Melatonin Prevents Depression but Not Anxiety-like Behavior Produced by the Chemotherapeutic Agent Temozolomide: Implication of Doublecortin Cells and Hilar Oligodendrocytes
Abstract
:1. Introduction
2. Results
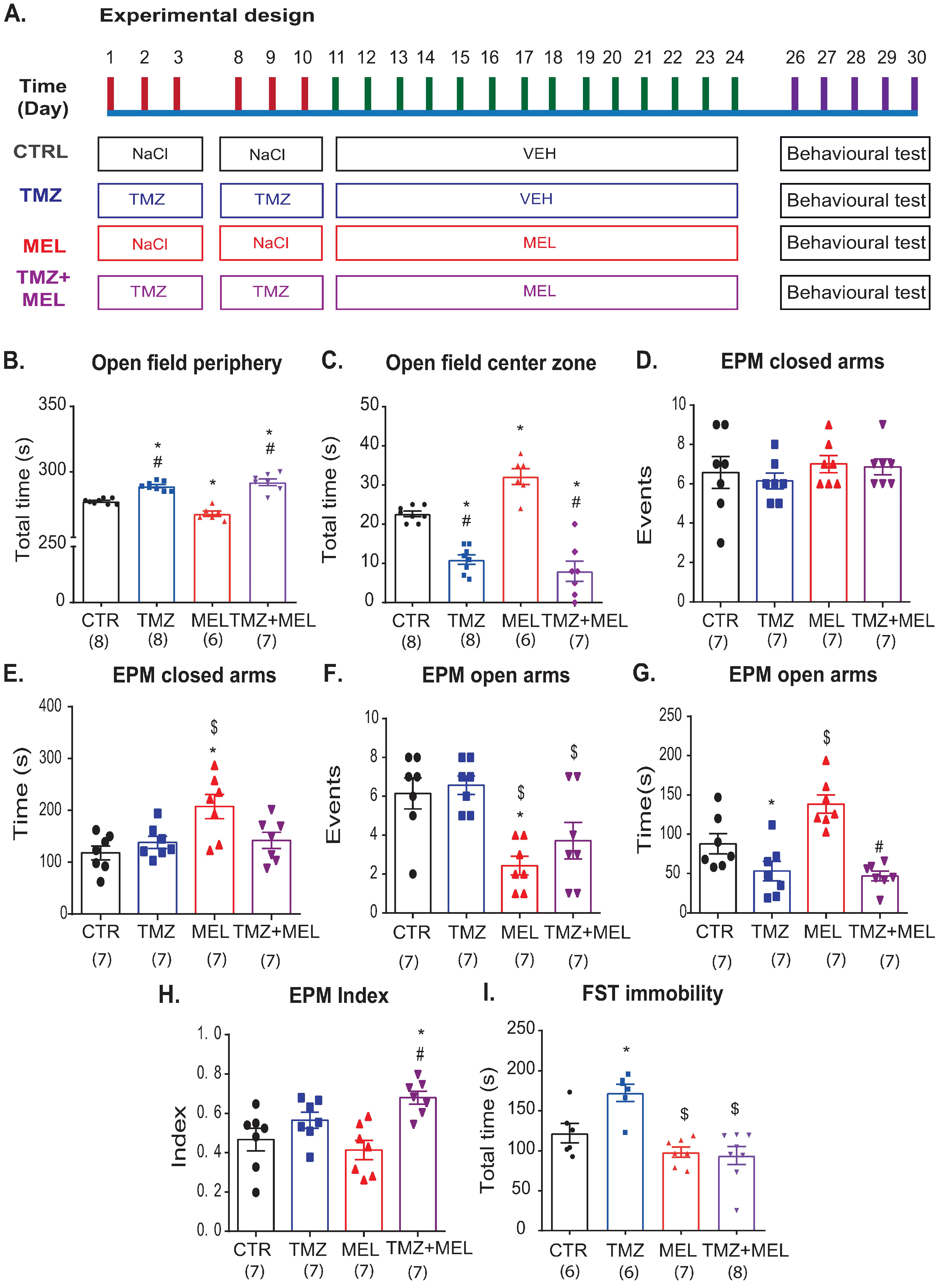
2.1. Temozolomide-Induced Anxiety-like Behavior, Which Melatonin Does Not Prevent
2.2. Temozolomide-Induced Depression-like Effects, Which Melatonin Prevents
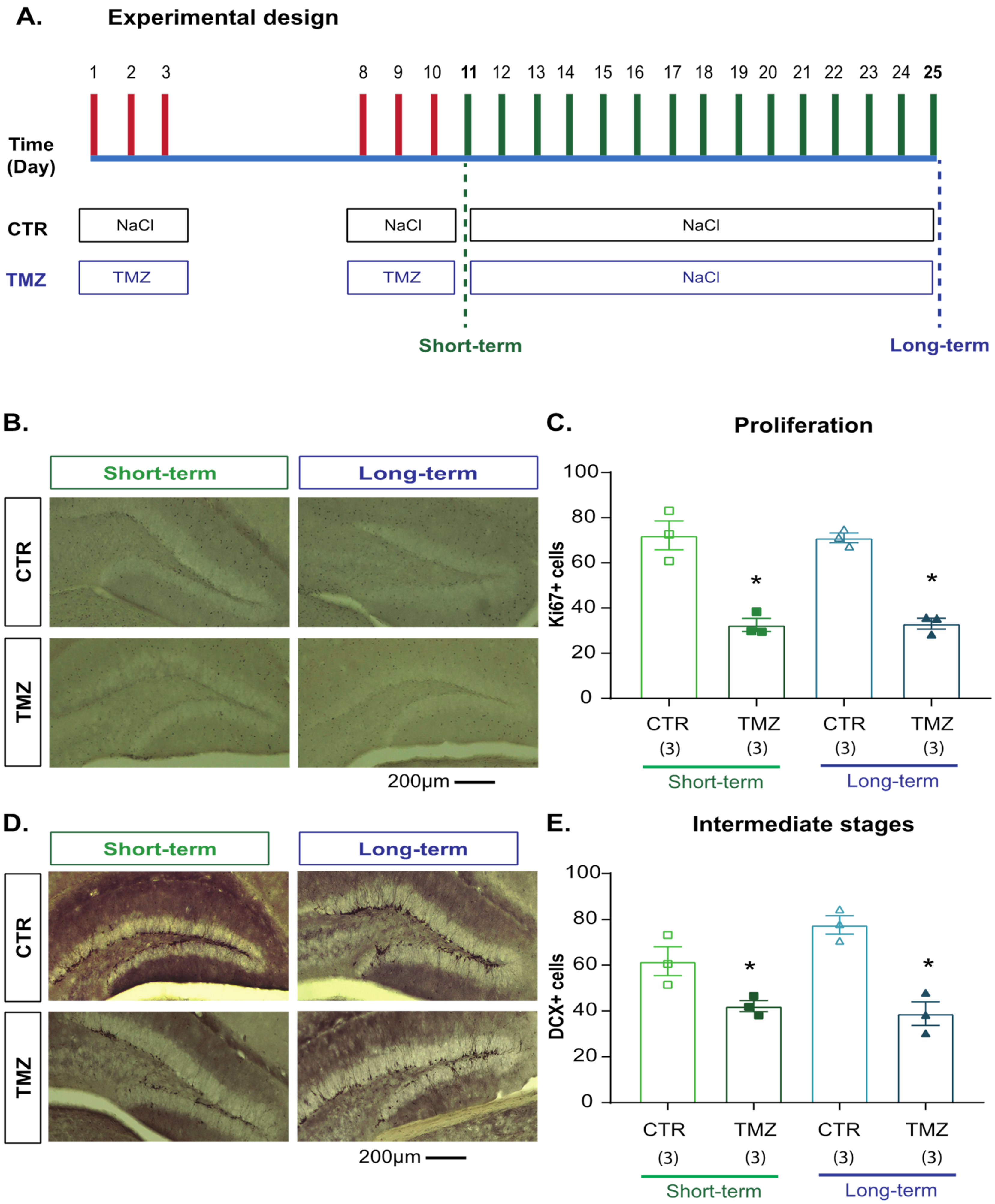
2.3. Temozolomide Induced Short- and Long-Term Effects on Cell Proliferation and Intermediate Stages of Hippocampal Neurogenesis
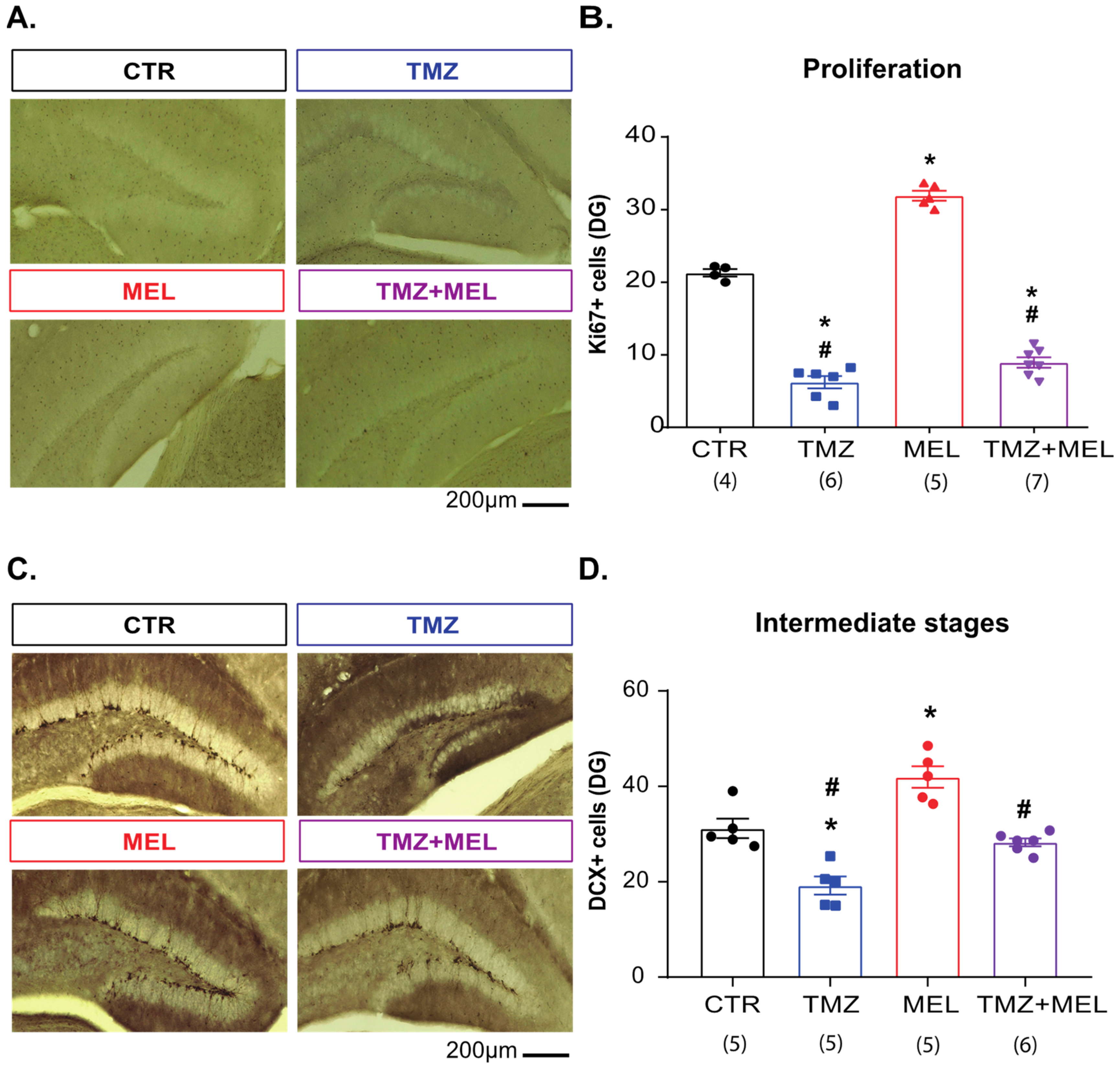
2.4. Melatonin Prevents Temozolomide’s Long-Term Effects on Intermediate Hippocampal Neurogenesis Stages
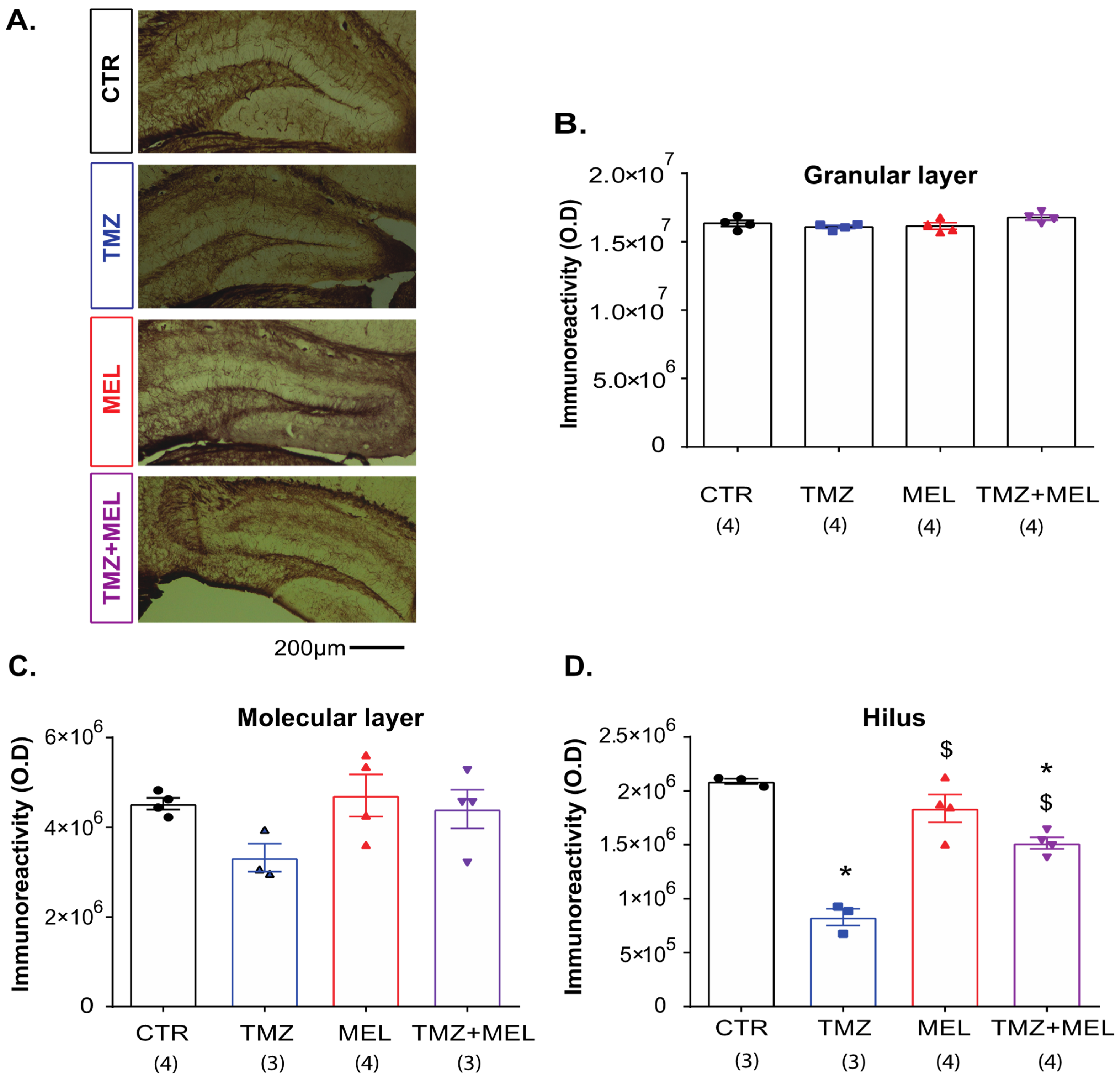
2.5. Melatonin Increased the Volume of the Infrapyramidal Mossy Fibers Tract in the Hilus of the Dentate Gyrus
2.6. Temozolomide Decreased CNPase Immunoreactivity in the Hilus but Not in the Granular Cell or the Dentate Gyrus’s Molecular Layer
2.7. Principal Component Analysis
3. Discussion
3.1. Temozolomide Induces Behavior Related to Depression and Anxiety, but Melatonin Only Prevented Depression-like Behavior
3.2. Melatonin Prevents the Decrement of Ki67 and Doublecortin Cells in the Granular Cell Layer and the CNPase Immunoreactivity in the Dentate Gyrus’s Hilus Caused by Temozolomide
3.3. Conclusions and Limitations
4. Materials and Methods
4.1. Animals
4.2. Melatonin, Temozolomide, and Experimental Design
4.2.1. Experiment 1 (Figure 1, Figure 3, Figure 4, Figure 5 and Figure 6)
4.2.2. Experiment 2 (Figure 2)
4.3. Behavior
4.3.1. Rotarod and Open Field Test
4.3.2. Elevated Plus Maze
4.3.3. Porsolt’s Forced Swimming Test
4.4. Brain Tissue Processing
4.5. Histology
4.6. Principal Component Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Perry, J.R.; Bélanger, K.; Mason, W.P.; Fulton, D.; Kavan, P.; Easaw, J.; Shields, C.; Kirby, S.; Macdonald, D.R.; Eisenstat, D.D.; et al. Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J. Clin. Oncol. 2010, 28, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.; Tabatabai, G.; Kästner, B.; Felsberg, J.; Steinbach, J.P.; Wick, A.; Schnell, O.; Hau, P.; Herrlinger, U.; Sabel, M.C.; et al. MGMT Promoter Methylation Is a Strong Prognostic Biomarker for Benefit from Dose-Intensified Temozolomide Rechallenge in Progressive Glioblastoma: The DIRECTOR Trial. Clin. Cancer Res. 2015, 21, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://gco.iarc.fr/today/fact-sheets-cancers (accessed on 3 July 2022).
- Available online: https://www.cancer.net/cancer-types/brain-tumor/types-treatment (accessed on 3 July 2022).
- McKinnon, C.; Nandhabalan, M.; Murray, S.A.; Plaha, P. Glioblastoma: Clinical presentation, diagnosis, and management. BMJ 2021, 374, n1560. [Google Scholar] [CrossRef] [PubMed]
- Bello-Alvarez, C.; Camacho-Arroyo, I. Impact of sex in the prevalence and progression of glioblastomas: The role of gonadal steroid hormones. Biol. Sex Differ. 2021, 12, 28. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Zárate, M.J.; Cittelly, D.M. Sex steroid hormone function in the brain niche: Implications for brain metastatic colonization and progression. Cancer Rep. 2022, 5, e1241. [Google Scholar] [CrossRef]
- Egeland, M.; Guinaudie, C.; Du Preez, A.; Musaelyan, K.; Zunszain, P.A.; Fernandes, C.; Pariante, C.M.; Thuret, S. Depletion of adult neurogenesis using the chemotherapy drug temozolomide in mice induces behavioural and biological changes relevant to depression. Transl. Psychiatry 2017, 7, e1101. [Google Scholar] [CrossRef]
- Leung, J.W.-H.; Cheung, K.-K.; Ngai, S.P.-C.; Tsang, H.W.-H.; Lau, B.W.-M. Protective Effects of Melatonin on Neurogenesis Impairment in Neurological Disorders and Its Relevant Molecular Mechanisms. Int. J. Mol. Sci. 2020, 21, 5645. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-López, L.; Pérez-Beltran, C.; Ramírez-Rodríguez, G. Chronic administration of a melatonin membrane receptor antagonist, luzindole, affects hippocampal neurogenesis without changes in hopelessness-like behavior in adult mice. Neuropharmacology 2016, 103, 211–221. [Google Scholar] [CrossRef]
- Patiño, P.; Parada, E.; Farré-Alins, V.; Molz, S.; Cacabelos, R.; Marco-Contelles, J.; López, M.G.; Tasca, C.I.; Ramos, E.; Romero, A.; et al. Melatonin protects against oxygen and glucose deprivation by decreasing extracellular glutamate and Nox-derived ROS in rat hippocampal slices. Neurotoxicology 2016, 57, 61–68. [Google Scholar] [CrossRef]
- Olivier, P.; Fontaine, R.H.; Loron, G.; Van Steenwinckel, J.; Biran, V.; Massonneau, V.; Kaindl, A.; Dalous, J.; Charriaut-Marlangue, C.; Aigrot, M.S.; et al. Melatonin Promotes Oligodendroglial Maturation of Injured White Matter in Neonatal Rats. PLoS ONE 2009, 4, e7128. [Google Scholar] [CrossRef]
- Sarlak, G.; Jenwitheesuk, A.; Chetsawang, B.; Govitrapong, P. Effects of melatonin on nervous system aging: Neurogenesis and neurodegeneration. J. Pharmacol. Sci. 2013, 123, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rodríguez, G.B.; Olvera-Hernández, S.; Vega-Rivera, N.M.; Ortiz-López, L. Melatonin Influences Structural Plasticity in the Axons of Granule Cells in the Dentate Gyrus of Balb/C Mice. Int. J. Mol. Sci. 2018, 20, 73. [Google Scholar] [CrossRef]
- Vega-Rivera, N.M.; Ortiz-López, L.; Granados-Juárez, A.; Estrada-Camarena, E.M.; Ramírez-Rodríguez, G.B. Melatonin Reverses the Depression-associated Behaviour and Regulates Microglia, Fractalkine Expression and Neurogenesis in Adult Mice Exposed to Chronic Mild Stress. Neuroscience 2020, 440, 316–366. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.; Vega-Rivera, N.M.; Benitez-King, G.; Castro-Garcia, M.; Ortiz-Lopez, L. Melatonin supplementation delays the decline of adult hippocampal neurogenesis during normal aging of mice. Neurosci. Lett. 2012, 530, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Nokia, M.S.; Anderson, M.L.; Shors, T.J. Chemotherapy disrupts learning, neurogenesis and theta activity in the adult brain. Eur. J. Neurosci. 2012, 36, 3521–3530. [Google Scholar] [CrossRef] [PubMed]
- Sekeres, M.J.; Bradley-Garcia, M.; Martinez-Canabal, A.; Winocur, G. Chemotherapy-Induced Cognitive Impairment and Hippocampal Neurogenesis: A Review of Physiological Mechanisms and Interventions. Int. J. Mol. Sci. 2021, 22, 12697. [Google Scholar] [CrossRef] [PubMed]
- Bumb, J.; Enning, F.; Mueller, J.; van der List, T.; Rohleder, C.; Findeisen, P.; Noelte, I.; Schwarz, E.; Leweke, F. Differential melatonin alterations in cerebrospinal fluid and serum of patients with major depressive disorder and bipolar disorder. Compr. Psychiatry 2016, 68, 34–39. [Google Scholar] [CrossRef]
- Li, K.; Shen, S.; Ji, Y.T.; Li, X.Y.; Zhang, L.S.; Wang, X.D. Melatonin Augments the Effects of Fluoxetine on Depression-Like Behavior and Hippocampal BDNF-TrkB Signaling. Neurosci. Bull. 2018, 34, 303–311. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and Microglia. Int. J. Mol. Sci. 2021, 22, 8296. [Google Scholar] [CrossRef]
- Villapol, S.; Fau, S.; Renolleau, S.; Biran, V.; Charriaut-Marlangue, C.; Baud, O. Melatonin Promotes Myelination by Decreasing White Matter Inflammation after Neonatal Stroke. Pediatr. Res. 2011, 69, 51–55. [Google Scholar] [CrossRef]
- Parkitny, L.; Maletic-Savatic, M. Glial PAMPering and DAMPening of Adult Hippocampal Neurogenesis. Brain Sci. 2021, 11, 1299. [Google Scholar] [CrossRef] [PubMed]
- Römer, B.; Krebs, J.; Overall, R.W.; Fabel, K.; Babu, H.; Overstreet-Wadiche, L.; Brandt, M.D.; Williams, R.W.; Jessberger, S.; Kempermann, G. Adult hippocampal neurogenesis and plasticity in the infrapyramidal bundle of the mossy fiber projection: I. Co-regulation by activity. Front. Neurosci. 2011, 5, 107. [Google Scholar] [CrossRef] [PubMed]
- Berggård, T.; Miron, S.; Önnerfjord, P.; Thulin, E.; Åkerfeldt, K.S.; Enghild, J.J.; Akke, M.; Linse, S. CalbindinD28k exhibits properties characteristic of a Ca2+ sensor. J. Biol. Chem. 2002, 277, 16662–16672. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro-Oncology 2020, 22, 1073–1113. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.L.; de Kloet, E.R. Forced swim stressor: Trends in usage and mechanistic consideration. Eur. J. Neurosci. 2022, 55, 2813–2831. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rodríguez, G.; Klempin, F.; Babu, H.; Benítez-King, G.; Kempermann, G. Melatonin modulates cell survival of new neurons in the hippocampus of adult mice. Neuropsychopharmacology 2009, 34, 2180–2191. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.-W.; Laudon, M.; Han, L.; Gao, J.; Huang, F.-L.; Yang, Y.-F.; Deng, H.-F. Antidepressant- and anxiolytic effects of the novel melatonin agonist Neu-P11 in rodent models. Acta Pharmacol. Sin. 2010, 31, 775–783. [Google Scholar] [CrossRef]
- Deacon, R.M. Measuring motor coordination in mice. J. Vis. Exp. 2013, 75, e2609. [Google Scholar] [CrossRef]
- Gould, T.D.; Dao, D.T.; Kovacsics, C.E. The Open Field Test. In Mood and Anxiety Related Phenotypes in Mice. In Neuromethods; Gould, T.D., Ed.; Humana Press: Totowa, NJ, USA, 2009; Volume 42, pp. 1–20. [Google Scholar]
- Komada, M.; Takao, K.; Miyakawa, T. Elevated plus maze for mice. J. Vis. Exp. 2008, 22, e1088. [Google Scholar]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. J. Vis. Exp. 2015, 96, e52434. [Google Scholar]
- Golombek, D.A.; Martini, M.; Cardinali, D.P. Melatonin as an anxiolytic in rats: Time dependence and interaction with the central GABAergic system. Eur. J. Pharmacol. 1993, 237, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Papp, M.; Litwa, E.; Gruca, P.; Mocaёr, E. Anxiolytic-like activity of agomelatine and melatonin in three animal models of anxiety. Behav. Pharmacol. 2006, 17, 9–18. [Google Scholar] [PubMed]
- Molendijk, M.L.; de Kloet, E.R. Coping with the forced swim stressor: Current state-of-the-art. Behav. Brain Res. 2019, 364, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lacoste, B.; Angeloni, D.; Dominguez-Lopez, S.; Calderoni, S.; Mauro, A.; Fraschini, F.; Descarries, L.; Gobbi, G. Anatomical and cellular localization of melatonin MT1 and MT2 receptors in the adult rat brain. J. Pineal Res. 2015, 58, 397–417. [Google Scholar] [CrossRef]
- Comai, S.; Ochoa-Sanchez, R.; Dominguez-Lopez, S.; Bambico, F.R.; Gobbi, G. Melancholic-Like behaviors and circadian neurobiological abnormalities in melatonin MT1 receptor knockout mice. Int. J. Neuropsychopharmacol. 2015, 18, pyu075. [Google Scholar] [CrossRef]
- Comai, S.; De Gregorio, D.; Posa, L.; Ochoa-Sanchez, R.; Bedini, A.; Gobbi, G. Dysfunction of serotonergic activity and emotional responses across the light-dark cycle in mice lacking melatonin MT2 receptors. J. Pineal Res. 2020, 69, e12653. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Sanchez, R.; Rainer, Q.; Comai, S.; Spadoni, G.; Bedini, A.; Rivara, S.; Fraschini, F.; Mor, M.; Tarzia, G.; Gobbi, G. Anxiolytic effects of the melatonin MT2 receptor partial agonist UCM765: Comparison with melatonin and diazepam. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 39, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Revest, J.-M.; Dupret, D.; Koehl, M.; Funk-Reiter, C.; Grosjean, N.; Piazza, P.-V.; Abrous, D.N. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol. Psychiatry 2009, 14, 959–967. [Google Scholar] [CrossRef]
- Dias, G.P.; Bevilaqua, M.C.; da Luz, A.C.; Fleming, R.L.; de Carvalho, L.A.; Cocks, G.; Beckman, D.; Hosken, L.C.; de Sant’Anna Machado, W.; Corrêa-e-Castro, A.C.; et al. Hippocampal biomarkers of fear memory in an animal model of generalized anxiety disorder. Behav. Brain Res. 2014, 263, 34–45. [Google Scholar] [CrossRef]
- Omeiza, N.A.; Abdulrahim, H.A.; Alagbonsi, A.I.; Ezurike, P.U.; Soluoku, T.K.; Isiabor, H.; Alli-Oluwafuyi, A.A. Melatonin salvages lead-induced neuro-cognitive shutdown, anxiety, and depressive-like symptoms via oxido-inflammatory and cholinergic mechanisms. Brain Behav. 2021, 11, e2227. [Google Scholar] [CrossRef]
- Kheirbek, M.A.; Klemenhagen, K.C.; Sahay, A.; Hen, R. Neurogenesis and generalization: A new approach to stratify and treat anxiety disorders. Nat. Neurosci. 2012, 15, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Garthe, A.; Behr, J.; Kempermann, G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS ONE 2009, 4, e5464. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Rodriguez, G.; Ortíz-López, L.; Domínguez-Alonso, A.; Benítez-King, G.A.; Kempermann, G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J. Pineal Res. 2011, 50, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Stefanova, N.A.; Maksimova, K.Y.; Kiseleva, E.; Rudnitskaya, E.A.; Muraleva, N.A.; Kolosova, N.G. Melatonin attenuates impairments of structural hippocampal neuroplasticity in OXYS rats during active progression ofAlzheimer’s disease-like pathology. J. Pineal Res. 2015, 59, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Ghareghani, M.; Sadeghi, H.; Zibara, K.; Danaei, N.; Azari, H.; Ghanbari, A. Melatonin Increases Oligodendrocyte Differentiation in Cultured Neural Stem Cells. Cell. Mol. Neurobiol. 2017, 37, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Schwegler, H.; Crusio, W.E.; Brust, I. Hippocampal mossy fibers and radial-maze learning in the mouse: A correlation with spatial working memory but not with non-spatial reference memory. Neuroscience 1990, 34, 293–298. [Google Scholar] [CrossRef]
- Toni, N.; Laplagne, D.A.; Zhao, C.; Lombardi, G.; Ribak, C.E.; Gage, F.H.; Schinder, A.F. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat. Neurosci. 2008, 11, 901–907. [Google Scholar] [CrossRef]
- Hoogenraad, C.C.; Bradke, F. Control of neuronal polarity and plasticity—A renaissance for microtubules? Trends Cell Biol. 2009, 19, 669–676. [Google Scholar] [CrossRef]
- Crusio, W.E.; Schwegler, H. Hippocampal mossy fiber distribution covaries with open-field habituation inthe mouse. Behav. Brain Res. 1987, 26, 153–158. [Google Scholar] [CrossRef]
- Kobayashi, K. Targeting the hippocampal mossy fiber synapse for the treatment of psychiatric disorders. Mol. Neurobiol. 2009, 39, 24–36. [Google Scholar] [CrossRef]
- Liu, D.; Wei, N.; Man, H.-Y.; Lu, Y.; Zhu, L.-Q.; Wang, J.-Z. The MT2 receptor stimulates axonogenesis and enhances synaptic transmission by activating Akt signaling. Cell Death Differ. 2015, 22, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Wellman, S.M.; Cambi, F.; Kozai, T.D. The role of oligodendrocytes and their progenitors on neural interface technology: A novel perspective on tissue regeneration and repair. Biomaterials 2018, 183, 200–217. [Google Scholar] [CrossRef] [PubMed]
- Wigley, R.; Hamilton, N.; Nishiyama, A.; Kirchhoff, F.; Butt, A.M. Morphological and physiological interactions of NG2-glia with astrocytes and neurons. J. Anat. 2007, 210, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Thornton, M.A.; Hughes, E.G. Neuron-oligodendroglia interactions: Activity-dependent regulation of cellular signaling. Neurosci. Lett. 2020, 727, 134916. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Zhu, Z.; Ransom, B.R.; Tong, X. Oligodendrocyte lineage cells and depression. Mol. Psychiatry 2021, 26, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Mendivil-Perez, M.; Soto-Mercado, V.; Guerra-Librero, A.; Fernandez-Gil, B.I.; Florido, J.; Shen, Y.-Q.; Tejada, M.A.; Capilla-Gonzalez, V.; Rusanova, I.; Garcia-Verdugo, J.M.; et al. Melatonin enhances neural stem cell differentiation and engraftment by increasing mitochondrial function. J. Pineal Res. 2017, 63, e12415. [Google Scholar] [CrossRef]
- Braun, P.E.; Sandillon, F.; Edwards, A.; Matthieu, J.M.; Privat, A. Immunocytochemical localization by electron microscopy of 2′3′-cyclic nucleotide 3′-phosphodiesterase in developing oligodendrocytes of normal and mutant brain. J. Neurosci. 1988, 8, 3057–3066. [Google Scholar] [CrossRef] [PubMed]
- Scherer, S.S.; Braun, P.E.; Grinspan, J.; Collarini, E.; Wang, D.Y.; Kamholz, J. Differential regulation of the 20,30-cyclic nucleotide 30-phosphodiesterase gene during oligodendrocyte development. Neuron 1994, 12, 1363–1375. [Google Scholar] [CrossRef]
- Cabrera-Muñoz, E.A.; Olvera-Hernández, S.; Vega-Rivera, N.M.; Meneses-San Juan, D.; Reyes-Haro, D.; Ortiz-López, L.; Ramírez Rodríguez, G.B. Environmental Enrichment Differentially Activates Neural Circuits in FVB/N Mice, Inducing Social Interaction in Females but Agonistic Behavior in Males. Neurochem. Res. 2022, 47, 781–794. [Google Scholar] [CrossRef]
- Yalcin, I.; Belzung, C.; Surget, A. Mouse strain differences in the unpredictable chronic mild stress: A four-antidepressant survey. Behav. Brain Res. 2008, 193, 140–143. [Google Scholar] [CrossRef]
- Cohen, H.; Liu, T.; Kozlovsky, N.; Kaplan, Z.; Zohar, J.; Mathé, A.A. The Neuropeptide Y (NPY)-ergic System is Associated with Behavioral Resilience to Stress Exposure in an Animal Model of Post-Traumatic Stress Disorder. Neuropsychopharmacology 2012, 37, 350–363. [Google Scholar] [CrossRef]
- Alboukadel Kassambara and Fabian Mundt (2020). Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R package version 1.0.7. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 23 August 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 23 August 2022).



| Principal Component | Eigenvalues |
|---|---|
| PC1 | 5.66 |
| PC2 | 1.71 |
| PC3 | 0.79 |
| PC4 | 0.42 |
| PC5 | 0.20 |
| PC6 | 0.135 |
| PC7 | 0.045 |
| PC8 | 0.015 |
| Measure | PC1 (%) | PC2 (%) |
|---|---|---|
| OF center time | 13.17 | 10.07 |
| OF periphery time | 13.17 | 10.07 |
| EPM open arms time | 7.69 | 16.68 |
| EPM close arms time | 8.21 | 12.49 |
| FST immobility time | 4.62 | 34.70 |
| Ki67-positive cells | 16.44 | 0.16 |
| DCX-positive cells | 12.62 | 10.95 |
| CB IFPMFT | 14.34 | 2.76 |
| CNPase immunoreactivity hilus | 9.70 | 2.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-Muñoz, E.A.; Ramírez-Rodríguez, G.B.; Díaz-Yañez, L.; Reyes-Galindo, V.; Meneses-San Juan, D.; Vega-Rivera, N.M. Melatonin Prevents Depression but Not Anxiety-like Behavior Produced by the Chemotherapeutic Agent Temozolomide: Implication of Doublecortin Cells and Hilar Oligodendrocytes. Int. J. Mol. Sci. 2023, 24, 13376. https://doi.org/10.3390/ijms241713376
Cabrera-Muñoz EA, Ramírez-Rodríguez GB, Díaz-Yañez L, Reyes-Galindo V, Meneses-San Juan D, Vega-Rivera NM. Melatonin Prevents Depression but Not Anxiety-like Behavior Produced by the Chemotherapeutic Agent Temozolomide: Implication of Doublecortin Cells and Hilar Oligodendrocytes. International Journal of Molecular Sciences. 2023; 24(17):13376. https://doi.org/10.3390/ijms241713376
Chicago/Turabian StyleCabrera-Muñoz, Edith Araceli, Gerardo Bernabé Ramírez-Rodríguez, Lizeth Díaz-Yañez, Verónica Reyes-Galindo, David Meneses-San Juan, and Nelly Maritza Vega-Rivera. 2023. "Melatonin Prevents Depression but Not Anxiety-like Behavior Produced by the Chemotherapeutic Agent Temozolomide: Implication of Doublecortin Cells and Hilar Oligodendrocytes" International Journal of Molecular Sciences 24, no. 17: 13376. https://doi.org/10.3390/ijms241713376





