Tailored Water-Soluble Covalent Organic Cages for Encapsulation of Pyrene and Information Encryption
Abstract
:1. Introduction
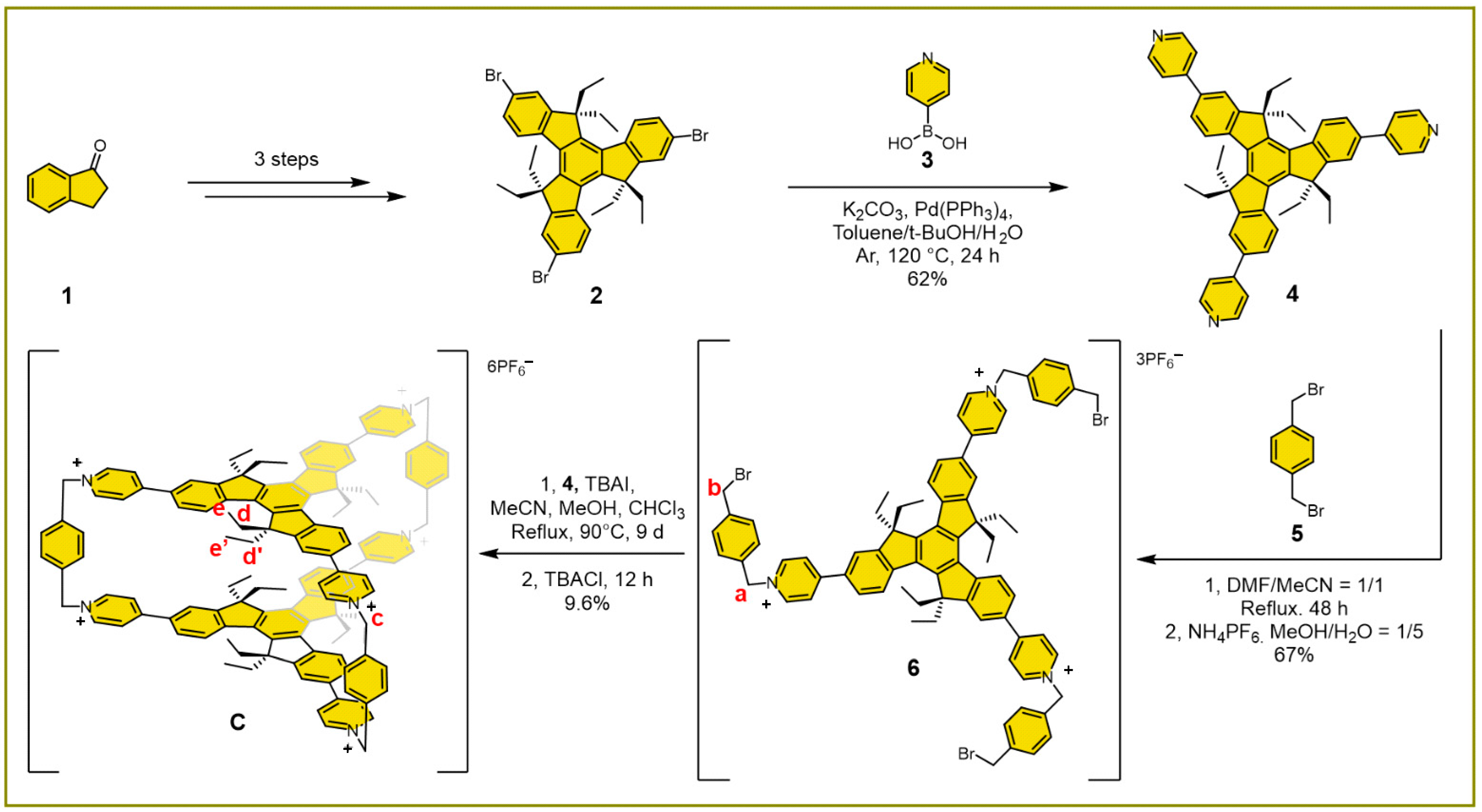
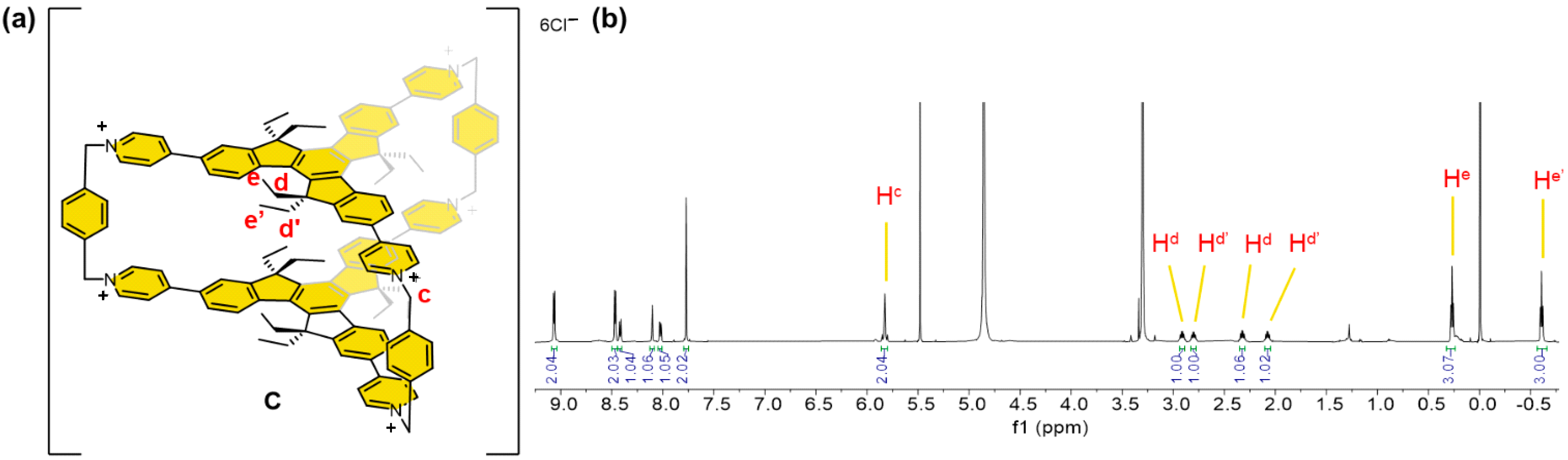
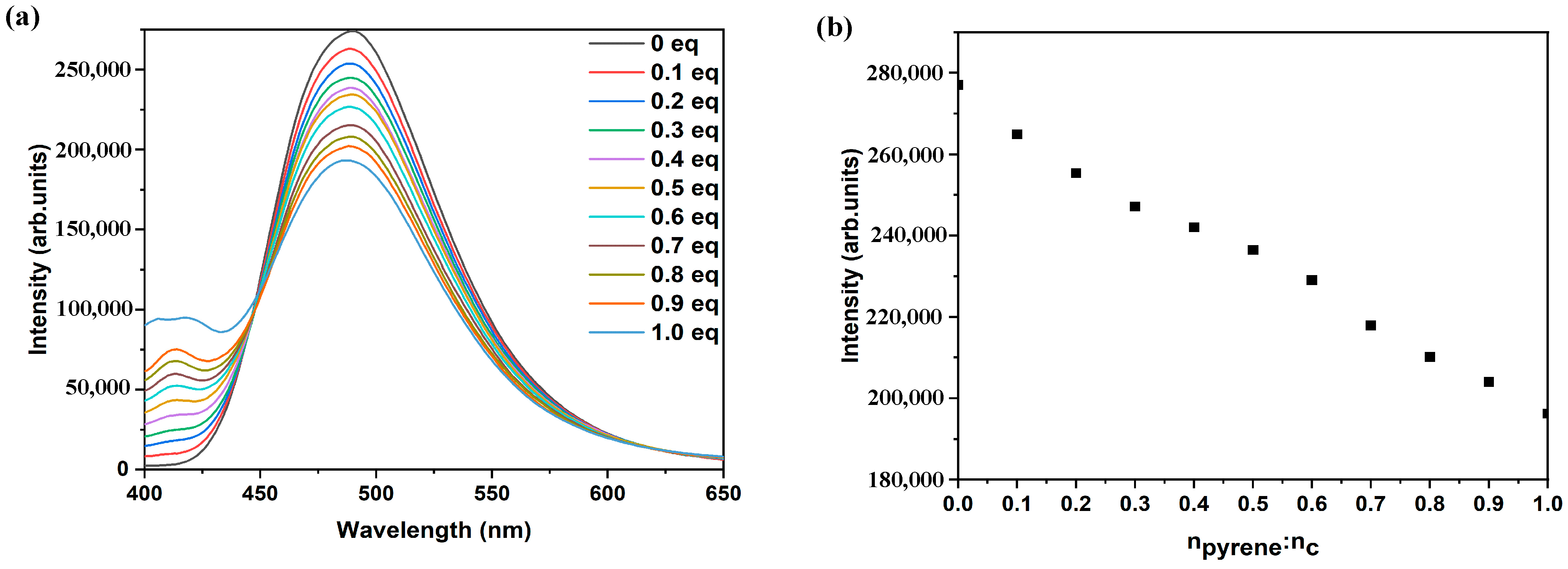
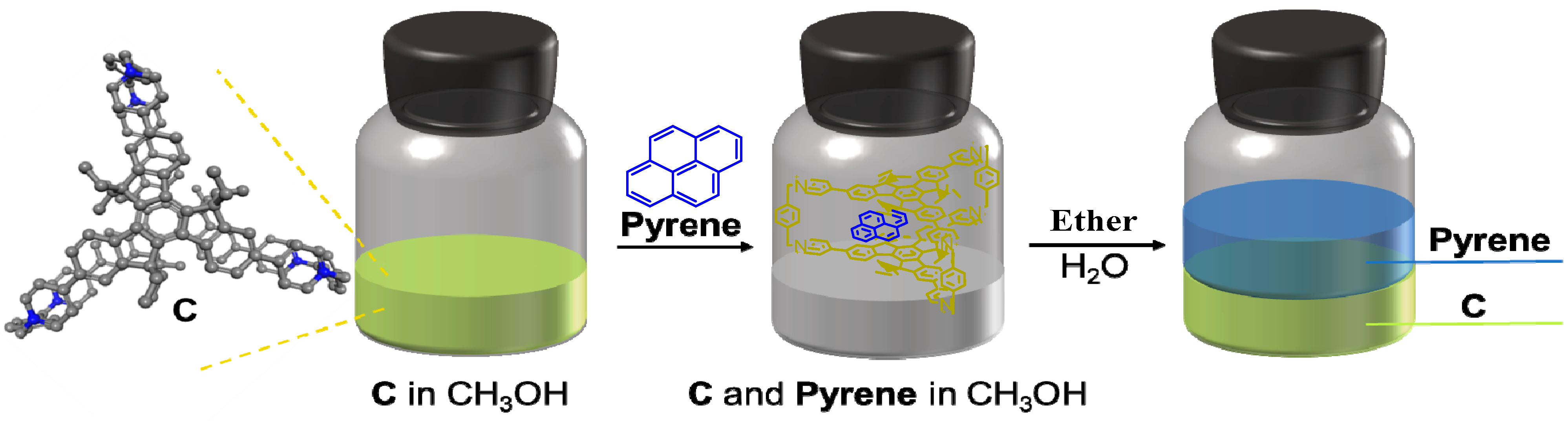
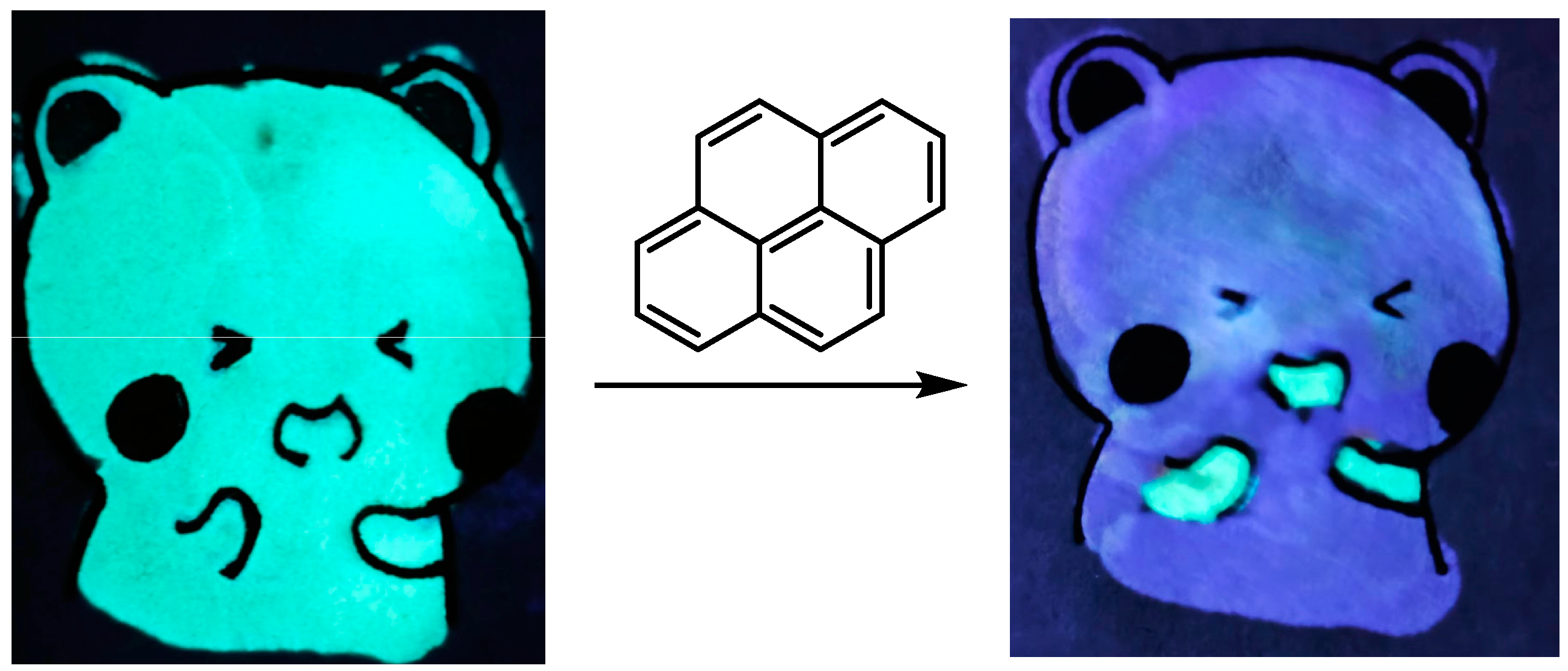
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Measurements
3.3. Materials Synthesis
3.3.1. Preparation of Compound 6
3.3.2. Preparation of Covalent Organic Cage C
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiester, M.J.; Ulmann, P.A.; Mirkin, C.A. Enzyme mimics based upon supramolecular coordination chemistry. Angew. Chem. Int. Ed. 2011, 50, 114–137. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Hirsch, A.K. Dynamic combinatorial chemistry: A tool to facilitate the identification of inhibitors for protein targets. Chem. Soc. Rev. 2015, 44, 2455–2488. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Olivares, D.; Kaoud, T.S.; Dalby, K.N.; Anslyn, E.V. In-situ generation of differential sensors that fingerprint kinases and the cellular response to their expression. J. Am. Chem. Soc. 2013, 135, 14814–14820. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.D.; Zou, Y.; Chen, Z.; Liu, S.; Yang, Y.; Pu, S. Finely regulated benzothiadiazole derivatives: Aggregation-induced emission (AIE), hypso- or bathochromic mechanofluorochromic behaviors, and multilevel information encryption applications. Dye. Pigment. 2023, 211, 111051. [Google Scholar] [CrossRef]
- Kolesnichenko, I.V.; Anslyn, E.V. Practical applications of supramolecular chemistry. Chem. Soc. Rev. 2017, 46, 2385–2390. [Google Scholar] [CrossRef]
- Zhang, X.H.; Shi, J.; Shen, G.Y.; Gou, F.; Cheng, J.H.; Zhou, X.G.; Xiang, H.F. Non-conjugated fluorescent molecular cages of salicylaldehyde-based tri-Schiff bases: AIE, enantiomers, mechanochromism, anion hosts/probes, and cell imaging properties. Mat. Chem. Front. 2017, 1, 1041–1050. [Google Scholar] [CrossRef]
- Liu, G.X.; Leng, J.H.; Zhou, Q.Y.; Deng, Z.; Shi, L.L.; Fan, C.L.; Xu, X.F.; Song, M.P. Fluorescence photoswitch of stiff-stilbene derivatives for anti-counterfeiting. Dye. Pigment. 2022, 203, 110361. [Google Scholar] [CrossRef]
- Han, X.; Hu, W.N.; Miao, L.L.; Hao, X.Q.; Shi, L.L.; Song, M.P. Structurally coordinated aggregation induced emission ionic supramolecular cages. Dye. Pigment. 2023, 211, 111078. [Google Scholar] [CrossRef]
- Zhu, J.L.; Zhang, D.; Ronson, T.K.; Wang, W.; Xu, L.; Yang, H.B.; Nitschke, J.R. A Cavity-Tailored Metal-Organic Cage Entraps Gases Selectively in Solution and the Amorphous Solid State. Angew. Chem. Int. Ed. 2021, 60, 11789–11792. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Fujita, M. Highly Selective Photomediated 1,4-Radical Addition too-Quinones Controlled by a Self-Assembled Cage. Angew. Chem. Int. Ed. 2008, 47, 2067–2069. [Google Scholar] [CrossRef]
- Ohara, K.; Inokuma, Y.; Fujita, M. The Catalytic Z to E Isomerization of Stilbenes in a Photosensitizing Porous Coordination Network. Angew. Chem. Int. Ed. 2010, 49, 5507–5509. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.L.; Wang, B.Y.; Lu, S.Y. Efficient bottom-up synthesis of graphene quantum dots at an atomically precise level. Matter 2023, 6, 728–760. [Google Scholar] [CrossRef]
- Cullen, W.; Takezawa, H.; Fujita, M. Demethylenation of Cyclopropanes via Photoinduced Guest-to-Host Electron Transfer in an M6 L4 Cage. Angew. Chem. Int. Ed. 2019, 58, 9171–9173. [Google Scholar] [CrossRef] [PubMed]
- Cullen, W.; Misuraca, M.C.; Hunter, C.A.; Williams, N.H.; Ward, M.D. Highly efficient catalysis of the Kemp elimination in the cavity of a cubic coordination cage. Nat. Chem. 2016, 8, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.J.; Nichol, G.S.; Lusby, P.J. Orthogonal Selection and Fixing of Coordination Self-Assembly Pathways for Robust Metallo-organic Ensemble Construction. J. Am. Chem. Soc. 2016, 138, 9308–9315. [Google Scholar] [CrossRef] [PubMed]
- Li, R.J.; Holstein, J.J.; Hiller, W.G.; Andreasson, J.; Clever, G.H. Mechanistic Interplay between Light Switching and Guest Binding in Photochromic [Pd2Dithienylethene4] Coordination Cages. J. Am. Chem. Soc. 2019, 141, 2097–2103. [Google Scholar] [CrossRef]
- Oldknow, S.; Martir, D.R.; Pritchard, V.E.; Blitz, M.A.; Fishwick, C.W.G.; Zysman-Colman, E.; Hardie, M.J. Structure-switching M3L2 Ir(iii) coordination cages with photo-isomerising azo-aromatic linkers. Chem. Sci. 2018, 9, 8150–8159. [Google Scholar] [CrossRef]
- Liu, W.X.; Liu, G.X.; Zhu, X.J.; Han, X.; Lu, A.T.; Lu, S.; Shi, L.L.; Hao, X.Q.; Song, M.P. Tailored metal–organic tetrahedral nanocages with aggregation-induced emission for an anti-counterfeiting ink and stimulus-responsive luminescence. New J. Chem. 2022, 46, 8062–8068. [Google Scholar] [CrossRef]
- Bao, S.J.; Xu, Z.M.; Ju, Y.; Song, Y.L.; Wang, H.; Niu, Z.; Li, X.; Braunstein, P.; Lang, J.P. The Covalent and Coordination Co-Driven Assembly of Supramolecular Octahedral Cages with Controllable Degree of Distortion. J. Am. Chem. Soc. 2020, 142, 13356–13361. [Google Scholar] [CrossRef]
- Shi, Q.; Zhou, X.; Yuan, W.; Su, X.; Neniskis, A.; Wei, X.; Taujenis, L.; Snarskis, G.; Ward, J.S.; Rissanen, K.; et al. Selective Formation of S4- and T-Symmetric Supramolecular Tetrahedral Cages and Helicates in Polar Media Assembled via Cooperative Action of Coordination and Hydrogen Bonds. J. Am. Chem. Soc. 2020, 142, 3658–3670. [Google Scholar] [CrossRef]
- Wang, H.; Wang, K.; Xu, Y.; Wang, W.; Chen, S.; Hart, M.; Wojtas, L.; Zhou, L.P.; Gan, L.; Yan, X.; et al. Hierarchical Self-Assembly of Nanowires on the Surface by Metallo-Supramolecular Truncated Cuboctahedra. J. Am. Chem. Soc. 2021, 143, 5826–5835. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhang, Z.; Lu, S.; Yuan, J.; Zhu, Q.; Chen, W.P.; Ling, S.; Li, X.; Zheng, Y.Z.; Zhu, K.; et al. Highly Emissive Perylene Diimide-Based Metallacages and Their Host-Guest Chemistry for Information Encryption. J. Am. Chem. Soc. 2020, 142, 18763–18768. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rajasree, S.S.; Lee, G.Y.; Yu, J.; Tang, J.-H.; Ni, R.; Li, G.; Houk, K.N.; Deria, P.; Stang, P.J. Anthracene–Triphenylamine-Based Platinum(II) Metallacages as Synthetic Light-Harvesting Assembly. J. Am. Chem. Soc. 2021, 143, 2908–2919. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hao, A.; Xing, P. XX Halogen Bond-Induced Supramolecular Helices. Angew. Chem. Int. Ed. 2022, 61, e202113786. [Google Scholar] [CrossRef] [PubMed]
- Dumele, O.; Schreib, B.; Warzok, U.; Trapp, N.; Schalley, C.A.; Diederich, F. Halogenverbrückte supramolekulare Kapseln im Festkörper, in Lösung und in der Gasphase. Angew. Chem. 2017, 129, 1172–1177. [Google Scholar] [CrossRef]
- Tozawa, T.; Jones, J.T.A.; Swamy, S.I.; Jiang, S.; Adams, D.J.; Shakespeare, S.; Clowes, R.; Bradshaw, D.; Hasell, T.; Chong, S.Y.; et al. Porous organic cages. Nat. Mater. 2009, 8, 973–978. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.; Chen, L.; Yang, D.; Li, X.; Wu, G.; Zeng, P.; Zhou, A.; Yin, Q.; Pan, Y.; Wu, B.; et al. Trapping White Phosphorus within a Purely Organic Molecular Container Produced by Imine Condensation. Angew. Chem. Int. Ed. 2017, 56, 14545–14550. [Google Scholar] [CrossRef]
- Ke, X.-S.; Kim, T.; He, Q.; Lynch, V.M.; Kim, D.; Sessler, J.L. Three-Dimensional Fully Conjugated Carbaporphyrin Cage. J. Am. Chem. Soc. 2018, 140, 16455–16459. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Q.; Long, H.; Zhang, W. A Highly C70 Selective Shape-Persistent Rectangular Prism Constructed through One-Step Alkyne Metathesis. J. Am. Chem. Soc. 2011, 133, 20995–21001. [Google Scholar] [CrossRef]
- Mastalerz, M.; Schneider, M.W.; Oppel, I.M.; Presly, O. A Salicylbisimine Cage Compound with High Surface Area and Selective CO2/CH4 Adsorption. Angew. Chem. Int. Ed. 2011, 50, 1046–1051. [Google Scholar] [CrossRef]
- Castilla, A.M.; Ousaka, N.; Bilbeisi, R.A.; Valeri, E.; Ronson, T.K.; Nitschke, J.R. High-Fidelity Stereochemical Memory in a FeII4L4 Tetrahedral Capsule. J. Am. Chem. Soc. 2013, 135, 17999–18006. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wang, C.; Wang, H.; Yang, L.; Jin, P.; Zhang, W.; Jiang, J. Multifunctional Tubular Organic Cage-Supported Ultrafine Palladium Nanoparticles for Sequential Catalysis. Angew. Chem. Int. Ed. 2019, 58, 18011–18016. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Mukherjee, P.S. Cage Encapsulated Gold Nanoparticles as Heterogeneous Photocatalyst for Facile and Selective Reduction of Nitroarenes to Azo Compounds. J. Am. Chem. Soc. 2018, 140, 12592–12601. [Google Scholar] [CrossRef] [PubMed]
- Brutschy, M.; Schneider, M.W.; Mastalerz, M.; Waldvogel, S.R. Porous Organic Cage Compounds as Highly Potent Affinity Materials for Sensing by Quartz Crystal Microbalances. Adv. Mater. 2012, 24, 6049–6052. [Google Scholar] [CrossRef]
- Shi, L.L.; Lu, S.Y. Precise carbon dots synthesis: Building bridges between organic chemistry and inorganic chemistry. Sci. Bull. 2022, 67, 2369–2371. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wang, Y.; Song, B.; Wang, H.J.; Zhou, J.; Sun, Y.; Jones, L.O.; Liu, W.; Zhang, L.; Zhang, X.; et al. A contorted nanographene shelter. Nat. Commun. 2021, 12, 5191. [Google Scholar] [CrossRef]
- Tong, L.; Zhu, D.; Chen, B.; Chen, Y.; Wu, G.; Zeng, F.; Li, H. Water-Soluble Cyclophanes Synthesized via the Zincke Reaction. Org. Lett. 2021, 23, 9343–9347. [Google Scholar] [CrossRef]
- Liu, W.; Tan, Y.; Jones, L.O.; Song, B.; Guo, Q.H.; Zhang, L.; Qiu, Y.; Feng, Y.; Chen, X.Y.; Schatz, G.C.; et al. PCage: Fluorescent Molecular Temples for Binding Sugars in Water. J. Am. Chem. Soc. 2021, 143, 15688–15700. [Google Scholar] [CrossRef]
- Thordarson, P. Available online: http://supramolecular.org (accessed on 28 October 2023).
- Wang, X.; Wang, Y.; Yang, H.; Fang, H.; Chen, R.; Sun, Y.; Zheng, N.; Tan, K.; Lu, X.; Tian, Z.; et al. Assembled molecular face-rotating polyhedra to transfer chirality from two to three dimensions. Nat. Commun. 2016, 7, 12469. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Guo, Y.; Zhang, G.; Shi, L. Tailored Water-Soluble Covalent Organic Cages for Encapsulation of Pyrene and Information Encryption. Int. J. Mol. Sci. 2023, 24, 17541. https://doi.org/10.3390/ijms242417541
Song H, Guo Y, Zhang G, Shi L. Tailored Water-Soluble Covalent Organic Cages for Encapsulation of Pyrene and Information Encryption. International Journal of Molecular Sciences. 2023; 24(24):17541. https://doi.org/10.3390/ijms242417541
Chicago/Turabian StyleSong, Haixin, Yujing Guo, Guorui Zhang, and Linlin Shi. 2023. "Tailored Water-Soluble Covalent Organic Cages for Encapsulation of Pyrene and Information Encryption" International Journal of Molecular Sciences 24, no. 24: 17541. https://doi.org/10.3390/ijms242417541



