Dual-Specificity Phosphatases in Regulation of Tumor-Associated Macrophage Activity
Abstract
:1. Introduction
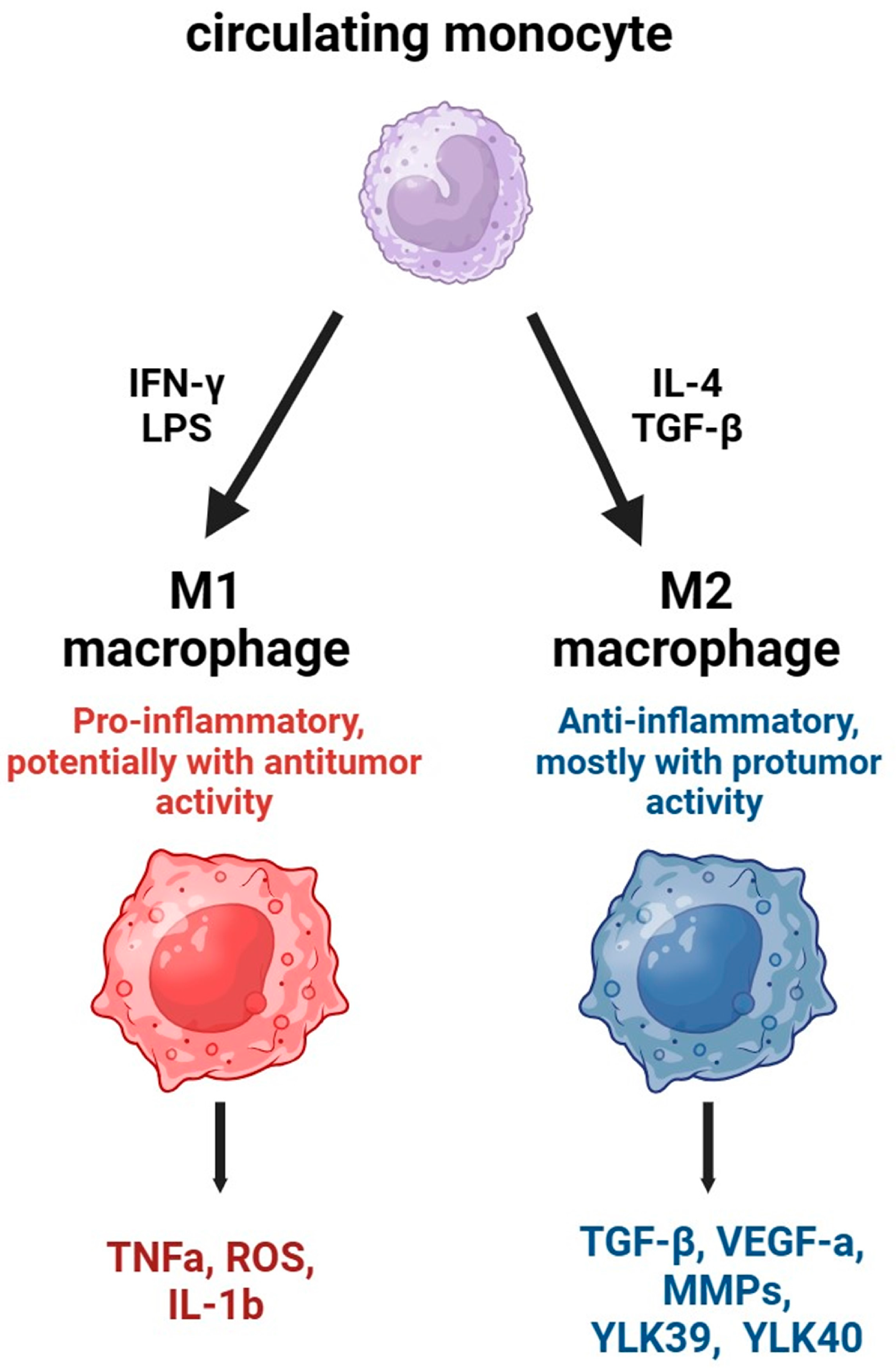
2. Tumor-Associated Macrophages and Its Precursors in Tumor Immunity
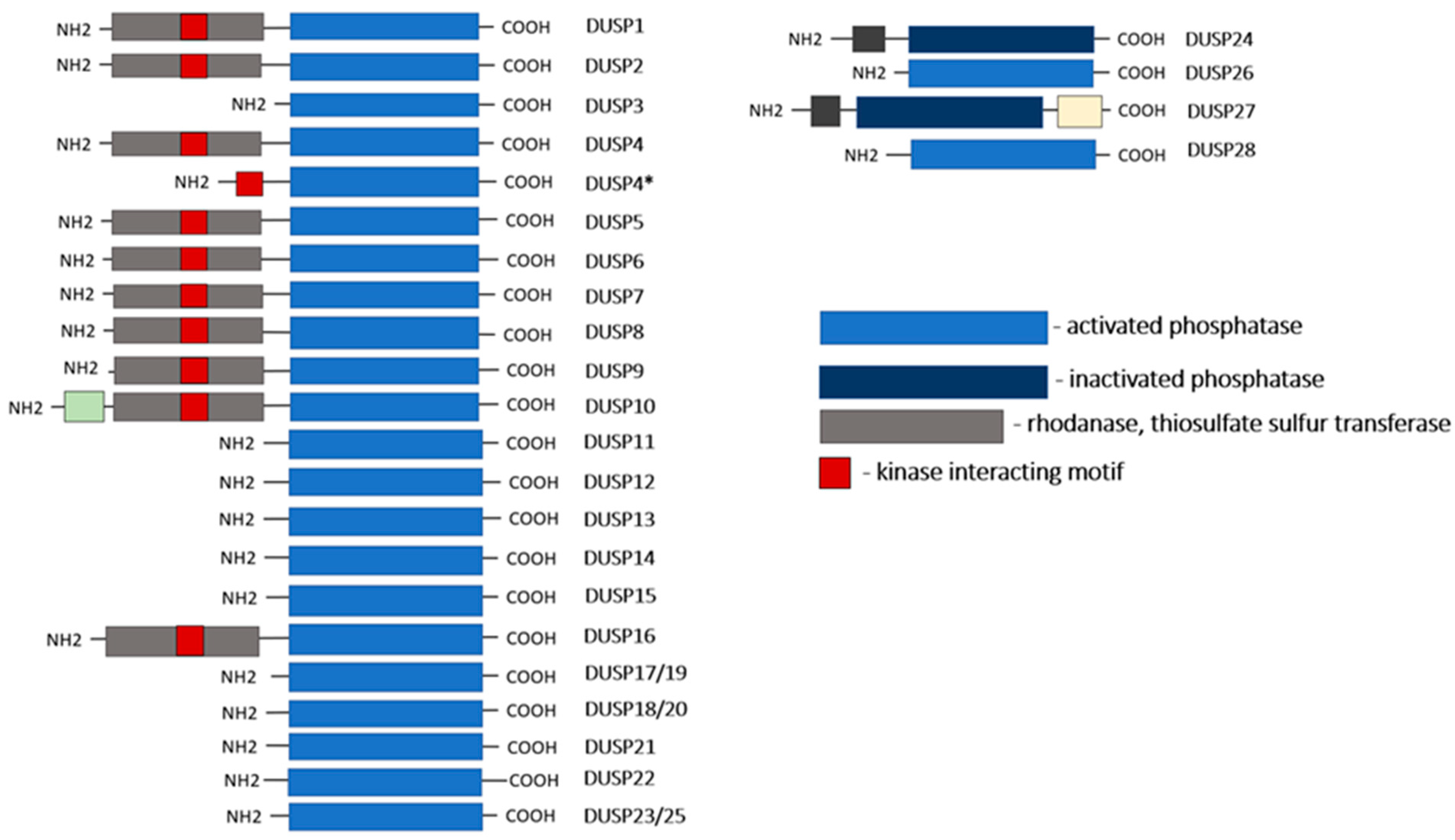
3. DUSPs: Structure and Function
4. DUSPs Interaction with Protein Kinases in Macrophage Signaling
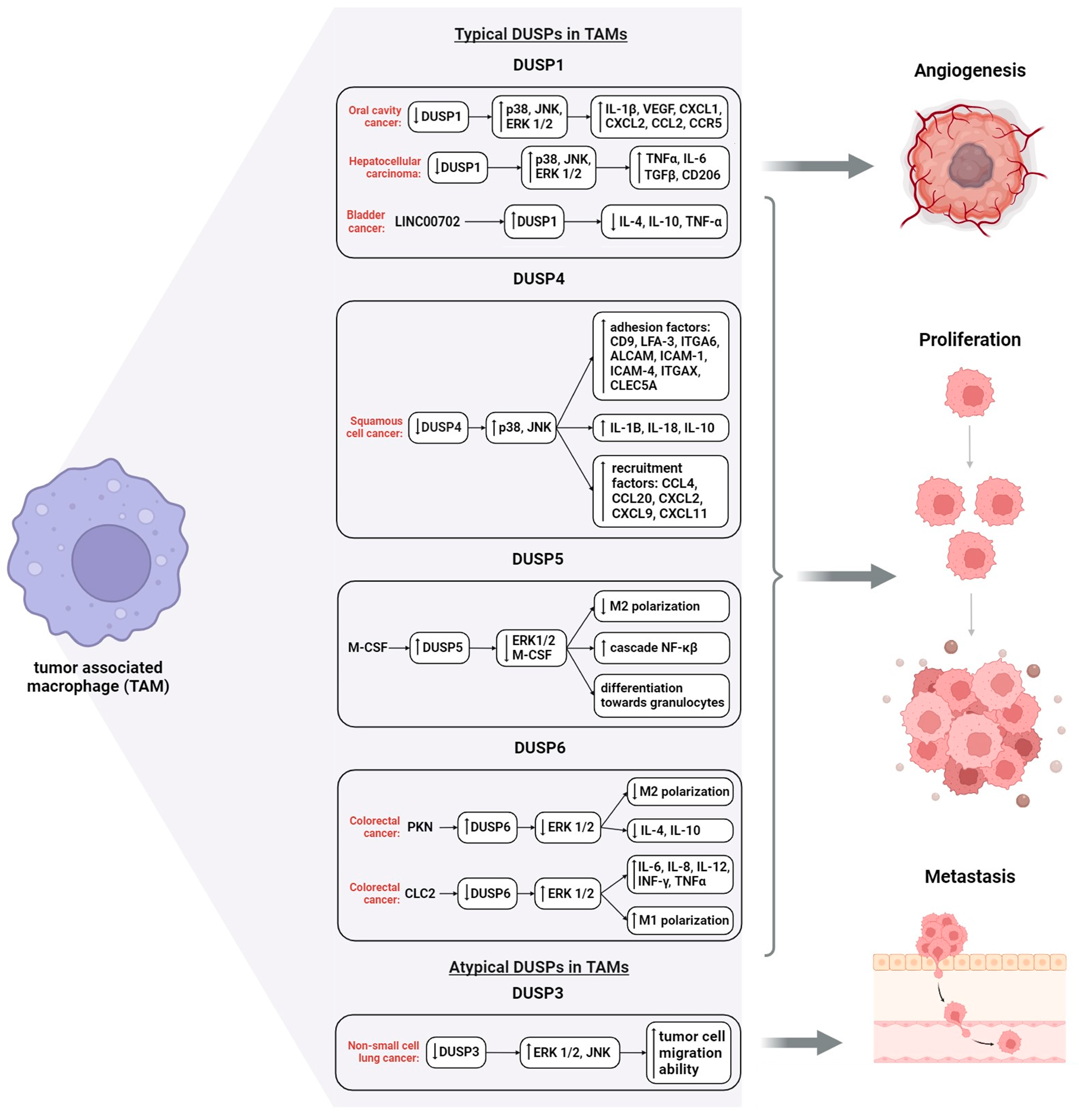
5. Regulation of TAM Functions by DUSPs
6. Epigenetic Regulation of DUSPs in Macrophages
- DNA Methylation
- Histone Code
- miRNA
7. Targeting DUSPs to TAM Therapeutic Modeling
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Akt | aktine |
| ALCAM | transmembrane glycoprotein CD166 |
| AP1 | activator protein 1 |
| ARG1 | arginase |
| ASK1 | apoptosis signal-regulating kinase 1 |
| ARG1 | arginase 1 |
| ATF2 | activating transcription factor |
| C3aR, C5aR | complement component 3a, 5a receptor 1 |
| CD9 | tetraspanin family protein |
| CHOP | C/EBP homologous protein |
| CLEC5A | C-type lectin domain family 5 member A |
| COX2 | COX-2 inhibitors |
| CREB | cAMP response elements beta |
| c-Src | Src cytoplasmic proto-oncogene-tyrosine-protein kinase |
| CXCL | chemokine (C-X-C motif) ligand |
| DLK | double leucine zipper kinase; MKK—MAPK kinases |
| DUSP | dual-specificity phosphatases |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| Elk1 | ETS Like-1 protein |
| ERK | extracellular signal-regulated kinase |
| FADD | Fas receptor death domain-interacting protein; MEKK, MEK kinase |
| FAK | focal adhesion kinase (proteintyrosine kinase) |
| FasL | ligand for Fas membrane molecule |
| FasR | receptor for Fas membrane molecule |
| FFA | saturated free fatty acid |
| GRB2 | protein with SH2 and SH3 domains |
| ICAM | intercellular adhesion molecule 1 |
| IGF1R | insulin-like growth factor receptor 1 |
| INFγ | interferon gamma |
| IRF1 | interferon regulatory factor 1 |
| ITGA6 | integrin alpha 6 |
| ITGAX | integrin, alpha X, CD11c |
| JNK | c-Jun N-terminal kinase |
| JNK | c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase |
| LITAF | lipopolysaccharide Induced TNF Factor |
| LFA3 | lymphocyte function-associated antigen 3 |
| LPS | lipopolysaccharides; GR, growth factors |
| MEK | MAPK/ERK kinase |
| MEF2 | myocyte Enhancer Factor 2 |
| mF | macrophages |
| MIP2 | major Intrinsic Protein 2 |
| MKP | mitogen-activated protein kinase phosphatase |
| MLK3 | mixed origin kinase 3, serine/threonine protein kinase |
| Mmd | monocyte to macrophage differentiation associated |
| MSK | mitogen and stress-activated protein kinases |
| NFAT | nuclear factor of activated T-cells |
| NO | nitric oxide |
| PGE2 | prostaglandin E2 |
| PKN2 | protein kinase N2 |
| Raf | protein belonging to the serine-threonine protein kinase family; |
| RAS | inactive membrane-bound small G protein; RAS-GTP, active RAS protein |
| ROS | reactive oxygen species |
| SOS | guanine nucleotide substitution factor |
| STAT | family of proteins that are intracellular transcription factors |
| SRF | serum Response Factor |
| TAB1 | mitogen-activated protein kinase, regulator of MAP3K7/TAK1 kinase kinase |
| TAK1 | transforming growth factor β-activated kinase 1, a member of the MAPK family |
| TGF-β | transforming growth factor beta |
| TNF-α | tumor necrosis factor α |
| TRAF6 | TNF receptor associated factor |
| TrkA | tyrosine kinase A |
| UV | ultraviolet radiation |
| VEGF | vascular endothelial growth factor |
References
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Cassetta, L.; Pollard, J.W. A timeline of tumour-associated macrophage biology. Nat. Rev. Cancer 2023, 23, 238–257. [Google Scholar] [CrossRef] [PubMed]
- Cortese, N.; Carriero, R.; Laghi, L.; Mantovani, A.; Marchesi, F. Prognostic significance of tumor-associated macrophages: Past, present and future. Semin. Immunol. 2020, 48, 101408. [Google Scholar] [CrossRef] [PubMed]
- Cherdyntseva, N.V.; Mitrofanova, I.V.; Buldakov, M.A.; Stakheeva, M.N.; Patysheva, M.R.; Zavjalova, M.V.; Kzhyshkowska, J.G. Macrophages and tumor progression: On the way to macrophage-specific therapy. Bull. Sib. Med. 2017, 16, 61–74. [Google Scholar] [CrossRef]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Larionova, I.; Kazakova, E.; Patysheva, M.; Kzhyshkowska, J. Transcriptional, Epigenetic and Metabolic Programming of Tumor-Associated Macrophages. Cancers 2020, 12, 1411. [Google Scholar] [CrossRef]
- Larionova, I.; Tuguzbaeva, G.; Ponomaryova, A.; Stakheyeva, M.; Cherdyntseva, N.; Pavlov, V.; Choinzonov, E.; Kzhyshkowska, J. Tumor-Associated Macrophages in Human Breast, Colorectal, Lung, Ovarian and Prostate Cancers. Front. Oncol. 2020, 10, 566511. [Google Scholar] [CrossRef]
- Patysheva, M.; Frolova, A.; Larionova, I.; Afanas’ev, S.; Tarasova, A.; Cherdyntseva, N.; Kzhyshkowska, J. Monocyte programming by cancer therapy. Front. Immunol. 2022, 13, 994319. [Google Scholar] [CrossRef]
- Sanchez, L.R.; Borriello, L.; Entenberg, D.; Condeelis, J.S.; Oktay, M.H.; Karagiannis, G.S. The emerging roles of macrophages in cancer metastasis and response to chemotherapy. J. Leukoc. Biol. 2019, 106, 259–274. [Google Scholar] [CrossRef]
- Beach, C.; MacLean, D.; Majorova, D.; Arnold, J.N.; Olcina, M.M. The effects of radiation therapy on the macrophage response in cancer. Front. Oncol. 2022, 12, 1020606. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Luo, Y. Targeting macrophages in cancer immunotherapy. Signal Transduct. Target. Ther. 2021, 6, 127. [Google Scholar] [CrossRef] [PubMed]
- Lloberas, J.; Valverde-Estrella, L.; Tur, J.; Vico, T.; Celada, A. Mitogen-Activated Protein Kinases and Mitogen Kinase Phosphatase 1: A Critical Interplay in Macrophage Biology. Front. Mol. Biosci. 2016, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Raffi, F.A.M. Dual-Specificity Phosphatases in Immunity and Infection: An Update. Int. J. Mol. Sci. 2019, 20, 2710. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.P.; Qi, X.W.; Sun, N.; Sun, Y.Y.; Zhang, Y.; Tan, X.N.; Ding, J.; Han, F.; Zhang, Y. The emerging roles of dual-specificity phosphatases and their specific characteristics in human cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188562. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.V.; Blagoev, B.; Gnad, F.; Macek, B.; Kumar, C.; Mortensen, P.; Mann, M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 2006, 127, 635–648. [Google Scholar] [CrossRef]
- Rao, K.M. MAP kinase activation in macrophages. J. Leukoc. Biol. 2001, 69, 3–10. [Google Scholar] [CrossRef]
- Neamatallah, T. Mitogen-Activated Protein Kinase Pathway: A Critical Regulator in Tumor-associated Macrophage Polarization. J. Microsc. Ultrastruct. 2019, 7, 53–56. [Google Scholar] [CrossRef]
- Hume, D.A.; Irvine, K.M.; Pridans, C. The Mononuclear Phagocyte System: The Relationship between Monocytes and Macrophages. Trends Immunol. 2019, 40, 98–112. [Google Scholar] [CrossRef]
- Ma, W.T.; Gao, F.; Gu, K.; Chen, D.K. The Role of Monocytes and Macrophages in Autoimmune Diseases: A Comprehensive Review. Front. Immunol. 2019, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
- Di Caro, G.; Cortese, N.; Castino, G.F.; Grizzi, F.; Gavazzi, F.; Ridolfi, C.; Capretti, G.; Mineri, R.; Todoric, J.; Zerbi, A.; et al. Dual prognostic significance of tumour-associated macrophages in human pancreatic adenocarcinoma treated or untreated with chemotherapy. Gut 2016, 65, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Saqib, U.; Sarkar, S.; Suk, K.; Mohammad, O.; Baig, M.S.; Savai, R. Phytochemicals as modulators of M1-M2 macrophages in inflammation. Oncotarget 2018, 9, 17937–17950. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.Q.; Waaijer, S.J.H.; Zwager, M.C.; de Vries, E.G.E.; van der Vegt, B.; Schröder, C.P. Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treat. Rev. 2018, 70, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Plüddemann, A. Tissue macrophages: Heterogeneity and functions. BMC Biol. 2017, 15, 53. [Google Scholar] [CrossRef]
- Ma, R.Y.; Black, A.; Qian, B.Z. Macrophage diversity in cancer revisited in the era of single-cell omics. Trends Immunol. 2022, 43, 546–563. [Google Scholar] [CrossRef]
- Esbona, K.; Yi, Y.; Saha, S.; Yu, M.; Van Doorn, R.R.; Conklin, M.W.; Graham, D.S.; Wisinski, K.B.; Ponik, S.M.; Eliceiri, K.W.; et al. The Presence of Cyclooxygenase 2, Tumor-Associated Macrophages, and Collagen Alignment as Prognostic Markers for Invasive Breast Carcinoma Patients. Am. J. Pathol. 2018, 188, 559–573. [Google Scholar] [CrossRef]
- Feng, Q.; Chang, W.; Mao, Y.; He, G.; Zheng, P.; Tang, W.; Wei, Y.; Ren, L.; Zhu, D.; Ji, M.; et al. Tumor-associated Macrophages as Prognostic and Predictive Biomarkers for Postoperative Adjuvant Chemotherapy in Patients with Stage II Colon Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2019, 25, 3896–3907. [Google Scholar] [CrossRef]
- Li, Z.; Maeda, D.; Yoshida, M.; Umakoshi, M.; Nanjo, H.; Shiraishi, K.; Saito, M.; Kohno, T.; Konno, H.; Saito, H.; et al. The intratumoral distribution influences the prognostic impact of CD68- and CD204-positive macrophages in non-small cell lung cancer. Lung Cancer 2018, 123, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Le Page, C.; Marineau, A.; Bonza, P.K.; Rahimi, K.; Cyr, L.; Labouba, I.; Madore, J.; Delvoye, N.; Mes-Masson, A.M.; Provencher, D.M.; et al. BTN3A2 expression in epithelial ovarian cancer is associated with higher tumor infiltrating T cells and a better prognosis. PLoS ONE 2012, 7, e38541. [Google Scholar] [CrossRef] [PubMed]
- Lundholm, M.; Hägglöf, C.; Wikberg, M.L.; Stattin, P.; Egevad, L.; Bergh, A.; Wikström, P.; Palmqvist, R.; Edin, S. Secreted Factors from Colorectal and Prostate Cancer Cells Skew the Immune Response in Opposite Directions. Sci. Rep. 2015, 5, 15651. [Google Scholar] [CrossRef] [PubMed]
- Tashireva, L.A.; Denisov, E.V.; Gerashchenko, T.S.; Pautova, D.N.; Buldakov, M.A.; Zavyalova, M.V.; Kzhyshkowska, J.; Cherdyntseva, N.V.; Perelmuter, V.M. Intratumoral heterogeneity of macrophages and fibroblasts in breast cancer is associated with the morphological diversity of tumor cells and contributes to lymph node metastasis. Immunobiology 2017, 222, 631–640. [Google Scholar] [CrossRef]
- Liu, T.; Larionova, I.; Litviakov, N.; Riabov, V.; Zavyalova, M.; Tsyganov, M.; Buldakov, M.; Song, B.; Moganti, K.; Kazantseva, P.; et al. Tumor-associated macrophages in human breast cancer produce new monocyte attracting and pro-angiogenic factor YKL-39 indicative for increased metastasis after neoadjuvant chemotherapy. Oncoimmunology 2018, 7, e1436922. [Google Scholar] [CrossRef]
- Laviron, M.; Boissonnas, A. Ontogeny of Tumor-Associated Macrophages. Front. Immunol. 2019, 10, 1799. [Google Scholar] [CrossRef]
- Franklin, R.A.; Liao, W.; Sarkar, A.; Kim, M.V.; Bivona, M.R.; Liu, K.; Pamer, E.G.; Li, M.O. The cellular and molecular origin of tumor-associated macrophages. Science 2014, 344, 921–925. [Google Scholar] [CrossRef]
- Castellheim, A.; Brekke, O.L.; Espevik, T.; Harboe, M.; Mollnes, T.E. Innate immune responses to danger signals in systemic inflammatory response syndrome and sepsis. Scand. J. Immunol. 2009, 69, 479–491. [Google Scholar] [CrossRef]
- Guilliams, M.; Mildner, A.; Yona, S. Developmental and Functional Heterogeneity of Monocytes. Immunity 2018, 49, 595–613. [Google Scholar] [CrossRef]
- Kiss, M.; Caro, A.A.; Raes, G.; Laoui, D. Systemic Reprogramming of Monocytes in Cancer. Front. Oncol. 2020, 10, 1399. [Google Scholar] [CrossRef]
- Cassetta, L.; Fragkogianni, S.; Sims, A.H.; Swierczak, A.; Forrester, L.M.; Zhang, H.; Soong, D.Y.H.; Cotechini, T.; Anur, P.; Lin, E.Y.; et al. Human Tumor-Associated Macrophage and Monocyte Transcriptional Landscapes Reveal Cancer-Specific Reprogramming, Biomarkers, and Therapeutic Targets. Cancer Cell 2019, 35, 588–602.e510. [Google Scholar] [CrossRef] [PubMed]
- Argyle, D.; Kitamura, T. Targeting Macrophage-Recruiting Chemokines as a Novel Therapeutic Strategy to Prevent the Progression of Solid Tumors. Front. Immunol. 2018, 9, 2629. [Google Scholar] [CrossRef] [PubMed]
- Tap, W.D.; Wainberg, Z.A.; Anthony, S.P.; Ibrahim, P.N.; Zhang, C.; Healey, J.H.; Chmielowski, B.; Staddon, A.P.; Cohn, A.L.; Shapiro, G.I.; et al. Structure-Guided Blockade of CSF1R Kinase in Tenosynovial Giant-Cell Tumor. N. Engl. J. Med. 2015, 373, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Lopez, L.; Kong, Y.W.; Sriram, G.; Patterson, J.C.; Rosenberg, S.; Morandell, S.; Haigis, K.M.; Yaffe, M.B. MAPKAP Kinase-2 Drives Expression of Angiogenic Factors by Tumor-Associated Macrophages in a Model of Inflammation-Induced Colon Cancer. Front. Immunol. 2020, 11, 607891. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, F.Y.; Kirkwood, K.L. The p38/MKP-1 signaling axis in oral cancer: Impact of tumor-associated macrophages. Oral Oncol. 2020, 103, 104591. [Google Scholar] [CrossRef] [PubMed]
- Baumann, D.; Drebant, J.; Hägele, T.; Burger, L.; Serger, C.; Lauenstein, C.; Dudys, P.; Erdmann, G.; Offringa, R. p38 MAPK signaling in M1 macrophages results in selective elimination of M2 macrophages by MEK inhibition. J. Immunother. Cancer 2021, 9, e002319. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB signaling in inflammation and cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef]
- Roux, P.P.; Blenis, J. ERK and p38 MAPK-activated protein kinases: A family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef]
- Kim, E.K.; Choi, E.J. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef]
- Subbannayya, Y.; Pinto, S.M.; Bösl, K.; Prasad, T.S.K.; Kandasamy, R.K. Dynamics of Dual Specificity Phosphatases and Their Interplay with Protein Kinases in Immune Signaling. Int. J. Mol. Sci. 2019, 20, 2086. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.; Hammer, M.; Mages, J. DUSP meet immunology: Dual specificity MAPK phosphatases in control of the inflammatory response. J. Immunol. 2006, 177, 7497–7504. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, K.; Nishida, E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim. Biophys. Acta-Mol. Cell Res. 2007, 1773, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Haagenson, K.K.; Wu, G.S. Mitogen activated protein kinase phosphatases and cancer. Cancer Biol. Ther. 2010, 9, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, J.; Barnes, J.; Kokkonen, G.C.; Lee, J.C.; Liu, Y. Restraint of proinflammatory cytokine biosynthesis by mitogen-activated protein kinase phosphatase-1 in lipopolysaccharide-stimulated macrophages. J. Immunol. 2002, 169, 6408–6416. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Shepherd, E.G.; Manson, M.E.; Nelin, L.D.; Sorokin, A.; Liu, Y. The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar macrophages to lipopolysaccharide: Attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. J. Biol. Chem. 2005, 280, 8101–8108. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.; Huotari, N.; Hömmö, T.; Leppänen, T.; Moilanen, E. The expression of interleukin-12 is increased by MAP kinase phosphatase-1 through a mechanism related to interferon regulatory factor 1. Mol. Immunol. 2012, 51, 219–226. [Google Scholar] [CrossRef]
- Abraham, S.M.; Lawrence, T.; Kleiman, A.; Warden, P.; Medghalchi, M.; Tuckermann, J.; Saklatvala, J.; Clark, A.R. Antiinflammatory effects of dexamethasone are partly dependent on induction of dual specificity phosphatase 1. J. Exp. Med. 2006, 203, 1883–1889. [Google Scholar] [CrossRef]
- Hammer, M.; Mages, J.; Dietrich, H.; Schmitz, F.; Striebel, F.; Murray, P.J.; Wagner, H.; Lang, R. Control of dual-specificity phosphatase-1 expression in activated macrophages by IL-10. Eur. J. Immunol. 2005, 35, 2991–3001. [Google Scholar] [CrossRef]
- Shepherd, E.G.; Zhao, Q.; Welty, S.E.; Hansen, T.N.; Smith, C.V.; Liu, Y. The function of mitogen-activated protein kinase phosphatase-1 in peptidoglycan-stimulated macrophages. J. Biol. Chem. 2004, 279, 54023–54031. [Google Scholar] [CrossRef] [PubMed]
- Salojin, K.V.; Owusu, I.B.; Millerchip, K.A.; Potter, M.; Platt, K.A.; Oravecz, T. Essential role of MAPK phosphatase-1 in the negative control of innate immune responses. J. Immunol. 2006, 176, 1899–1907. [Google Scholar] [CrossRef] [PubMed]
- Wong, H.R.; Dunsmore, K.E.; Page, K.; Shanley, T.P. Heat shock-mediated regulation of MKP-1. Am. J. Physiol. Cell Physiol. 2005, 289, C1152–C1158. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, K.L.; Brummer, T.; Rolph, M.S.; Liu, S.M.; Callejas, N.A.; Grumont, R.J.; Gillieron, C.; Mackay, F.; Grey, S.; Camps, M.; et al. Positive regulation of immune cell function and inflammatory responses by phosphatase PAC-1. Nat. Immunol. 2006, 7, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Neamatallah, T.; Jabbar, S.; Tate, R.; Schroeder, J.; Shweash, M.; Alexander, J.; Plevin, R. Whole Genome Microarray Analysis of DUSP4-Deletion Reveals A Novel Role for MAP Kinase Phosphatase-2 (MKP-2) in Macrophage Gene Expression and Function. Int. J. Mol. Sci. 2019, 20, 3434. [Google Scholar] [CrossRef] [PubMed]
- Al-Mutairi, M.S.; Cadalbert, L.C.; McGachy, H.A.; Shweash, M.; Schroeder, J.; Kurnik, M.; Sloss, C.M.; Bryant, C.E.; Alexander, J.; Plevin, R. MAP kinase phosphatase-2 plays a critical role in response to infection by Leishmania mexicana. PLoS Pathog. 2010, 6, e1001192. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Tang, P.; Zhang, Y. MAP kinase phosphatase 2 regulates macrophage-adipocyte interaction. PLoS ONE 2015, 10, e0120755. [Google Scholar] [CrossRef]
- Seo, H.; Cho, Y.C.; Ju, A.; Lee, S.; Park, B.C.; Park, S.G.; Kim, J.H.; Kim, K.; Cho, S. Dual-specificity phosphatase 5 acts as an anti-inflammatory regulator by inhibiting the ERK and NF-κB signaling pathways. Sci. Rep. 2017, 7, 17348. [Google Scholar] [CrossRef]
- Grasset, M.F.; Gobert-Gosse, S.; Mouchiroud, G.; Bourette, R.P. Macrophage differentiation of myeloid progenitor cells in response to M-CSF is regulated by the dual-specificity phosphatase DUSP5. J. Leukoc. Biol. 2010, 87, 127–135. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhu, Y.; Xu, J.; Yang, M.; Chen, P.; Xu, W.; Zhao, J.; Geng, L.; Gong, S. PKN2 in colon cancer cells inhibits M2 phenotype polarization of tumor-associated macrophages via regulating DUSP6-Erk1/2 pathway. Mol. Cancer 2018, 17, 13. [Google Scholar] [CrossRef]
- Carson, W.F.t.; Salter-Green, S.E.; Scola, M.M.; Joshi, A.; Gallagher, K.A.; Kunkel, S.L. Enhancement of macrophage inflammatory responses by CCL2 is correlated with increased miR-9 expression and downregulation of the ERK1/2 phosphatase Dusp6. Cell. Immunol. 2017, 314, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Nyunoya, T.; Monick, M.M.; Powers, L.S.; Yarovinsky, T.O.; Hunninghake, G.W. Macrophages survive hyperoxia via prolonged ERK activation due to phosphatase down-regulation. J. Biol. Chem. 2005, 280, 26295–26302. [Google Scholar] [CrossRef] [PubMed]
- Matsuguchi, T.; Musikacharoen, T.; Johnson, T.R.; Kraft, A.S.; Yoshikai, Y. A novel mitogen-activated protein kinase phosphatase is an important negative regulator of lipopolysaccharide-mediated c-Jun N-terminal kinase activation in mouse macrophage cell lines. Mol. Cell. Biol. 2001, 21, 6999–7009. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-B.; Tang, C.-X.; He, L.; Cheng, W.; Jiang, P.; Wang, X.-Y. DUSP8 inhibits LPS-induced acute lung injury by regulating macrophage response. Life Res. 2021, 4, 23. [Google Scholar] [CrossRef]
- Gao, L.; Zeng, H.; Zhang, T.; Mao, C.; Wang, Y.; Han, Z.; Chen, K.; Zhang, J.; Fan, Y.; Gu, J.; et al. MicroRNA-21 deficiency attenuated atherogenesis and decreased macrophage infiltration by targeting Dusp-8. Atherosclerosis 2019, 291, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, Z.; Luo, Y.; Yang, H.; Yang, M. DUSP9 alleviates hepatic ischemia/reperfusion injury by restraining both mitogen-activated protein kinase and IKK in an apoptosis signal-regulating kinase 1-dependent manner. Acta Biochim. Biophys. Sin. 2022, 54, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Blattman, J.N.; Kennedy, N.J.; Duong, J.; Nguyen, T.; Wang, Y.; Davis, R.J.; Greenberg, P.D.; Flavell, R.A.; Dong, C. Regulation of innate and adaptive immune responses by MAP kinase phosphatase 5. Nature 2004, 430, 793–797. [Google Scholar] [CrossRef]
- Qian, F.; Deng, J.; Gantner, B.N.; Flavell, R.A.; Dong, C.; Christman, J.W.; Ye, R.D. Map kinase phosphatase 5 protects against sepsis-induced acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 302, L866–L874. [Google Scholar] [CrossRef]
- Hömmö, T.; Pesu, M.; Moilanen, E.; Korhonen, R. Regulation of Inflammatory Cytokine Production by MKP-5 in Macrophages. Basic Clin. Pharmacol. Toxicol. 2015, 117, 96–104. [Google Scholar] [CrossRef]
- Niedzielska, M.; Bodendorfer, B.; Münch, S.; Eichner, A.; Derigs, M.; da Costa, O.; Schweizer, A.; Neff, F.; Nitschke, L.; Sparwasser, T.; et al. Gene trap mice reveal an essential function of dual specificity phosphatase Dusp16/MKP-7 in perinatal survival and regulation of Toll-like receptor (TLR)-induced cytokine production. J. Biol. Chem. 2014, 289, 2112–2126. [Google Scholar] [CrossRef]
- Singh, P.; Dejager, L.; Amand, M.; Theatre, E.; Vandereyken, M.; Zurashvili, T.; Singh, M.; Mack, M.; Timmermans, S.; Musumeci, L.; et al. DUSP3 Genetic Deletion Confers M2-like Macrophage-Dependent Tolerance to Septic Shock. J. Immunol. 2015, 194, 4951–4962. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Chuang, H.C.; Tsai, C.Y.; Xiao, Y.Z.; Yang, J.Y.; Huang, R.H.; Shih, Y.C.; Tan, T.H. DUSP11 Attenuates Lipopolysaccharide-Induced Macrophage Activation by Targeting TAK1. J. Immunol. 2020, 205, 1644–1652. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.M.; Kincaid, R.P.; Nottingham, R.M.; Lambowitz, A.M.; Sullivan, C.S. DUSP11 activity on triphosphorylated transcripts promotes Argonaute association with noncanonical viral microRNAs and regulates steady-state levels of cellular noncoding RNAs. Genes Dev. 2016, 30, 2076–2092. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.S.L.; Han, J.; James, S.J.; Png, C.W.; Weerasooriya, M.; Alonso, S.; Zhang, Y. Dual-Specificity Phosphatase 12 Targets p38 MAP Kinase to Regulate Macrophage Response to Intracellular Bacterial Infection. Front. Immunol. 2017, 8, 1259. [Google Scholar] [CrossRef] [PubMed]
- Huiyun Seo, S.C. Dual-Specificity Protein Phosphatase 26 (DUSP26) Inhibits LPS-Induced TNF-α Production. Bull. Korean Chem. Soc. 2010, 31, 2692–2694. [Google Scholar] [CrossRef]
- Seternes, O.M.; Kidger, A.M.; Keyse, S.M. Dual-specificity MAP kinase phosphatases in health and disease. Biochim. Biophys. Acta-Mol. Cell Res. 2019, 1866, 124–143. [Google Scholar] [CrossRef]
- Huang, C.Y.; Tan, T.H. DUSPs, to MAP kinases and beyond. Cell Biosci. 2012, 2, 24. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, Y.; Yu, H.; Shen, B.; Liang, Y.; Jin, R.; Liu, X.; Shi, L.; Cai, X. Role of DUSP1/MKP1 in tumorigenesis, tumor progression and therapy. Cancer Med. 2016, 5, 2061–2068. [Google Scholar] [CrossRef]
- Abraham, S.M.; Clark, A.R. Dual-specificity phosphatase 1: A critical regulator of innate immune responses. Biochem. Soc. Trans. 2006, 34, 1018–1023. [Google Scholar] [CrossRef]
- Wang, J.; Ford, H.R.; Grishin, A.V. NF-κB-mediated expression of MAPK phosphatase-1 is an early step in desensitization to TLR ligands in enterocytes. Mucosal Immunol. 2010, 3, 523–534. [Google Scholar] [CrossRef]
- Xu, Y.R.; Lei, C.Q. TAK1-TABs Complex: A Central Signalosome in Inflammatory Responses. Front. Immunol. 2020, 11, 608976. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.F.; Chuang, H.C.; Tan, T.H. Regulation of Dual-Specificity Phosphatase (DUSP) Ubiquitination and Protein Stability. Int. J. Mol. Sci. 2019, 20, 2668. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, E.; Taketomi, A.; Gion, T.; Kuroda, Y.; Endo, K.; Watanabe, A.; Nakashima, H.; Aishima, S.; Kohnoe, S.; Maehara, Y. Suppressed MKP-1 is an independent predictor of outcome in patients with hepatocellular carcinoma. Oncology 2005, 69, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Manzano, R.G.; Martinez-Navarro, E.M.; Forteza, J.; Brugarolas, A. Microarray phosphatome profiling of breast cancer patients unveils a complex phosphatase regulatory role of the MAPK and PI3K pathways in estrogen receptor-negative breast cancers. Int. J. Oncol. 2014, 45, 2250–2266. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Jang, S.M.; Ahn, H.; Sim, J.; Yi, K.; Chung, Y.; Han, H.; Rehman, A.; Chung, M.S.; Jang, K.; et al. Clinicopathological significance of dual-specificity protein phosphatase 4 expression in invasive ductal carcinoma of the breast. J. Breast Cancer 2015, 18, 1–7. [Google Scholar] [CrossRef]
- Russo, L.C.; Farias, J.O.; Ferruzo, P.Y.M.; Monteiro, L.F.; Forti, F.L. Revisiting the roles of VHR/DUSP3 phosphatase in human diseases. Clinics 2018, 73, e466s. [Google Scholar] [CrossRef]
- Karakashev, S.V.; Reginato, M.J. Hypoxia/HIF1α induces lapatinib resistance in ERBB2-positive breast cancer cells via regulation of DUSP2. Oncotarget 2015, 6, 1967–1980. [Google Scholar] [CrossRef]
- Dong, W.; Li, N.; Pei, X.; Wu, X. Differential expression of DUSP2 in left- and right-sided colon cancer is associated with poor prognosis in colorectal cancer. Oncol. Lett. 2018, 15, 4207–4214. [Google Scholar] [CrossRef]
- Saigusa, S.; Inoue, Y.; Tanaka, K.; Toiyama, Y.; Okugawa, Y.; Shimura, T.; Hiro, J.; Uchida, K.; Mohri, Y.; Kusunoki, M. Decreased expression of DUSP4 is associated with liver and lung metastases in colorectal cancer. Med. Oncol. 2013, 30, 620. [Google Scholar] [CrossRef]
- Yan, X.; Liu, L.; Li, H.; Huang, L.; Yin, M.; Pan, C.; Qin, H.; Jin, Z. Dual specificity phosphatase 5 is a novel prognostic indicator for patients with advanced colorectal cancer. Am. J. Cancer Res. 2016, 6, 2323–2333. [Google Scholar]
- Yu, D.; Li, Z.; Gan, M.; Zhang, H.; Yin, X.; Tang, S.; Wan, L.; Tian, Y.; Zhang, S.; Zhu, Y.; et al. Decreased expression of dual specificity phosphatase 22 in colorectal cancer and its potential prognostic relevance for stage IV CRC patients. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2015, 36, 8531–8535. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Chen, J.Y.; Han, Z.D.; He, H.C.; Chen, J.H.; Chen, Y.R.; Yang, S.B.; Wu, Y.D.; Zeng, Y.R.; Zou, J.; et al. Down-regulation of dual-specificity phosphatase 5 predicts poor prognosis of patients with prostate cancer. Int. J. Clin. Exp. Med. 2015, 8, 4186–4194. [Google Scholar] [PubMed]
- Lin, H.P.; Ho, H.M.; Chang, C.W.; Yeh, S.D.; Su, Y.W.; Tan, T.H.; Lin, W.J. DUSP22 suppresses prostate cancer proliferation by targeting the EGFR-AR axis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 14653–14667. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Green, J.A.; Wong, H.; VanderBurg, M.E.; Crook, T. DUSP7 and DUSP8 promoter hypermethylations: Predictors of clinical outcomes in advanced epithelial ovarian carcinoma. J. Clin. Oncol. 2007, 25, 5501. [Google Scholar] [CrossRef]
- Liu, W.; Tian, X.; Ding, X.; Zhang, L. Expression of Dual-Specificity Phosphatase 2 (DUSP2) in Patients with Serous Ovarian Carcinoma and in SKOV3 and OVCAR3 Cells In Vitro. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 10180–10189. [Google Scholar] [CrossRef]
- Chen, X.J.; Wu, S.; Yan, R.M.; Fan, L.S.; Yu, L.; Zhang, Y.M.; Wei, W.F.; Zhou, C.F.; Wu, X.G.; Zhong, M.; et al. The role of the hypoxia-Nrp-1 axis in the activation of M2-like tumor-associated macrophages in the tumor microenvironment of cervical cancer. Mol. Carcinog. 2019, 58, 388–397. [Google Scholar] [CrossRef]
- Wang, H.Y.; Cheng, Z.; Malbon, C.C. Overexpression of mitogen-activated protein kinase phosphatases MKP1, MKP2 in human breast cancer. Cancer Lett. 2003, 191, 229–237. [Google Scholar] [CrossRef]
- Liu, F.; Gore, A.J.; Wilson, J.L.; Korc, M. DUSP1 is a novel target for enhancing pancreatic cancer cell sensitivity to gemcitabine. PLoS ONE 2014, 9, e84982. [Google Scholar] [CrossRef]
- Kim, H.S.; Ullevig, S.L.; Zamora, D.; Lee, C.F.; Asmis, R. Redox regulation of MAPK phosphatase 1 controls monocyte migration and macrophage recruitment. Proc. Natl. Acad. Sci. USA 2012, 109, E2803–E2812. [Google Scholar] [CrossRef]
- Zhang, X.; Hyer, J.M.; Yu, H.; D’Silva, N.J.; Kirkwood, K.L. DUSP1 phosphatase regulates the proinflammatory milieu in head and neck squamous cell carcinoma. Cancer Res. 2014, 74, 7191–7197. [Google Scholar] [CrossRef]
- Lin, S.C.; Chien, C.W.; Lee, J.C.; Yeh, Y.C.; Hsu, K.F.; Lai, Y.Y.; Lin, S.C.; Tsai, S.J. Suppression of dual-specificity phosphatase-2 by hypoxia increases chemoresistance and malignancy in human cancer cells. J. Clin. Investig. 2011, 121, 1905–1916. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; He, W.; Li, Y.; Xu, N.; Zhu, X.; Lin, Y.; Gou, X. Loss of DUSP2 predicts a poor prognosis in patients with bladder cancer. Hum. Pathol. 2019, 85, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Lo Nigro, C.; Vivenza, D.; Denaro, N.; Lattanzio, L.; Fortunato, M.; Crook, T.; Merlano, M.C. DUSP2 methylation is a candidate biomarker of outcome in head and neck cancer. Ann. Transl. Med. 2018, 6, 271. [Google Scholar] [CrossRef] [PubMed]
- Henkens, R.; Delvenne, P.; Arafa, M.; Moutschen, M.; Zeddou, M.; Tautz, L.; Boniver, J.; Mustelin, T.; Rahmouni, S. Cervix carcinoma is associated with an up-regulation and nuclear localization of the dual-specificity protein phosphatase VHR. BMC Cancer 2008, 8, 147. [Google Scholar] [CrossRef] [PubMed]
- Arnoldussen, Y.J.; Lorenzo, P.I.; Pretorius, M.E.; Waehre, H.; Risberg, B.; Maelandsmo, G.M.; Danielsen, H.E.; Saatcioglu, F. The mitogen-activated protein kinase phosphatase vaccinia H1-related protein inhibits apoptosis in prostate cancer cells and is overexpressed in prostate cancer. Cancer Res. 2008, 68, 9255–9264. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Yeh, C.L.; Chou, H.C.; Yang, C.H.; Fu, Y.N.; Chen, Y.T.; Cheng, H.W.; Huang, C.Y.; Liu, H.P.; Huang, S.F.; et al. Vaccinia H1-related phosphatase is a phosphatase of ErbB receptors and is down-regulated in non-small cell lung cancer. J. Biol. Chem. 2011, 286, 10177–10184. [Google Scholar] [CrossRef] [PubMed]
- Vandereyken, M.; Jacques, S.; Van Overmeire, E.; Amand, M.; Rocks, N.; Delierneux, C.; Singh, P.; Singh, M.; Ghuysen, C.; Wathieu, C.; et al. Dusp3 deletion in mice promotes experimental lung tumour metastasis in a macrophage dependent manner. PLoS ONE 2017, 12, e0185786. [Google Scholar] [CrossRef]
- Hasegawa, T.; Enomoto, A.; Kato, T.; Kawai, K.; Miyamoto, R.; Jijiwa, M.; Ichihara, M.; Ishida, M.; Asai, N.; Murakumo, Y.; et al. Roles of induced expression of MAPK phosphatase-2 in tumor development in RET-MEN2A transgenic mice. Oncogene 2008, 27, 5684–5695. [Google Scholar] [CrossRef]
- Yip-Schneider, M.T.; Lin, A.; Marshall, M.S. Pancreatic tumor cells with mutant K-ras suppress ERK activity by MEK-dependent induction of MAP kinase phosphatase-2. Biochem. Biophys. Res. Commun. 2001, 280, 992–997. [Google Scholar] [CrossRef]
- Sieben, N.L.; Oosting, J.; Flanagan, A.M.; Prat, J.; Roemen, G.M.; Kolkman-Uljee, S.M.; van Eijk, R.; Cornelisse, C.J.; Fleuren, G.J.; van Engeland, M. Differential gene expression in ovarian tumors reveals Dusp 4 and Serpina 5 as key regulators for benign behavior of serous borderline tumors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 7257–7264. [Google Scholar] [CrossRef]
- Zhao, Y.; Cai, H.; Ding, X.; Zhou, X. An integrative analysis of the single-cell transcriptome identifies DUSP4 as an exhaustion-associated gene in tumor-infiltrating CD8+ T cells. Funct. Integr. Genom. 2023, 23, 136. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Park, S.Y.; Kang, G.H. Down-regulation of dual-specificity phosphatase 5 in gastric cancer by promoter CpG island hypermethylation and its potential role in carcinogenesis. Am. J. Pathol. 2013, 182, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Aurtenetxe, O.; Zaldumbide, L.; Erramuzpe, A.; López, R.; López, J.I.; Cortés, J.M.; Pulido, R.; Nunes-Xavier, C.E. DUSP5 expression associates with poor prognosis in human neuroblastoma. Exp. Mol. Pathol. 2018, 105, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Song, L.; Ritchie, A.M.; Melton, D.W. Increased levels of DUSP6 phosphatase stimulate tumourigenesis in a molecularly distinct melanoma subtype. Pigment. Cell Melanoma Res. 2012, 25, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yu, X.; Guo, L.; Lu, S.H. DUSP6, a tumor suppressor, is involved in differentiation and apoptosis in esophageal squamous cell carcinoma. Oncol. Lett. 2013, 6, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Han, Q.; Shan, Z.; Qu, X.; Guo, L.; Zhou, Y. Dual specificity phosphatase 6 suppresses the growth and metastasis of prostate cancer cells. Mol. Med. Rep. 2014, 10, 3052–3058. [Google Scholar] [CrossRef]
- Furukawa, T.; Sunamura, M.; Motoi, F.; Matsuno, S.; Horii, A. Potential tumor suppressive pathway involving DUSP6/MKP-3 in pancreatic cancer. Am. J. Pathol. 2003, 162, 1807–1815. [Google Scholar] [CrossRef]
- Messina, S.; Frati, L.; Leonetti, C.; Zuchegna, C.; Di Zazzo, E.; Calogero, A.; Porcellini, A. Dual-specificity phosphatase DUSP6 has tumor-promoting properties in human glioblastomas. Oncogene 2011, 30, 3813–3820. [Google Scholar] [CrossRef]
- Degl’Innocenti, D.; Romeo, P.; Tarantino, E.; Sensi, M.; Cassinelli, G.; Catalano, V.; Lanzi, C.; Perrone, F.; Pilotti, S.; Seregni, E.; et al. DUSP6/MKP3 is overexpressed in papillary and poorly differentiated thyroid carcinoma and contributes to neoplastic properties of thyroid cancer cells. Endocr.-Relat. Cancer 2013, 20, 23–37. [Google Scholar] [CrossRef]
- Xu, S.; Furukawa, T.; Kanai, N.; Sunamura, M.; Horii, A. Abrogation of DUSP6 by hypermethylation in human pancreatic cancer. J. Hum. Genet. 2005, 50, 159–167. [Google Scholar] [CrossRef]
- Chen, H.Y.; Yu, S.L.; Chen, C.H.; Chang, G.C.; Chen, C.Y.; Yuan, A.; Cheng, C.L.; Wang, C.H.; Terng, H.J.; Kao, S.F.; et al. A five-gene signature and clinical outcome in non-small-cell lung cancer. N. Engl. J. Med. 2007, 356, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.C.; Chen, H.; Ko, J.M.; Chan, K.W.; Chan, Y.P.; Law, S.; Chua, D.; Kwong, D.L.; Lung, H.L.; Srivastava, G.; et al. Tumor suppressor dual-specificity phosphatase 6 (DUSP6) impairs cell invasion and epithelial-mesenchymal transition (EMT)-associated phenotype. Int. J. Cancer 2012, 130, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Song, M.; Jiao, R.; Li, W.; Zhao, J.; Xiao, M.; Jin, M.; Zhang, Z.; Deng, H. DUSP7 inhibits cervical cancer progression by inactivating the RAS pathway. J. Cell. Mol. Med. 2021, 25, 9306–9318. [Google Scholar] [CrossRef] [PubMed]
- Gao, X. Identification of DUSP7 as an RNA Marker for Prognostic Stratification in Acute Myeloid Leukemia: Evidence from Large Population Cohorts. Genet. Res. 2023, 2023, 4348290. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, Y.; Sun, L.; Zhang, Z.; Jiang, Z.; Qin, Z.; Han, H.; Liu, Z.; Li, X.; Tang, A.; et al. Decreased expression of dual-specificity phosphatase 9 is associated with poor prognosis in clear cell renal cell carcinoma. BMC Cancer 2011, 11, 413. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Lv, T.; Chen, G.; Ye, H.; Wu, W.; Li, G.; Zhi, F.C. Epigenetic silencing of DUSP9 induces the proliferation of human gastric cancer by activating JNK signaling. Oncol. Rep. 2015, 34, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martínez, M.; Stamatakis, K.; Fresno, M. The Dual-Specificity Phosphatase 10 (DUSP10): Its Role in Cancer, Inflammation, and Immunity. Int. J. Mol. Sci. 2019, 20, 1626. [Google Scholar] [CrossRef]
- Erica, L.C.; Alexander, B. Emerging Roles of Atypical Dual Specificity Phosphatases in Cancer. In Oncogene and Cancer; Yahwardiah, S., Ed.; IntechOpen: Rijeka, Croatia, 2013; Chapter 4. [Google Scholar]
- Kresse, S.H.; Berner, J.M.; Meza-Zepeda, L.A.; Gregory, S.G.; Kuo, W.L.; Gray, J.W.; Forus, A.; Myklebost, O. Mapping and characterization of the amplicon near APOA2 in 1q23 in human sarcomas by FISH and array CGH. Mol. Cancer 2005, 4, 39. [Google Scholar] [CrossRef]
- Hoornaert, I.; Marynen, P.; Goris, J.; Sciot, R.; Baens, M. MAPK phosphatase DUSP16/MKP-7, a candidate tumor suppressor for chromosome region 12p12-13, reduces BCR-ABL-induced transformation. Oncogene 2003, 22, 7728–7736. [Google Scholar] [CrossRef]
- Cui, Z.; He, S.; Wen, F.; Xu, X.; Li, Y.; Lu, L.; Wu, S. Potential Biomarkers of Colon Adenocarcinoma Based on Weighted Gene Co-Expression Network Analysis. 2021; preprint. [Google Scholar] [CrossRef]
- Pu, J.; Qin, Z.; Fang, Q.; Huang, Y.; Lu, Y.; Li, W.; Wang, J.; Tang, Q.; Zeng, D.; Wei, H. Hypoxia-induced HIF1A activates DUSP18-mediated MAPK14 dephosphorylation to promote hepatocellular carcinoma cell migration and invasion. Pathol. Res. Pract. 2022, 237, 153955. [Google Scholar] [CrossRef]
- Sekine, Y.; Ikeda, O.; Hayakawa, Y.; Tsuji, S.; Imoto, S.; Aoki, N.; Sugiyama, K.; Matsuda, T. DUSP22/LMW-DSP2 regulates estrogen receptor-alpha-mediated signaling through dephosphorylation of Ser-118. Oncogene 2007, 26, 6038–6049. [Google Scholar] [CrossRef] [PubMed]
- Mélard, P.; Idrissi, Y.; Andrique, L.; Poglio, S.; Prochazkova-Carlotti, M.; Berhouet, S.; Boucher, C.; Laharanne, E.; Chevret, E.; Pham-Ledard, A.; et al. Molecular alterations and tumor suppressive function of the DUSP22 (Dual Specificity Phosphatase 22) gene in peripheral T-cell lymphoma subtypes. Oncotarget 2016, 7, 68734–68748. [Google Scholar] [CrossRef] [PubMed]
- Parrilla Castellar, E.R.; Jaffe, E.S.; Said, J.W.; Swerdlow, S.H.; Ketterling, R.P.; Knudson, R.A.; Sidhu, J.S.; Hsi, E.D.; Karikehalli, S.; Jiang, L.; et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood 2014, 124, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.I.; Brummer, T.; Daly, R.J.; O’Brien, P.M. DUSP26 negatively affects the proliferation of epithelial cells, an effect not mediated by dephosphorylation of MAPKs. Biochim. Biophys. Acta-Mol. Cell Res. 2010, 1803, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Imoto, I.; Inoue, J.; Onda, M.; Emi, M.; Inazawa, J. A novel amplification target, DUSP26, promotes anaplastic thyroid cancer cell growth by inhibiting p38 MAPK activity. Oncogene 2007, 26, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Guler, S.; Zik, B.; Yalcin, A. Upregulation of Dual-Specificity Phosphatase-26 is Required for Transforming Growth Factor β1(TGFβ1)-induced Epithelial-Mesenchymal Transition in A549 and PANC1 Cells. Mol. Biol. Rep. 2022, 49, 10195–10204. [Google Scholar] [CrossRef]
- Thompson, E.M.; Stoker, A.W. A Review of DUSP26: Structure, Regulation and Relevance in Human Disease. Int. J. Mol. Sci. 2021, 22, 776. [Google Scholar] [CrossRef]
- Kerneur, C.; Cano, C.E.; Olive, D. Major pathways involved in macrophage polarization in cancer. Front. Immunol. 2022, 13, 1026954. [Google Scholar] [CrossRef]
- Wang, S.J.; Li, R.; Ng, T.S.C.; Luthria, G.; Oudin, M.J.; Prytyskach, M.; Kohler, R.H.; Weissleder, R.; Lauffenburger, D.A.; Miller, M.A. Efficient blockade of locally reciprocated tumor-macrophage signaling using a TAM-avid nanotherapy. Sci. Adv. 2020, 6, eaaz8521. [Google Scholar] [CrossRef]
- Boyer, S.; Lee, H.J.; Steele, N.; Zhang, L.; Sajjakulnukit, P.; Andren, A.; Ward, M.H.; Singh, R.; Basrur, V.; Zhang, Y.; et al. Multiomic characterization of pancreatic cancer-associated macrophage polarization reveals deregulated metabolic programs driven by the GM-CSF-PI3K pathway. eLife 2022, 11, e73796. [Google Scholar] [CrossRef]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, V.; Jakymiw, A.; Van Tubergen, E.A.; D’Silva, N.J.; Kirkwood, K.L. Control of cytokine mRNA expression by RNA-binding proteins and microRNAs. J. Dent. Res. 2012, 91, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Yu, S.; Zhang, J.; Wu, S. Dysregulated tumor-associated macrophages in carcinogenesis, progression and targeted therapy of gynecological and breast cancers. J. Hematol. Oncol. 2021, 14, 181. [Google Scholar] [CrossRef] [PubMed]
- Delprat, V.; Tellier, C.; Demazy, C.; Raes, M.; Feron, O.; Michiels, C. Cycling hypoxia promotes a pro-inflammatory phenotype in macrophages via JNK/p65 signaling pathway. Sci. Rep. 2020, 10, 882. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Tang, C.; Lu, X.; Liu, R.; Zhou, M.; He, D.; Zheng, D.; Sun, C.; Wu, Z. MiR-101 targets DUSP1 to regulate the TGF-β secretion in sorafenib inhibits macrophage-induced growth of hepatocarcinoma. Oncotarget 2015, 6, 18389–18405. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Liu, F.Y.; Li, P.; He, J.; Kirkwood, C.L.; Sohn, J.; Chan, J.M.; Magner, W.J.; Kirkwood, K.L. MKP-1 is required to limit myeloid-cell mediated oral squamous cell carcinoma progression and regional extension. Oral Oncol. 2021, 120, 105401. [Google Scholar] [CrossRef] [PubMed]
- Khor, G.H.; Froemming, G.R.; Zain, R.B.; Abraham, M.T.; Omar, E.; Tan, S.K.; Tan, A.C.; Vincent-Chong, V.K.; Thong, K.L. DNA methylation profiling revealed promoter hypermethylation-induced silencing of p16, DDAH2 and DUSP1 in primary oral squamous cell carcinoma. Int. J. Med. Sci. 2013, 10, 1727–1739. [Google Scholar] [CrossRef]
- Pan, W.; Han, J.; Wei, N.; Wu, H.; Wang, Y.; Sun, J. LINC00702-mediated DUSP1 transcription in the prevention of bladder cancer progression: Implications in cancer cell proliferation and tumor inflammatory microenvironment. Genomics 2022, 114, 110428. [Google Scholar] [CrossRef]
- Yu, W.; Li, D.; Ding, X.; Sun, Y.; Liu, Y.; Cong, J.; Yang, J.; Sun, J.; Ning, X.; Wang, H.; et al. LINC00702 suppresses proliferation and invasion in non-small cell lung cancer through regulating miR-510/PTEN axis. Aging 2019, 11, 1471–1485. [Google Scholar] [CrossRef]
- Yu, D.; Wang, X.Y.; Jin, Z.L. Linc00702 inhibits cell growth and metastasis through regulating PTEN in colorectal cancer. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3624–3632. [Google Scholar] [CrossRef]
- Nirschl, T.R.; El Asmar, M.; Ludwig, W.W.; Ganguly, S.; Gorin, M.A.; Johnson, M.H.; Pierorazio, P.M.; Drake, C.G.; Allaf, M.E.; Zarif, J.C. Transcriptional profiling of tumor associated macrophages in human renal cell carcinoma reveals significant heterogeneity and opportunity for immunomodulation. Am. J. Clin. Exp. Urol. 2020, 8, 48–58. [Google Scholar] [PubMed]
- Stephens, K.E.; Miaskowski, C.A.; Levine, J.D.; Pullinger, C.R.; Aouizerat, B.E. Epigenetic regulation and measurement of epigenetic changes. Biol. Res. Nurs. 2013, 15, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.W.; Huang, K.; Yang, C.; Kang, C.S. Non-coding RNAs as regulators in epigenetics (Review). Oncol. Rep. 2017, 37, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.; Pattarayan, D.; Rajaguru, P.; Sudhakar Gandhi, P.S.; Thimmulappa, R.K. MicroRNA Regulation of Acute Lung Injury and Acute Respiratory Distress Syndrome. J. Cell. Physiol. 2016, 231, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Ampomah, P.B.; Cai, B.; Sukka, S.R.; Gerlach, B.D.; Yurdagul, A., Jr.; Wang, X.; Kuriakose, G.; Darville, L.N.F.; Sun, Y.; Sidoli, S.; et al. Macrophages use apoptotic cell-derived methionine and DNMT3A during efferocytosis to promote tissue resolution. Nat. Metab. 2022, 4, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.L.; Tai, J.J.; Wong, W.C.; Han, H.; Sem, X.; Yeap, W.H.; Kourilsky, P.; Wong, S.C. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood 2011, 118, e16–e31. [Google Scholar] [CrossRef]
- Musikacharoen, T.; Yoshikai, Y.; Matsuguchi, T. Histone acetylation and activation of cAMP-response element-binding protein regulate transcriptional activation of MKP-M in lipopolysaccharide-stimulated macrophages. J. Biol. Chem. 2003, 278, 9167–9175. [Google Scholar] [CrossRef]
- Subuddhi, A.; Kumar, M.; Majumder, D.; Sarkar, A.; Ghosh, Z.; Vasudevan, M.; Kundu, M.; Basu, J. Unraveling the role of H3K4 trimethylation and lncRNA HOTAIR in SATB1 and DUSP4-dependent survival of virulent Mycobacterium tuberculosis in macrophages. Tuberculosis 2020, 120, 101897. [Google Scholar] [CrossRef]
- Ying, H.; Kang, Y.; Zhang, H.; Zhao, D.; Xia, J.; Lu, Z.; Wang, H.; Xu, F.; Shi, L. MiR-127 modulates macrophage polarization and promotes lung inflammation and injury by activating the JNK pathway. J. Immunol. 2015, 194, 1239–1251. [Google Scholar] [CrossRef]
- Xiao, J.; Tang, J.; Chen, Q.; Tang, D.; Liu, M.; Luo, M.; Wang, Y.; Wang, J.; Zhao, Z.; Tang, C.; et al. miR-429 regulates alveolar macrophage inflammatory cytokine production and is involved in LPS-induced acute lung injury. Biochem. J. 2015, 471, 281–291. [Google Scholar] [CrossRef]
- Zeng, H.S.; Lin, Y.J.; Gao, L.; Zhang, T.T.; Cao, J.T.; Fan, Y.Q.; Yin, Z.F.; Gu, J.; Wang, C.Q. Influence of ox-LDL on migration function of THP-1 macrophages and microRNA21 expression and MAPK pathway phosphorylation. Chin. J. Cardiovasc. Rehabil. Med. 2018, 27, 241–246. [Google Scholar] [CrossRef]
- Singh, G.B.; Khanna, S.; Raut, S.K.; Sharma, S.; Sharma, R.; Khullar, M. DUSP-1 gene expression is not regulated by promoter methylation in diabetes-associated cardiac hypertrophy. Ther. Adv. Cardiovasc. Dis. 2017, 11, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Cheong, J.-G.; Ravishankar, A.; Sharma, S.; Parkhurst, C.; Nehar-Belaid, D.; Ma, S.; Paddock, L.; Fatou, B.; Karakaslar, O.; Thibodeau, A.; et al. Epigenetic Memory of COVID-19 in Innate Immune Cells and Their Progenitors. 2022; preprint. [Google Scholar] [CrossRef]
- Jeong, Y.; Du, R.; Zhu, X.; Yin, S.; Wang, J.; Cui, H.; Cao, W.; Lowenstein, C.J. Histone deacetylase isoforms regulate innate immune responses by deacetylating mitogen-activated protein kinase phosphatase-1. J. Leukoc. Biol. 2014, 95, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Lo Muzio, L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Zandi, Z.; Kashani, B.; Alishahi, Z.; Pourbagheri-Sigaroodi, A.; Esmaeili, F.; Ghaffari, S.H.; Bashash, D.; Momeny, M. Dual-specificity phosphatases: Therapeutic targets in cancer therapy resistance. J. Cancer Res. Clin. Oncol. 2022, 148, 57–70. [Google Scholar] [CrossRef]
- Bakan, A.; Lazo, J.S.; Wipf, P.; Brummond, K.M.; Bahar, I. Toward a molecular understanding of the interaction of dual specificity phosphatases with substrates: Insights from structure-based modeling and high throughput screening. Curr. Med. Chem. 2008, 15, 2536–2544. [Google Scholar] [CrossRef]
- Vainonen, J.P.; Momeny, M.; Westermarck, J. Druggable cancer phosphatases. Sci. Transl. Med. 2021, 13, eabe2967. [Google Scholar] [CrossRef]
- Molina, G.; Vogt, A.; Bakan, A.; Dai, W.; Queiroz de Oliveira, P.; Znosko, W.; Smithgall, T.E.; Bahar, I.; Lazo, J.S.; Day, B.W.; et al. Zebrafish chemical screening reveals an inhibitor of Dusp6 that expands cardiac cell lineages. Nat. Chem. Biol. 2009, 5, 680–687. [Google Scholar] [CrossRef]
- Korotchenko, V.N.; Saydmohammed, M.; Vollmer, L.L.; Bakan, A.; Sheetz, K.; Debiec, K.T.; Greene, K.A.; Agliori, C.S.; Bahar, I.; Day, B.W.; et al. In vivo structure-activity relationship studies support allosteric targeting of a dual specificity phosphatase. Chembiochem A Eur. J. Chem. Biol. 2014, 15, 1436–1445. [Google Scholar] [CrossRef]
- Kaltenmeier, C.T.; Vollmer, L.L.; Vernetti, L.A.; Caprio, L.; Davis, K.; Korotchenko, V.N.; Day, B.W.; Tsang, M.; Hulkower, K.I.; Lotze, M.T.; et al. A Tumor Cell-Selective Inhibitor of Mitogen-Activated Protein Kinase Phosphatases Sensitizes Breast Cancer Cells to Lymphokine-Activated Killer Cell Activity. J. Pharmacol. Exp. Ther. 2017, 361, 39–50. [Google Scholar] [CrossRef]
- Zhang, F.; Tang, B.; Zhang, Z.; Xu, D.; Ma, G. DUSP6 Inhibitor (E/Z)-BCI Hydrochloride Attenuates Lipopolysaccharide-Induced Inflammatory Responses in Murine Macrophage Cells via Activating the Nrf2 Signaling Axis and Inhibiting the NF-κB Pathway. Inflammation 2019, 42, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.; Zheng, L.; Du, J.; Wei, S.; Zhu, X.; Xiong, J.W. A DUSP6 inhibitor suppresses inflammatory cardiac remodeling and improves heart function after myocardial infarction. Dis. Models Mech. 2023, 16, dmm049662. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; He, Y.; Yang, C.; Lu, N.; Bao, J.; Gao, S.; Hosyanto, F.F.; He, X.; Fu, H.; Yan, H.; et al. Methylprednisolone Promotes Mycobacterium smegmatis Survival in Macrophages through NF-κB/DUSP1 Pathway. Microorganisms 2023, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Lazo, J.S.; Nunes, R.; Skoko, J.J.; Queiroz de Oliveira, P.E.; Vogt, A.; Wipf, P. Novel benzofuran inhibitors of human mitogen-activated protein kinase phosphatase-1. Bioorganic Med. Chem. 2006, 14, 5643–5650. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Cooley, K.A.; Brisson, M.; Tarpley, M.G.; Wipf, P.; Lazo, J.S. Cell-active dual specificity phosphatase inhibitors identified by high-content screening. Drug Discov. Today 2005, 10, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Tamewitz, A.; Skoko, J.; Sikorski, R.P.; Giuliano, K.A.; Lazo, J.S. The benzo[c]phenanthridine alkaloid, sanguinarine, is a selective, cell-active inhibitor of mitogen-activated protein kinase phosphatase-1. J. Biol. Chem. 2005, 280, 19078–19086. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, G.; Zhang, P.F.; Zhang, J.; Huang, Y.X.; Lu, Y.M.; Da, W.; Sun, Q.; Zhu, J.S. Sanguinarine inhibits growth and invasion of gastric cancer cells via regulation of the DUSP4/ERK pathway. J. Cell. Mol. Med. 2017, 21, 1117–1127. [Google Scholar] [CrossRef]
- Cui, Y.; Luo, Y.; Qian, Q.; Tian, J.; Fang, Z.; Wang, X.; Zeng, Y.; Wu, J.; Li, Y. Sanguinarine Regulates Tumor-Associated Macrophages to Prevent Lung Cancer Angiogenesis through the WNT/β-Catenin Pathway. Front. Oncol. 2022, 12, 732860. [Google Scholar] [CrossRef]
- Günzl, P.; Bauer, K.; Hainzl, E.; Matt, U.; Dillinger, B.; Mahr, B.; Knapp, S.; Binder, B.R.; Schabbauer, G. Anti-inflammatory properties of the PI3K pathway are mediated by IL-10/DUSP regulation. J. Leukoc. Biol. 2010, 88, 1259–1269. [Google Scholar] [CrossRef]
- Jia, Y.; Wei, Y. Modulators of MicroRNA Function in the Immune System. Int. J. Mol. Sci. 2020, 21, 2357. [Google Scholar] [CrossRef]
- Wu, Q.; Allouch, A.; Martins, I.; Modjtahedi, N.; Deutsch, E.; Perfettini, J.L. Macrophage biology plays a central role during ionizing radiation-elicited tumor response. Biomed. J. 2017, 40, 200–211. [Google Scholar] [CrossRef] [PubMed]

| Isoforms and Cellular Localization | Inductors | Pathway | Effect of DUSPs in Macrophages |
|---|---|---|---|
| MAPK-specific phosphatases/typical DUSPs | |||
| DUSP1 Nuclear | LPS | p38, JNK | ↓ p38 and JNK, ↓ TNF-α, IL-6, IL-10; ↑ IL-12 and IRF-1 (↑ expression of IL-12 by enhancing IRF1 expression) [56,57,58] |
| IL-10 + DEX | p38 | ↓ IL-6, IL-12, p38 (↑ prolonged expression DUSP1) [59,60] | |
| DEX | JNK > p38 | ↓ JNK, TNF-α, COX2, IL-1 [56,59] | |
| Peptidoglycan, Zymosan, poly(I:C), Flagellin | JNK, p38 | ↓ JNK, p38, TNF-α [61] In DUSP1−/− mF ↑ p38, ↑ TNF-α, IL-10, CD86 and CD40 [62] | |
| Heat shock | p38 | ↑ activity of heat shock elements (HSE) in the MKP-1 promoter, stability of MKP-1 mRNA in mF [63] | |
| DUSP2 Nuclear | LPS | ERK, p38 > JNK | In DUSP2 −/− mF ↓ ERK and p38, Elk1 and NFAT-AP-1 activation ↑ JNK, ↓ TNF-α, IL-6, IL-12α, COX2, IL-1β, C5aR, C3aR + ↓ PGE2, NO [64] |
| DUSP4 Nuclear | M-CSF | ERK | In DUSP4−/− mF, ↑ ERK ↓ Mmd, Csf2, expression of surface proteins CD115, CD34 [65] |
| LPS | JNK, p38 | In DUSP4−/− mF ↑ JNK and p38, ↑ ARG-1, IL-6, TNFα, IL-12, PGE2 and ↓ expression of inducible nitric oxide synthase (iNOS) and IL-10, + ↑ DUSP1 as a result of increased ERK signaling [66] | |
| FFA | JNK, p38 | ↓ JNK, TNF-α, IL-6, IL-12; ↓ macrophage M1 activation through JNK and p38 [67] | |
| IL-4 | JNK, p38 | ↑ macrophage M2 activation [67] | |
| DUSP5 Nuclear/cytoplasmic | LPS | ERK | ↓ ERK1/2, ↓ ERK1/2 phosphorylation, ↑ NF-κB activity [68] |
| M-CSF | ERK | ↓ ERK1/2, block macrophage differentiation => differentiate towards to granulocytes [69] | |
| DUSP6 Cytoplasmic | PKN2 | ERK | ↓ ERK1/2, IL-4, IL-10 [70] |
| CCL2+LPS | ERK | ↑ ERK1/2 phosphorylation [71] | |
| Hyperoxia | ERK | ↑ ERK [72] | |
| DUSP7 Cytoplasmic | No information | ||
| DUSP8 Nuclear/cytoplasmic | LPS | JNK | ↓ JNK, TNF-α, IL-1β, IL-6 [73,74] |
| miR-21 | JNK, p38 | ↑ p38, JNK + ↑ macrophage migration and macrophage adhesion to endothelium [75] | |
| DUSP9 Cytoplasmic | hypoxia/reoxygenation | JNK, p38 | ↓ JNK, p38 ↓ ASK1 phosphorylation, TRAF6, IKKβ (NF− κB pathway), K63 ubiquitination, TNF-α, IL-1β, IL-6 [76] |
| DUSP10 Nuclear/cytoplasmic | LPS, peptidoglycan, poly(I:C) | JNK [77] + JNK, ERK, p38 [78] | ↓ AP-1, TNF-α, IL-6, JNK activity [77] in DUSP10−/− mF ↑ JNK, ERK, p38 phosphorylation, ↑ TNF-α, IL-6, MIP-2, ↑iNOS, ROS production [78] |
| Inhibition by siRNA of pharmacological inhibitor AS077234-4 | p38 | ↓ p38, TNF-α и IL-6 [79] | |
| DUSP16 Nuclear/cytoplasmic | LPS | JNK1/2 | In DUSP16−/− mF ↑ produce IL-12, IRF-1, ↑ JNK 1/2 phosphorylation [80] |
| Atypical DUSPs | |||
| DUSP3 Cytoplasmic | LPS | ERK | In DUSP3−/− mF ↓ ERK1/2 phosphorylation, Akt, TNF, IL-6 [81] |
| DUSP11 Nuclear/cytoplasmic | LPS | TAK1 | In DUSP−/− mF, ↑ TAK1 phosphorylation (TGF-β–activated kinase 1), TNF-α, IL-6 и IL-1β [82,83] |
| DUSP12 Nuclear | LPS | JNK, p38 | ↓ JNK, p38 activation, ↓ expression of AP-1, TNF-α, IL-6, IL-1β, CCL2, ↑ IL-10 [84] |
| DUSP14 Cytoplasmic | No information | ||
| DUSP18 Nuclear/cytoplasmic/mitochondrial intermembrane space | No information | ||
| DUSP22 Nuclear | No information | ||
| DUSP26 Cytoplasmic | LPS | p38, JNK | ↓ TNF-α, p38 significantly and JNK slightly [85] |
| Isoform | Features in Solid Tumor | Correlation with Cancer Progression Parameters |
|---|---|---|
| DUSP1 | ↑ breast cancer [16,106,107] ↑ prostate cancer [108] ↓ prostate cancer [109], lung cancer by oxidation [106], head and neck squamous cell carcinoma [110] | negative correlation with OS in ovarian cancer [16] positive correlation with DFS in hepatocellular carcinoma [93] |
| DUSP2 | ↓ lung, breast, colorectal, prostate, ovarian [111], ↓ ovarian carcinoma [105] ↓ bladder cancer [112] hypermethylation CpG island in head and neck cancer [113] | positive correlation with RFS in Her2+ breast cancer [97] positive correlation with OS and DMFS in colorectal cancer [98] positive correlation with OS in ovarian cancer [105] positive correlation with OS and RFS in bladder cancer [112] |
| DUSP3 | ↑ cervical carcinoma, prostate cancer [114,115] ↓ non-small lung cancer [116] | positive correlation with OS for lung, kidney, sarcoma, lymphoma, and breast cancer/negative correlation with OS for lung, breast, and brain cancer for other groups [96] negative correlation with metastasis in non-small lung cancer mice model [117] |
| DUSP4 | ↑ medullary thyroid carcinoma, pancreatic cancer, breast cancer, colorectal cancer, rectal cancer, melanoma [99,107,118,119] ↓ serous ovarian carcinoma [120] | negative correlation with OS and DFS in early breast cancer [95] positive correlation with RFS in colorectal cancer [99] positive correlation with OS in lung, pancreatic cancer, gastric, and clear cell renal cell cancer [121] hypermethylation predicts a negative survival factor in B-cell lymphoma [95] |
| DUSP5 | ↓ colorectal cancer, prostate cancer, gastric cancer [100,102,122] hypermethylation of CpG islands in gastric cancer [122] | positive correlation with DFS and DSS in colorectal cancer [100] negative correlation with high Gleason score, biochemical recurrence and metastasis in prostate cancer [102] positive correlation with OS in gastric cancer [122] negative correlation with OS in neuroblastoma [123] |
| DUSP6 | ↓ melanoma [124] ↓ esophageal cancer [125] ↓ prostate cancer [126] ↓ pancreatic cancer [127] ↑ glioblastoma [128] ↑ thyroid carcinoma [129] hypermethylation of gene promoters in pancreatic cancer [130] | positive correlation with OS, RFS in non-small cell lung cancer [131] positive correlation with OS in esophageal cancer, nasopharyngeal [132] |
| DUSP7 | ↓ ovarian cancer [133] ↑ acute and myeloid leukemia [134] hypermethylation of gene promoters in ovarian cancer [104] | methylation-dependent positive correlation with PFS and OS in ovarian cancer [104] |
| DUSP8 | hypermethylation of gene promoters in ovarian cancer [104] | methylation-dependent positive correlation with PFS and OS in ovarian cancer [104] |
| DUSP9 | ↓ renal carcinoma [135] ↑ gastric cancer [136] hypomethylation of gene promoters in gastric cancer [136] | positive correlation in renal cancer with OS [135] |
| DUSP10 | ↑ ER- breast cancer [94] ↑ prostate cancer [137] ↑ hepatocellular carcinoma, melanoma, lung cancer, colorectal cancer, prostate cancer, glioblastoma [137,138] | |
| DUSP12 | ↑ sarcoma, neuroblastoma, retinoblastoma, intracranial ependymoma, chronic myeloid leukemia [139] ↑ DUSP12 positive correlate with c-met and itga, cell migration and genomic instability [138] | |
| DUSP16 | ↑ hepatocellular carcinoma [137] ↑ leukemia [140] | |
| DUSP18 | ↑ colon cancer [141] ↑ hepatocellular carcinoma [142] | positive correlation with OS in colon cancer [141] |
| DUSP22 | ↓ breast cancer [143] ↓ colorectal cancer [101] ↓ peripheral T-cell lymphoma [144] ↓ prostate cancer [103] | positive correlation with OS in IV stage colorectal cancer [101] positive correlation with PFS and DFS in prostate cancer [103] negative correlation OS in anaplastic large cell lymphoma [145] |
| DUSP26 | ↓ ovarian cancer, neuroblastoma, medulloblastoma and glioblastoma [146] ↑ thyroid carcinoma [147] ↑ for TGFβ1-promoted EMT in pancreatic, lung cancers cell lines [148] | positive correlation with DFS in neuroblastoma [149] |
| Isoforms | Epigenetic Changes | Key Mechanism | Effects in Macrophages | References |
|---|---|---|---|---|
| DUSP4 | DNA Methylation | Efferocating ↑ DNMT3A ↓ methylation of DUSP4 promoter ↓ DUSP4 mRNA expression | ↑ PGE2, TGF-β production | [167] |
| DUSP1 | Histone code | ↓ chromatin activity ↑ H3 and H4 histones acetylation ↑ mRNA DUSP1 expression | --- | [168] |
| DUSP1 | Histone code | ↑ acetylation of histones H3 and H4 at the DUSP1 promoter | --- | [169] |
| DUSP4 | Histone code | ↑ methylation of H3K4me3 histones | ↓ CXCL1, CXCL2, CXCL3 | [170] |
| DUSP1 | miRNA | ↑ miR-127 ↓ DUSP1 mRNA expression | ↑ IL-6, TNF-α, IL-1β ↑ NOS | [171] |
| DUSP1 | miRNA | miR-429, miR-200b, and miR-200c ↓ DUSP1 mRNA expression | ↑ IL-6, TNF-α, IL-1β | [172] |
| DUSP6 | miRNA | ↑ miR-9 ↓ DUSP6 mRNA expression | ↓ NOS (after 6 h of CCL2 induction) ↑ TNF-α | [71] |
| DUSP8 | miRNA | ↓ miR-21 ↑ DUSP8 mRNA expression | ↓ CCL2-induced migration ↓ macrophage-endothelium interaction | [16] |
| DUSP8 | miRNA | ↑ Ox-LDL ↑ miR-21 ↑ DUSP8 mRNA expression | ↑ migration | [173] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patysheva, M.R.; Prostakishina, E.A.; Budnitskaya, A.A.; Bragina, O.D.; Kzhyshkowska, J.G. Dual-Specificity Phosphatases in Regulation of Tumor-Associated Macrophage Activity. Int. J. Mol. Sci. 2023, 24, 17542. https://doi.org/10.3390/ijms242417542
Patysheva MR, Prostakishina EA, Budnitskaya AA, Bragina OD, Kzhyshkowska JG. Dual-Specificity Phosphatases in Regulation of Tumor-Associated Macrophage Activity. International Journal of Molecular Sciences. 2023; 24(24):17542. https://doi.org/10.3390/ijms242417542
Chicago/Turabian StylePatysheva, Marina R., Elizaveta A. Prostakishina, Arina A. Budnitskaya, Olga D. Bragina, and Julia G. Kzhyshkowska. 2023. "Dual-Specificity Phosphatases in Regulation of Tumor-Associated Macrophage Activity" International Journal of Molecular Sciences 24, no. 24: 17542. https://doi.org/10.3390/ijms242417542





