRNAs into Extracellular Vesicles. Embo Rep. 2022, 23, e54312. [Google Scholar] [CrossRef] [PubMed]
- Bachurski, D.; Schuldner, M.; Nguyen, P.-H.; Malz, A.; Reiners, K.S.; Grenzi, P.C.; Babatz, F.; Schauss, A.C.; Hansen, H.P.; Hallek, M.; et al. Extracellular Vesicle Measurements with Nanoparticle Tracking Analysis—An Accuracy and Repeatability Comparison between NanoSight NS300 and ZetaView. J. Extracell Vesicles 2019, 8, 1596016. [Google Scholar] [CrossRef]
- Tiozzo, C.; Bustoros, M.; Lin, X.; Mejia, C.M.D.; Gurzenda, E.; Chavez, M.; Hanna, I.; Aguiari, P.; Perin, L.; Hanna, N. Placental Extracellular Vesicles–Associated MicroRNA-519c Mediates Endotoxin Adaptation in Pregnancy. Am. J. Obstet. Gynecol. 2021, 225, 681.e1–681.e20. [Google Scholar] [CrossRef]
- Daaboul, G.G.; Gagni, P.; Benussi, L.; Bettotti, P.; Ciani, M.; Cretich, M.; Freedman, D.S.; Ghidoni, R.; Ozkumur, A.Y.; Piotto, C.; et al. Digital Detection of Exosomes by Interferometric Imaging. Sci. Rep. 2016, 6, 37246. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.; Araújo-Filho, I.; Piffoux, M.; Nicolás-Boluda, A.; Grangier, A.; Boucenna, I.; Real, C.C.; Marques, F.L.N.; Faria, D.d.P.; do Rego, A.C.M.; et al. Local Administration of Stem Cell-Derived Extracellular Vesicles in a Thermoresponsive Hydrogel Promotes a pro-Healing Effect in a Rat Model of Colo-Cutaneous Post-Surgical Fistula. Nanoscale 2020, 13, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Hardij, J.; Cecchet, F.; Berquand, A.; Gheldof, D.; Chatelain, C.; Mullier, F.; Chatelain, B.; Dogné, J. Characterisation of Tissue Factor-bearing Extracellular Vesicles with AFM: Comparison of Air-tapping-mode AFM and Liquid Peak Force AFM. J. Extracell Vesicles 2013, 2, 21045. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Kao, Y.; Zhou, Q.; Wuethrich, A.; Stark, M.S.; Schaider, H.; Soyer, H.P.; Lin, L.L.; Trau, M. An Integrated Microfluidic-SERS Platform Enables Sensitive Phenotyping of Serum Extracellular Vesicles in Early Stage Melanomas. Adv. Funct. Mater. 2022, 32, 2010296. [Google Scholar] [CrossRef]
- Zadka, Ł.; Buzalewicz, I.; Ulatowska-Jarża, A.; Rusak, A.; Kochel, M.; Ceremuga, I.; Dzięgiel, P. Label-Free Quantitative Phase Imaging Reveals Spatial Heterogeneity of Extracellular Vesicles in Select Colon Disorders. Am. J. Pathol. 2021, 191, 2147–2171. [Google Scholar] [CrossRef]
- Guo, Y.; Vickers, K.; Xiong, Y.; Zhao, S.; Sheng, Q.; Zhang, P.; Zhou, W.; Flynn, C.R. Comprehensive Evaluation of Extracellular Small RNA Isolation Methods from Serum in High Throughput Sequencing. Bmc Genom. 2017, 18, 50. [Google Scholar] [CrossRef] [Green Version]
- Kroh, E.M.; Parkin, R.K.; Mitchell, P.S.; Tewari, M. Analysis of Circulating MicroRNA Biomarkers in Plasma and Serum Using Quantitative Reverse Transcription-PCR (QRT-PCR). Methods 2010, 50, 298–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, W.; Su, X.; Gao, X.; Dai, Z.; Zou, X. A Label-Free and PCR-Free Electrochemical Assay for Multiplexed MicroRNA Profiles by Ligase Chain Reaction Coupling with Quantum Dots Barcodes. Biosens. Bioelectron. 2014, 53, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Pall, G.S.; Hamilton, A.J. Improved Northern Blot Method for Enhanced Detection of Small RNA. Nat. Protoc. 2008, 3, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Zöllner, H.; Hahn, S.A.; Maghnouj, A. Quantitative RT-PCR Specific for Precursor and Mature MiRNAs. Methods Mol. Biol. Clifton N. J. 2013, 1095, 121–134. [Google Scholar] [CrossRef]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-Time Quantification of MicroRNAs by Stem–Loop RT–PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Kappel, A.; Keller, A. MiRNA Assays in the Clinical Laboratory: Workflow, Detection Technologies and Automation Aspects. Clin. Chem. Lab. Med. Cclm 2017, 55, 636–647. [Google Scholar] [CrossRef]
- Shi, R.; Chiang, V.L. Facile Means for Quantifying MicroRNA Expression by Real-Time PCR. Biotechniques 2005, 39, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, S.D.; Carletti, M.Z.; Christenson, L.K. Quantitative RT-PCR Methods for Mature MicroRNA Expression Analysis. Methods Mol. Biology Clifton N. J. 2010, 630, 49–64. [Google Scholar] [CrossRef]
- Carrascosa, L.G.; Huertas, C.S.; Lechuga, L.M. Prospects of Optical Biosensors for Emerging Label-Free RNA Analysis. Trac. Trends Anal. Chem. 2016, 80, 177–189. [Google Scholar] [CrossRef]
- Giuliano, K.A.; Taylor, D.L. Fluorescent-Protein Biosensors: New Tools for Drug Discovery. Trends Biotechnol. 1998, 16, 135–140. [Google Scholar] [CrossRef]
- Sang, S.; Wang, Y.; Feng, Q.; Wei, Y.; Ji, J.; Zhang, W. Progress of New Label-Free Techniques for Biosensors: A Review. Crit. Rev. Biotechnol. 2016, 36, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A. Optical Biosensors in Drug Discovery. Nat. Rev. Drug Discov. 2002, 1, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive Optical Biosensors for Unlabeled Targets: A Review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Su, W.; Liu, S.; Huang, C.; Ghalandari, B.; Divsalar, A.; Ding, X. Recent Progresses in Electrochemical DNA Biosensors for MicroRNA Detection. Phenomics 2022, 2, 18–32. [Google Scholar] [CrossRef]
- Dave, V.P.; Ngo, T.A.; Pernestig, A.-K.; Tilevik, D.; Kant, K.; Nguyen, T.; Wolff, A.; Bang, D.D. MicroRNA Amplification and Detection Technologies: Opportunities and Challenges for Point of Care Diagnostics. Lab. Investig. 2019, 99, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Cacheux, J.; Bancaud, A.; Leichlé, T.; Cordelier, P. Technological Challenges and Future Issues for the Detection of Circulating MicroRNAs in Patients With Cancer. Front. Chem. 2019, 7, 815. [Google Scholar] [CrossRef] [Green Version]
- Lai, M.; Slaughter, G. Label-Free MicroRNA Optical Biosensors. Nanomaterials 2019, 9, 1573. [Google Scholar] [CrossRef] [Green Version]
- Denning, T.W.; Bhatia, A.M.; Kane, A.F.; Patel, R.M.; Denning, P.W. Pathogenesis of NEC: Role of the Innate and Adaptive Immune Response. Semin. Perinatol. 2017, 41, 15–28. [Google Scholar] [CrossRef] [Green Version]
- Hunter, C.J.; Plaen, I.G.D. Inflammatory Signaling in NEC: Role of NF-ΚB, Cytokines and Other Inflammatory Mediators. Pathophysiol 2014, 21, 55–65. [Google Scholar] [CrossRef]
- D’Angelo, G.; Impellizzeri, P.; Marseglia, L.; Montalto, A.S.; Russo, T.; Salamone, I.; Falsaperla, R.; Corsello, G.; Romeo, C.; Gitto, E. Current Status of Laboratory and Imaging Diagnosis of Neonatal Necrotizing Enterocolitis. Ital. J. Pediatr. 2018, 44, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunasekaran, A.; Devette, C.; Levin, S.; Chaaban, H. Biomarkers of Necrotizing Enterocolitis: The Search Continues. Clin. Perinatol. 2022, 49, 181–194. [Google Scholar] [CrossRef]
- Galley, J.D.; Mar, P.; Wang, Y.; Han, R.; Rajab, A.; Besner, G.E. Urine-Derived Extracellular Vesicle MiRNAs as Possible Biomarkers for and Mediators of Necrotizing Enterocolitis: A Proof of Concept Study. J. Pediatr. Surg. 2021, 56, 1966–1975. [Google Scholar] [CrossRef]
- Sannigrahi, M.K.; Sharma, R.; Singh, V.; Panda, N.K.; Rattan, V.; Khullar, M. Role of Host MiRNA Hsa-MiR-139-3p in HPV-16–Induced Carcinomas. Clin. Cancer Res. 2017, 23, 3884–3895. [Google Scholar] [CrossRef] [Green Version]
- Go, H.; Maeda, H.; Miyazaki, K.; Maeda, R.; Kume, Y.; Namba, F.; Momoi, N.; Hashimoto, K.; Otsuru, S.; Kawasaki, Y.; et al. Extracellular Vesicle MiRNA-21 Is a Potential Biomarker for Predicting Chronic Lung Disease in Premature Infants. Am J. Physiol.-lung. C 2020, 318, L845–L851. [Google Scholar] [CrossRef]
- Zhong, X.; Yan, Q.; Chen, Z.; Jia, C.; Li, X.; Liang, Z.; Gu, J.; Wei, H.; Lian, C.; Zheng, J.; et al. Umbilical Cord Blood-Derived Exosomes From Very Preterm Infants With Bronchopulmonary Dysplasia Impaired Endothelial Angiogenesis: Roles of Exosomal MicroRNAs. Front. Cell Dev. Biol. 2021, 9, 637248. [Google Scholar] [CrossRef] [PubMed]
- Lal, C.V.; Olave, N.; Travers, C.; Rezonzew, G.; Dolma, K.; Simpson, A.; Halloran, B.; Aghai, Z.; Das, P.; Sharma, N.; et al. Exosomal MicroRNA Predicts and Protects against Severe Bronchopulmonary Dysplasia in Extremely Premature Infants. Jci Insight 2018, 3, e93994. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, J.; Yuan, R.; Deng, Z.; Wu, X. Bone Marrow Mesenchymal Stem Cell-Derived Exosomes Alleviate Hyperoxia-Induced Lung Injury via the Manipulation of MicroRNA-425. Arch. Biochem. Biophys. 2021, 697, 108712. [Google Scholar] [CrossRef] [PubMed]
- Peeples, E.S.; Sahar, N.; Snyder, W.; Mirnics, K. Temporal Brain MicroRNA Expression Changes in a Mouse Model of Neonatal Hypoxic–Ischemic Injury. Pediatr. Res. 2022, 91, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Casey, S.; Goasdoue, K.; Miller, S.M.; Brennan, G.P.; Cowin, G.; O’Mahony, A.G.; Burke, C.; Hallberg, B.; Boylan, G.B.; Sullivan, A.M.; et al. Temporally Altered MiRNA Expression in a Piglet Model of Hypoxic Ischemic Brain Injury. Mol. Neurobiol. 2020, 57, 4322–4344. [Google Scholar] [CrossRef]
- Lawson, A.; Snyder, W.; Peeples, E.S. Intranasal Administration of Extracellular Vesicles Mitigates Apoptosis in a Mouse Model of Neonatal Hypoxic-Ischemic Brain Injury. Neonatology 2022, 119, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Yang, P.; Lu, Y. MicroRNA-410 Serves as a Candidate Biomarker in Hypoxic-ischemic Encephalopathy Newborns and Provides Neuroprotection in Oxygen-glucose Deprivation-injured PC12 and SH-SY5Y Cells. Brain Behav. 2021, 11, e2293. [Google Scholar] [CrossRef] [PubMed]
- Min, W.; Wu, Y.; Fang, Y.; Hong, B.; Dai, D.; Zhou, Y.; Liu, J.; Li, Q. Bone Marrow Mesenchymal Stem Cells-Derived Exosomal MicroRNA-124-3p Attenuates Hypoxic-Ischemic Brain Damage through Depressing Tumor Necrosis Factor Receptor Associated Factor 6 in Newborn Rats. Bioengineered 2022, 13, 3195–3207. [Google Scholar] [CrossRef]
- Thébaud, B.; Goss, K.N.; Laughon, M.; Whitsett, J.A.; Abman, S.H.; Steinhorn, R.H.; Aschner, J.L.; Davis, P.G.; McGrath-Morrow, S.A.; Soll, R.F.; et al. Bronchopulmonary Dysplasia. Nat. Rev. Dis. Prim. 2019, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.G.; Procianoy, R.S.; Neto, E.C.; Silveira, R.C. Preterm Neonates with Respiratory Distress Syndrome: Ventilator-Induced Lung Injury and Oxidative Stress. J. Immunol. Res. 2018, 2018, 6963754. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Zhao, J.; Tao, Y.; Guo, M.; Ya, Z.; Chen, C.; Qin, N.; Zheng, J.; Luo, J.; Xu, L. MicroRNA-21: A Promising Biomarker for the Prognosis and Diagnosis of Non-Small Cell Lung Cancer. Oncol. Lett. 2018, 16, 2777–2782. [Google Scholar] [CrossRef] [PubMed]
- Wei Wei Li, J.; Wei, L.; Han, Z.; Chen, Z. Mesenchymal Stromal Cells-Derived Exosomes Alleviate Ischemia/Reperfusion Injury in Mouse Lung by Transporting Anti-Apoptotic MiR-21-5p. Eur. J. Pharmacol. 2019, 852, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Genschmer, K.R.; Russell, D.W.; Lal, C.; Szul, T.; Bratcher, P.E.; Noerager, B.D.; Roda, M.A.; Xu, X.; Rezonzew, G.; Viera, L.; et al. Activated PMN Exosomes: Pathogenic Entities Causing Matrix Destruction and Disease in the Lung. Cell 2019, 176, 113–126.e15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Chen, C.; Zhang, X.; Weng, X.; Sheng, A.; Zhu, Y.; Chen, S.; Zheng, X.; Lu, C. High Neutrophil-to-Lymphocyte Ratio Is an Early Predictor of Bronchopulmonary Dysplasia. Front. Pediatr. 2019, 7, 464. [Google Scholar] [CrossRef] [PubMed]
- Douglas-Escobar, M.; Weiss, M.D. Hypoxic-Ischemic Encephalopathy: A Review for the Clinician. Jama Pediatr. 2015, 169, 397–403. [Google Scholar] [CrossRef]
- Bonifacio, S.L.; Chalak, L.F.; Meurs, K.P.V.; Laptook, A.R.; Shankaran, S. Neuroprotection for Hypoxic-Ischemic Encephalopathy: Contributions from the Neonatal Research Network. Semin. Perinatol. 2022, 46, 151639. [Google Scholar] [CrossRef] [PubMed]
- Sabir, H.; Bonifacio, S.L.; Gunn, A.J.; Thoresen, M.; Chalak, L.F.; Committee, N.B.S.G. and P. Unanswered Questions Regarding Therapeutic Hypothermia for Neonates with Neonatal Encephalopathy. Semin. Fetal Neonatal Med. 2021, 26, 101257. [Google Scholar] [CrossRef] [PubMed]
- Ponnusamy, V.; Yip, P.K. The Role of MicroRNAs in Newborn Brain Development and Hypoxic Ischaemic Encephalopathy. Neuropharmacology 2019, 149, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yang, S.; Hao, X.; Zhang, B.; Zhang, H.; Xin, C.; Hao, Y. Extracellular Vesicle-Derived MicroRNA-410 From Mesenchymal Stem Cells Protects Against Neonatal Hypoxia-Ischemia Brain Damage Through an HDAC1-Dependent EGR2/Bcl2 Axis. Front. Cell Dev. Biol. 2021, 8, 579236. [Google Scholar] [CrossRef] [PubMed]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef] [PubMed]
- Royo, F.; Théry, C.; Falcón-Pérez, J.M.; Nieuwland, R.; Witwer, K.W. Methods for Separation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey Performed by the ISEV Rigor and Standardization Subcommittee. Cells 2020, 9, 1955. [Google Scholar] [CrossRef]
- Shi, L.; Kuhnell, D.; Borra, V.J.; Langevin, S.M.; Nakamura, T.; Esfandiari, L. Rapid and Label-Free Isolation of Small Extracellular Vesicles from Biofluids Utilizing a Novel Insulator Based Dielectrophoretic Device. Lab. Chip. 2019, 19, 3726–3734. [Google Scholar] [CrossRef]
- Tkach, M.; Kowal, J.; Théry, C. Why the Need and How to Approach the Functional Diversity of Extracellular Vesicles. Philos. Trans. R. Soc B Biological Sci. 2018, 373, 20160479. [Google Scholar] [CrossRef]
- Hartjes, T.A.; Mytnyk, S.; Jenster, G.W.; van Steijn, V.; Royen, M.E. van Extracellular Vesicle Quantification and Characterization: Common Methods and Emerging Approaches. Bioengineering 2019, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Simonsen, J.B. What Are We Looking At? Extracellular Vesicles, Lipoproteins, or Both? Circ. Res. 2017, 121, 920–922. [Google Scholar] [CrossRef]
- Osteikoetxea, X.; Sódar, B.; Németh, A.; Szabó-Taylor, K.; Pálóczi, K.; Vukman, K.V.; Tamási, V.; Balogh, A.; Kittel, Á.; Pállinger, É.; et al. Differential Detergent Sensitivity of Extracellular Vesicle Subpopulations. Org. Biomol. Chem. 2015, 13, 9775–9782. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, X.; Deng, Y.; Yang, C. An Update on Isolation Methods for Proteomic Studies of Extracellular Vesicles in Biofluids. Molecules 2019, 24, 3516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- SZATANEK, R.; BARAN, J.; SIEDLAR, M.; BAJ-KRZYWORZEKA, M. Isolation of Extracellular Vesicles: Determining the Correct Approach (Review). Int. J. Mol. Med. 2015, 36, 11–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Northrop-Albrecht, E.J.; Taylor, W.R.; Huang, B.Q.; Kisiel, J.B.; Lucien, F. Assessment of Extracellular Vesicle Isolation Methods from Human Stool Supernatant. J. Extracell Vesicles 2022, 11, e12208. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.C.; Chan, K.Y.Y.; Lam, H.S.; Wong, R.P.O.; Ma, T.P.Y.; Sit, T.; Leung, K.T.; Chan, L.C.N.; Pang, Y.L.I.; Cheung, H.M.; et al. A Prospective Cohort Study of Fecal MiR-223 and MiR-451a as Noninvasive and Specific Biomarkers for Diagnosis of Necrotizing Enterocolitis in Preterm Infants. Neonatology 2021, 117, 555–561. [Google Scholar] [CrossRef]
- Turunen, J.; Tejesvi, M.V.; Suokas, M.; Virtanen, N.; Paalanne, N.; Kaisanlahti, A.; Reunanen, J.; Tapiainen, T. Bacterial Extracellular Vesicles in the Microbiome of First-Pass Meconium in Newborn Infants. Pediatr. Res. 2022, 1–10. [Google Scholar] [CrossRef]
- Erdbrügger, U.; Blijdorp, C.J.; Bijnsdorp, I.V.; Borràs, F.E.; Burger, D.; Bussolati, B.; Byrd, J.B.; Clayton, A.; Dear, J.W.; Falcón-Pérez, J.M.; et al. Urinary Extracellular Vesicles: A Position Paper by the Urine Task Force of the International Society for Extracellular Vesicles. J. Extracell Vesicles 2021, 10, e12093. [Google Scholar] [CrossRef]
- Barreiro, K.; Dwivedi, O.P.; Valkonen, S.; Groop, P.; Tuomi, T.; Holthofer, H.; Rannikko, A.; Yliperttula, M.; Siljander, P.; Laitinen, S.; et al. Urinary Extracellular Vesicles: Assessment of Pre-analytical Variables and Development of a Quality Control with Focus on Transcriptomic Biomarker Research. J. Extracell Vesicles 2021, 10, e12158. [Google Scholar] [CrossRef] [PubMed]
- Cheshmi, B.; Cheshomi, H. Salivary Exosomes: Properties, Medical Applications, and Isolation Methods. Mol. Biol. Rep. 2020, 47, 6295–6307. [Google Scholar] [CrossRef]
- Han, Y.; Jia, L.; Zheng, Y.; Li, W. Salivary Exosomes: Emerging Roles in Systemic Disease. Int. J. Biol. Sci. 2018, 14, 633–643. [Google Scholar] [CrossRef]
- Iyengar, A.; Maron, J.L. Detecting Infection in Neonates: Promises and Challenges of a Salivary Approach. Clin. Ther. 2015, 37, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Khanna, P.; Johnson, K.; Maron, J. Optimal Reference Genes for RT-QPCR Normalization in the Newborn. Biotech. Histochem. 2017, 92, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Cadamuro, J.; Ibarz, M.; Cornes, M.; Nybo, M.; Haschke-Becher, E.; von Meyer, A.; Lippi, G.; Simundic, A.-M. Managing Inappropriate Utilization of Laboratory Resources. Diagnosis 2019, 6, 5–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mrazek, C.; Simundic, A.-M.; Salinas, M.; von Meyer, A.; Cornes, M.; Bauçà, J.M.; Nybo, M.; Lippi, G.; Haschke-Becher, E.; Keppel, M.H.; et al. Inappropriate Use of Laboratory Tests: How Availability Triggers Demand—Examples across Europe. Clin. Chim. Acta 2020, 505, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Kiefer, D. A Translational View of Biomarkers in Preterm Labor. Am J Reprod Immunol 2012, 67, 268–272. [Google Scholar] [CrossRef]
- Singh, H.; Cho, S.J.; Gupta, S.; Kaur, R.; Sunidhi, S.; Saluja, S.; Pandey, A.K.; Bennett, M.V.; Lee, H.C.; Das, R.; et al. Designing a Bed-Side System for Predicting Length of Stay in a Neonatal Intensive Care Unit. Sci. Rep. 2021, 11, 3342. [Google Scholar] [CrossRef]
- Ding, L.; Wang, H.; Geng, H.; Cui, N.; Huang, F.; Zhu, X.; Zhu, X. Prediction of Bronchopulmonary Dysplasia in Preterm Infants Using Postnatal Risk Factors. Front. Pediatr. 2020, 8, 349. [Google Scholar] [CrossRef]
- Lure, A.C.; Du, X.; Black, E.W.; Irons, R.; Lemas, D.J.; Taylor, J.A.; Lavilla, O.; de la Cruz, D.; Neu, J. Using Machine Learning Analysis to Assist in Differentiating between Necrotizing Enterocolitis and Spontaneous Intestinal Perforation: A Novel Predictive Analytic Tool. J. Pediatr. Surg. 2021, 56, 1703–1710. [Google Scholar] [CrossRef]
- López-Martínez, F.; Núñez-Valdez, E.R.; Gomez, J.L.; García-Díaz, V. A Neural Network Approach to Predict Early Neonatal Sepsis. Comput. Electr. Eng. 2019, 76, 379–388. [Google Scholar] [CrossRef]
- Gehle, D.B.; Chapman, A.; Gregoski, M.; Brunswick, M.; Anderson, E.; Ramakrishnan, V.; Muhammad, L.N.; Head, W.; Lesher, A.P.; Ryan, R.M. A Predictive Model for Preterm Babies Born < 30 Weeks Gestational Age Who Will Not Attain Full Oral Feedings. J. Perinatol. 2022, 42, 126–131. [Google Scholar] [CrossRef] [PubMed]
| Condition | Study | Study Population (n) | EV Source | EV Isolation (I) & Analysis (A) | EV-miRNA Isolation | miRNA | Statistical Performance |
|---|---|---|---|---|---|---|---|
| NEC | [64] | Neonates < 34 weeks GA NEC (12) Age-matched healthy controls (22) | Urine | I: ExoUrine EV Isolation Kit (System Biosciences) A: NTA, western blot, TEM | ExoRNEasy Midi Kits & Qiagen Qiaquick small RNA Kit (Qiagen Inc.) | 139-3p 604 5186 5703 | p < 0.05 p < 0.05 p < 0.05 p < 0.05 |
| BPD | [66] | Neonates < 32 weeks GA, DOL 28 BPD (39) Non-BPD controls (34) Neonatal Mice Exposed to hyperoxia (4) Exposed to air (controls) (3) | Serum | I: ExoQuick precipitation solution (System Biosciences) A: NTA & ExoScreen, western blot | mirVana miRNA Isolation kit (Ambion Applied Biosystems) | 21 | p = 0.001 AUC = 0.850 p < 0.01 |
| [67] | Neonates < 32 weeks GA BPD (12) Non-BPD controls (14) | UC Serum | I: PEG precipitation A: NTA & ExoScreen, western blot, TEM | SeraMir Exosome RNA Purification Kit (System Biosciences) | 17-5p 20b-5p 103a-3p 185-5p 200a-3p 765 | p < 0.05 p < 0.05 p < 0.05 p < 0.05 p < 0.05 p < 0.05 | |
| [68] | Neonates 36 weeks PMA with BPD (25) GA-matched, FT controls, intubated for surgery (25) Neonates < 28 weeks GA BPD (15) Non-BPD controls (15) Neonatal Mice Exposed to hyperoxia (5-7) Exposed to air (controls) (5-7) | TA TA BALF | I: Ultracentrifugation A: NTA | miRCURY RNA Isolation Kit Cell and Plant with miRNA primers (Exiqon) | 876-3p | p = 0.001 p < 0.05 AUC = 0.917 p < 0.05 | |
| [69] | Neonatal Rats Exposed to hyperoxia (10) Exposed to air (controls) (10) | Lung Homogenate | I: Total exosome isolation reagent (Thermo Scientific) A: NTA, western blot, TEM | Trizol kits with miR primer (Beijing Dingguo Changsheng Biotechnology Co.) | 425 | p < 0.01 | |
| HIBD | [70] | Neonatal Mice, 24 hours post-surgery Unilateral carotid ligation + hypoxia (HIBD) (12) Sham surgery + normoxia (controls) (12) | Brain Homogenate | NA | NA | 182-5p * 342-3p * | p < 0.05 p < 0.05 |
| [71] | Neonates, FT Moderate to severe HIBD (7) Healthy control (7) | UC Serum | NA | NA | 92b-3p * 342-3p * | p = 0.016793 p = 0.00059 | |
| [72] | Neonatal Mice Hypoxia-preconditioned (3) | Brain homogenate | I: Ultracentrifugation and Sucrose Step Gradient A: NTA, western blot, electron microscopy | RNeasy Lipid Tissue Mini Kit (Qiagen) miRNA-Seq with NEXTFLEX small RNA kit (PerkinElmer) | 92b-3p 182-5p 342-3p | NA | |
| [73] | Neonates, FT HIBD (102) Healthy controls (60) | Serum | NA | NA | 410 * | p < 0.01 AUC = 0.886 | |
| [74] | Neonatal Rats Unilateral carotid ligation + hypoxia (HIBD) (12) Sham surgery + normoxia (controls) (12) | Brain homogenate | NA | NA | 124-3p * | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiller, E.A.; Cohen, K.; Lin, X.; El-Khawam, R.; Hanna, N. Extracellular Vesicle-microRNAs as Diagnostic Biomarkers in Preterm Neonates. Int. J. Mol. Sci. 2023, 24, 2622. https://doi.org/10.3390/ijms24032622
Schiller EA, Cohen K, Lin X, El-Khawam R, Hanna N. Extracellular Vesicle-microRNAs as Diagnostic Biomarkers in Preterm Neonates. International Journal of Molecular Sciences. 2023; 24(3):2622. https://doi.org/10.3390/ijms24032622
Chicago/Turabian StyleSchiller, Emily A., Koral Cohen, Xinhua Lin, Rania El-Khawam, and Nazeeh Hanna. 2023. "Extracellular Vesicle-microRNAs as Diagnostic Biomarkers in Preterm Neonates" International Journal of Molecular Sciences 24, no. 3: 2622. https://doi.org/10.3390/ijms24032622





