Antimicrobial Activity and Toxicity of Newly Synthesized 4-[4-(benzylamino)butoxy]-9H-carbazole Derivatives
Abstract
:1. Introduction
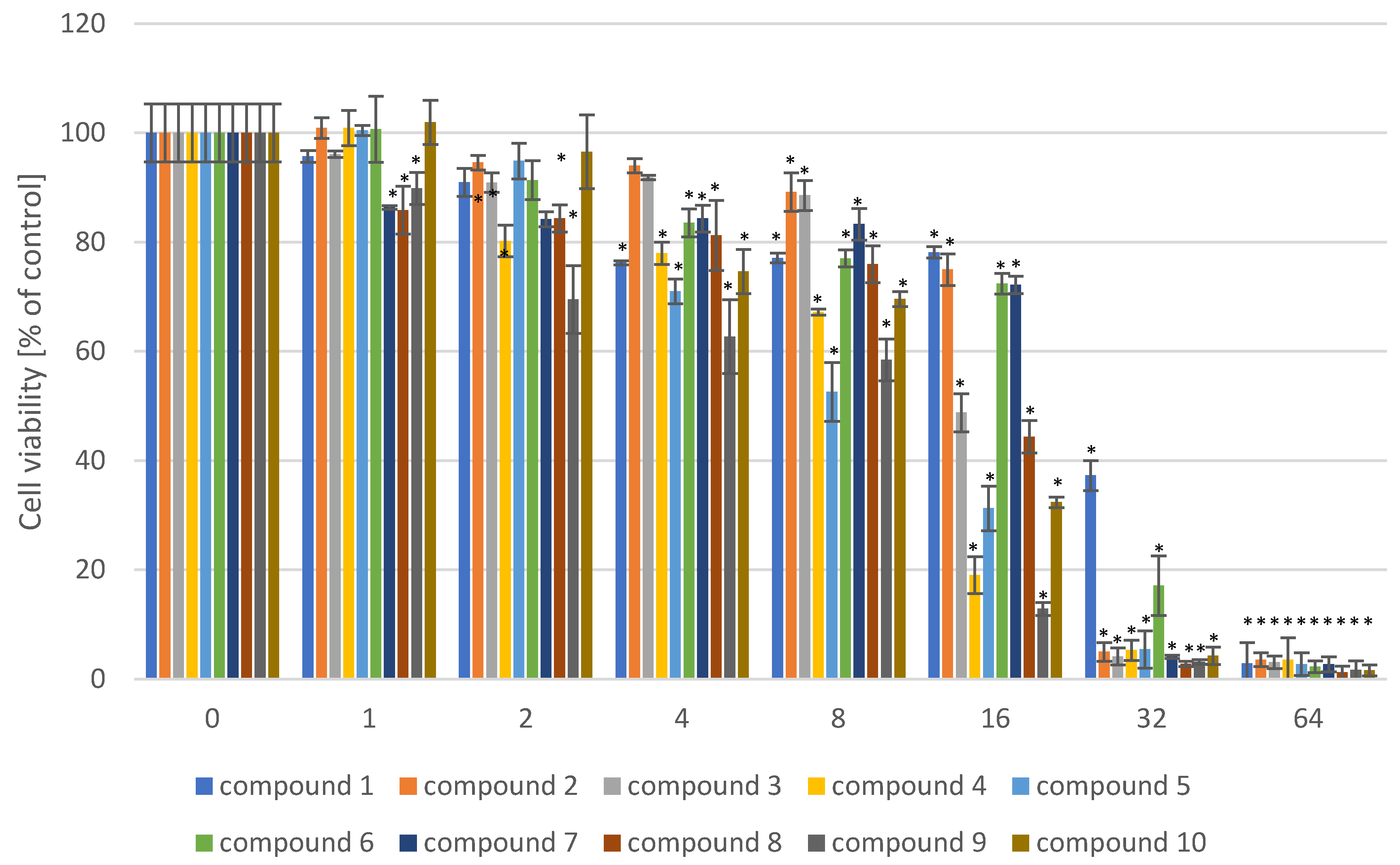
2. Results
3. Discussion
4. Materials and Methods
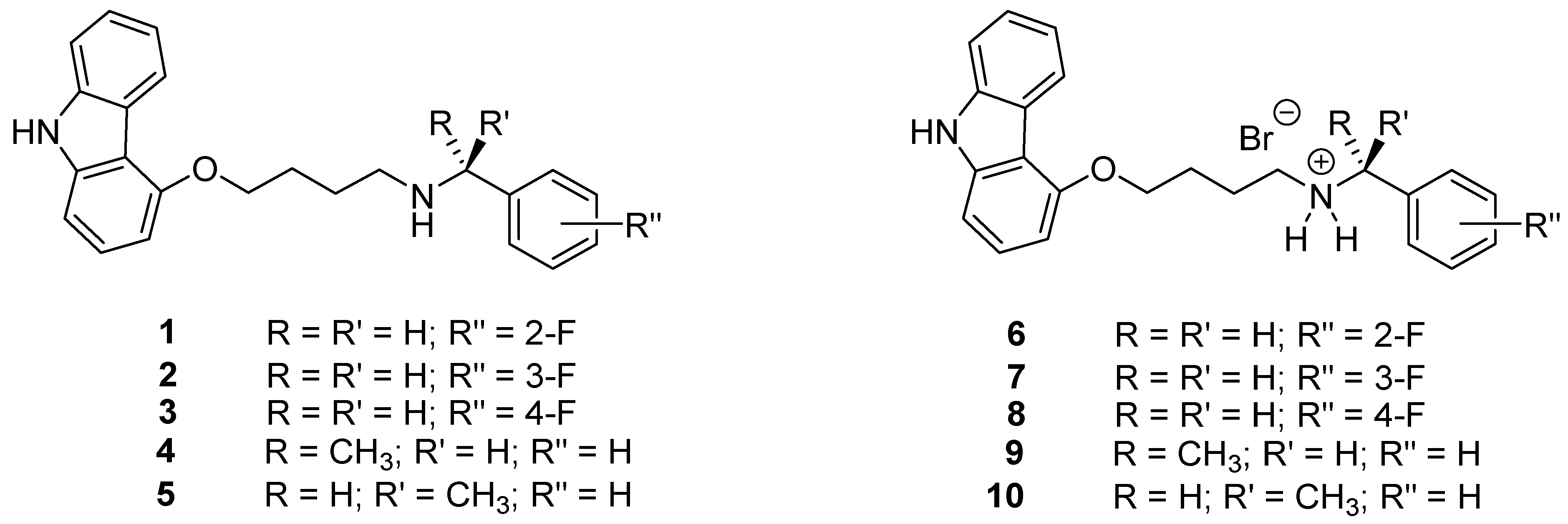
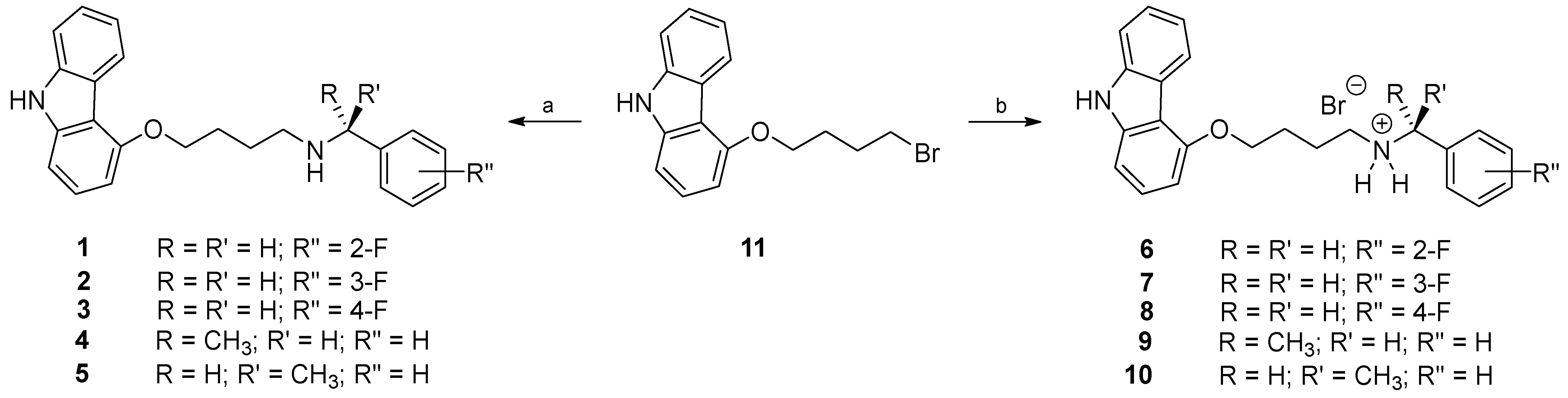
4.1. Synthesis of 4-[4-(benzylamino)butoxy]-9H-carbazole Derivatives 1–10
4.1.1. General Procedure for the Preparation of Carbazole Derivatives 1–5
- 4-[4-(2-fluorophenylmethylamino)butoxy]-9H-carbazole 1
- 4-[4-(3-fluorophenylmethylamino)butoxy]-9H-carbazole 2
- 4-[4-(4-fluorophenylmethylamino)butoxy]-9H-carbazole 3
- (R)-4-[4-(1-phenylethylamino)butoxy]-9H-carbazole 4
- (S)-4-[4-(1-phenylethylamino)butoxy]-9H-carbazole 5 (enantiomer of 4)
4.1.2. General Procedure for the Preparation of Hydrobromides 6–10
- 4-[4-(2-fluorophenylmethylamino)butoxy]-9H-carbazole hydrobromide 6
- 4-[4-(3-fluorophenylmethylamino)butoxy]-9H-carbazole hydrobromide 7
- 4-[4-(4-fluorophenylmethylamino)butoxy]-9H-carbazole hydrobromide 8
- (R)-4-[4-(1-phenylethylamino)butoxy]-9H-carbazole hydrobromide 9
- (S)-4-[4-(1-phenylethylamino)butoxy]-9H-carbazole hydrobromide 10 (enantiomer of 9)
4.2. Determination of Antimicrobial Activity
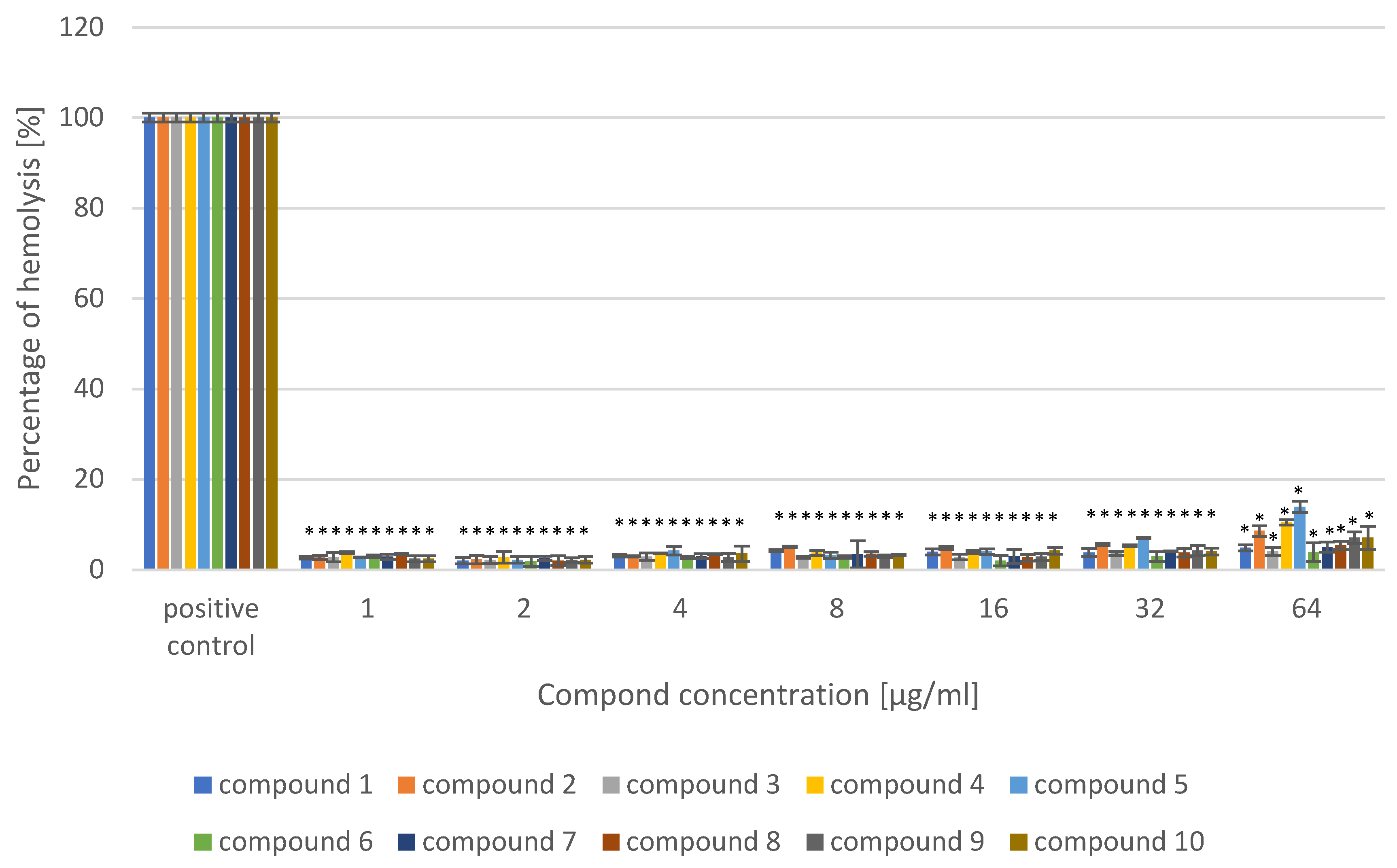
4.3. Evaluation of the Hemolytic Activity of 4-[4-(benzylamino)butoxy]-9H-carbazole Derivatives
4.4. Determination of the Cytotoxic Activity of 4-[4-(benzylamino)butoxy]-9H-carbazole Derivatives
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Antimicrobial Resistance Collaborators. Global mortality associated with 33 bacterial pathogens in 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2023, 400, 2221–2248. [Google Scholar]
- Patil, S.A.; Patil, S.A.; Ble-González, E.A.; Isbel, S.R.; Hampton, S.M.; Bugarin, A. Carbazole Derivatives as Potential Antimicrobial Agents. Molecules 2022, 27, 6575. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, K.; Bernat, P.; Felczak, A.; Różalska, S.; Lisowska, K. Antibacterial activity of high concentrations of carvedilol against Gram-positive and Gram-negative bacteria. Int. J. Antimicrob. Agents 2018, 51, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, K.; Felczak, A.; Głowacka, I.E.; Piotrowska, D.G.; Lisowska, K. Evaluation of the antimicrobial potential and toxicity of a newly synthesised 4-(4-(benzylamino)butoxy)-9H-carbazole. Int. J. Mol. Sci. 2021, 22, 12796. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.; Kirkwood, N.K.; Kenyon, E.J.; Huckvale, R.; Cantillon, D.M.; Waddell, S.J.; Ward, S.E.; Richardson, G.P.; Kros, C.J.; Derudas, M. Design, synthesis, and biological evaluation of a new series of carvedilol derivatives that protect sensory hair cells from aminoglycoside-induced damage by blocking the mechanoelectrical transducer channel. J. Med. Chem. 2019, 62, 5312–5329. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Hao, Y.; Zhang, G.; Wang, S.; Miao, T.; Zhang, K. Synthesis, in vitro antimicrobial and cytotoxic activities of new carbazole derivatives of ursolic acid. Bioorg. Med. Chem. Lett. 2015, 25, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.P.; Ansari, M.F.; Zhang, S.L.; Zhou, C.H. Novel carbazole-oxadiazoles as potential Staphylococcus aureus germicides. Pestic. Biochem. Physiol. 2021, 175, 104849. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.P.; Sangaraiah, N.; Meng, J.P.; Zhou, C.H. Unique Carbazole-oxadiazole derivatives as new potential antibiotics for combating Gram-Positive and -Negative bacteria. J. Med. Chem. 2022, 65, 6171–6190. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, K.; Nowak, M.; Piwoński, I.; Lisowska, K. The Synergy of ciprofloxacin and carvedilol against Staphylococcus aureus-Prospects of a New Treatment Strategy? Molecules 2019, 24, 4104. [Google Scholar] [CrossRef] [PubMed]
- Jasass, R.S.; Alshehrei, F.; Farghaly, T.A. Microwave-assisted synthesis of antimicrobial agents containing carbazole and thiazole moieties. J. Heterocycl. Chem. 2018, 55, 2099–2106. [Google Scholar] [CrossRef]
- Gu, W.; Qiao, C.; Wang, S.F.; Hao, Y.; Miao, T.T. Synthesis and biological evaluation of novel N-substituted 1H-dibenzo[a,c]carbazole derivatives of dehydroabietic acid as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2014, 24, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Salih, N.; Salimon, J.; Yousif, E. Synthesis and antimicrobial activities of 9H-carbazole derivatives. Arab. J. Chem. 2016, 9, S781–S786. [Google Scholar] [CrossRef]
- Hagmann, W.K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef]
- Pattanashetty, S.H.; Hosamani, K.M.; Shettar, A.K.; Mohammed Shafeeulla, R. Design, Synthesis and Computational Studies of Novel Carbazole N-phenylacetamide Hybrids as Potent Antibacterial, Anti-inflammatory, and Antioxidant Agents. J. Heterocycl. Chem. 2018, 55, 1765–1774. [Google Scholar] [CrossRef]
- Merzouki, O.; Arrousse, N.; El Barnossi, A.; Ech-chihbi, E.; Fernine, Y.; Iraqi Housseini, A.; Rais, Z.; Taleb, M. Eco-friendly synthesis, characterization, in-silico ADMET and molecular docking analysis of novel carbazole derivatives as antibacterial and antifungal agents. J. Mol. Struct. 2023, 1271, 133966. [Google Scholar] [CrossRef]
- Ding, Y.-G.; Chen, A.-Q.; Wang, N.; Long, Z.-Q.; Liu, H.-W.; Xie, J.; Liu, S.-T.; Qi, P.-Y.; Zhou, X.; Liu, L.-W.; et al. Discovery of novel 3-(piperazin-1-yl)propan-2-ol decorated carbazole derivatives as new membrane-targeting antibacterial agents. Arab. J. Chem. 2023, 16, 104991. [Google Scholar] [CrossRef]
- Gupta, D.; Bhatia, D.; Dave, V.; Sutariya, V.; Varghese Gupta, S. Salts of Therapeutic Agents: Chemical, Physicochemical, and Biological Considerations. Molecules 2018, 23, 1719. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.J.; Li, M.Y.; Jin, X.J.; Zheng, C.J.; Piao, H.R. Design, synthesis and evaluation of carbazole derivatives as potential antimicrobial agents. J. Enzyme Inhib. Med. Chem. 2021, 36, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Lipiec, T.; Szmal, Z. Chemia Analityczna z Elementami Analizy Instrumentalnej; Państwowy Zakład Wydawnictw Lekarskich: Warszawa, Poland, 1997. [Google Scholar]




| MIC (µg/mL) | MBC/MFC (µg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | S. aureus | S. epidermidis | E. coli | P. aeruginosa | A. flavus | C. albicans | S. aureus | S. epidermidis | E. coli | P. aeruginosa | A. flavus | C. albicans |
| Compound 1 | 64 | 64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| Compound 2 | 32 | 32 | >64 | >64 | >64 | >64 | 32 | 32 | >64 | >64 | >64 | >64 |
| Compound 3 | 32 | 32 | >64 | >64 | >64 | >64 | >64 | 64 | >64 | >64 | >64 | >64 |
| Compound 4 | 32 | 32 | >64 | >64 | >64 | >64 | 32 | 32 | >64 | >64 | >64 | >64 |
| Compound 5 | 32 | 32 | >64 | >64 | >64 | >64 | 64 | 64 | >64 | >64 | >64 | >64 |
| Compound 6 | 64 | 64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| Compound 7 | 32 | 32 | >64 | >64 | >64 | >64 | 64 | 64 | >64 | >64 | >64 | >64 |
| Compound 8 | 32 | 32 | >64 | >64 | >64 | >64 | 64 | 64 | >64 | >64 | >64 | >64 |
| Compound 9 | 32 | 32 | >64 | >64 | >64 | >64 | 64 | 64 | >64 | >64 | >64 | >64 |
| Compound 10 | 32 | 32 | >64 | >64 | >64 | >64 | 64 | 64 | >64 | >64 | >64 | >64 |
| Ciprofloxacin | 0.5 | 0.25 | 0.125 | 0.5 | - | - | 2 | 0.5 | 0.125 | 1 | - | - |
| Amphotericin B | - | - | - | - | 4 | 1 | - | - | - | - | 16 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niedziałkowska, K.; Felczak, A.; Głowacka, I.E.; Piotrowska, D.G.; Lisowska, K. Antimicrobial Activity and Toxicity of Newly Synthesized 4-[4-(benzylamino)butoxy]-9H-carbazole Derivatives. Int. J. Mol. Sci. 2023, 24, 13722. https://doi.org/10.3390/ijms241813722
Niedziałkowska K, Felczak A, Głowacka IE, Piotrowska DG, Lisowska K. Antimicrobial Activity and Toxicity of Newly Synthesized 4-[4-(benzylamino)butoxy]-9H-carbazole Derivatives. International Journal of Molecular Sciences. 2023; 24(18):13722. https://doi.org/10.3390/ijms241813722
Chicago/Turabian StyleNiedziałkowska, Katarzyna, Aleksandra Felczak, Iwona E. Głowacka, Dorota G. Piotrowska, and Katarzyna Lisowska. 2023. "Antimicrobial Activity and Toxicity of Newly Synthesized 4-[4-(benzylamino)butoxy]-9H-carbazole Derivatives" International Journal of Molecular Sciences 24, no. 18: 13722. https://doi.org/10.3390/ijms241813722






