Magnesium Nutrient Application Induces Metabolomics and Physiological Responses in Mulberry (Morus alba) Plants
Abstract
:1. Introduction
2. Results
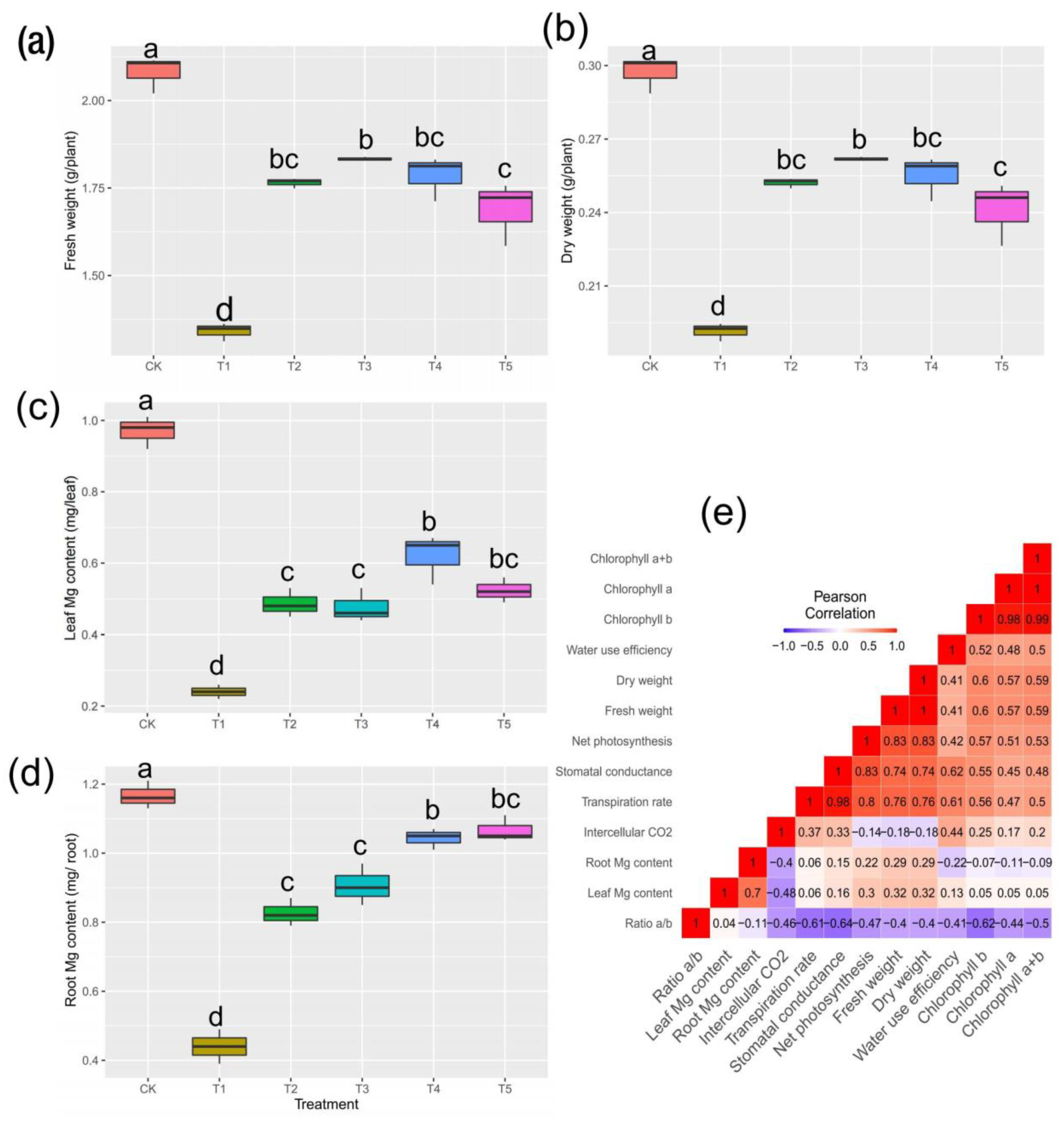
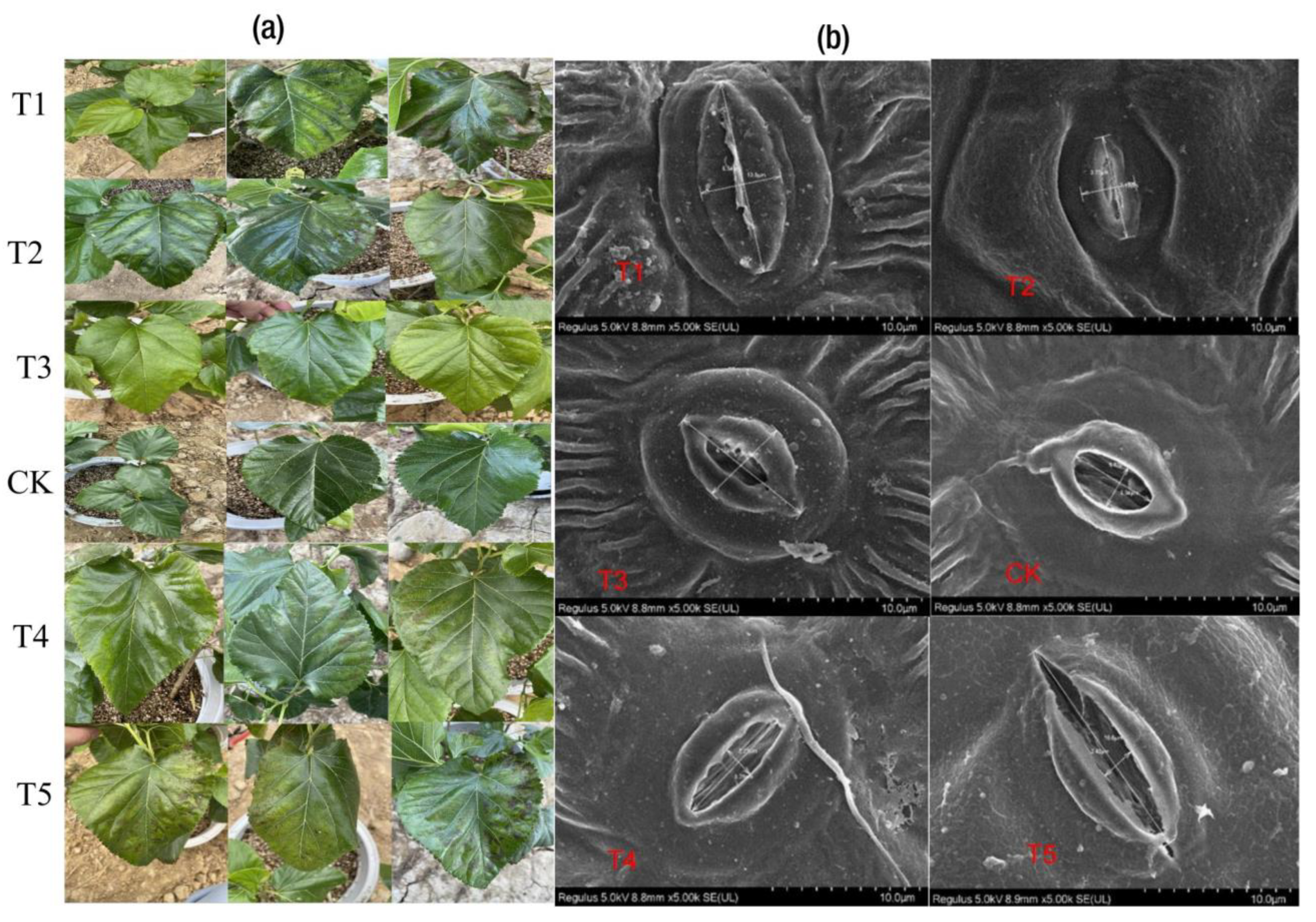
2.1. Plant Biomass Production and Mg Content in Tissues
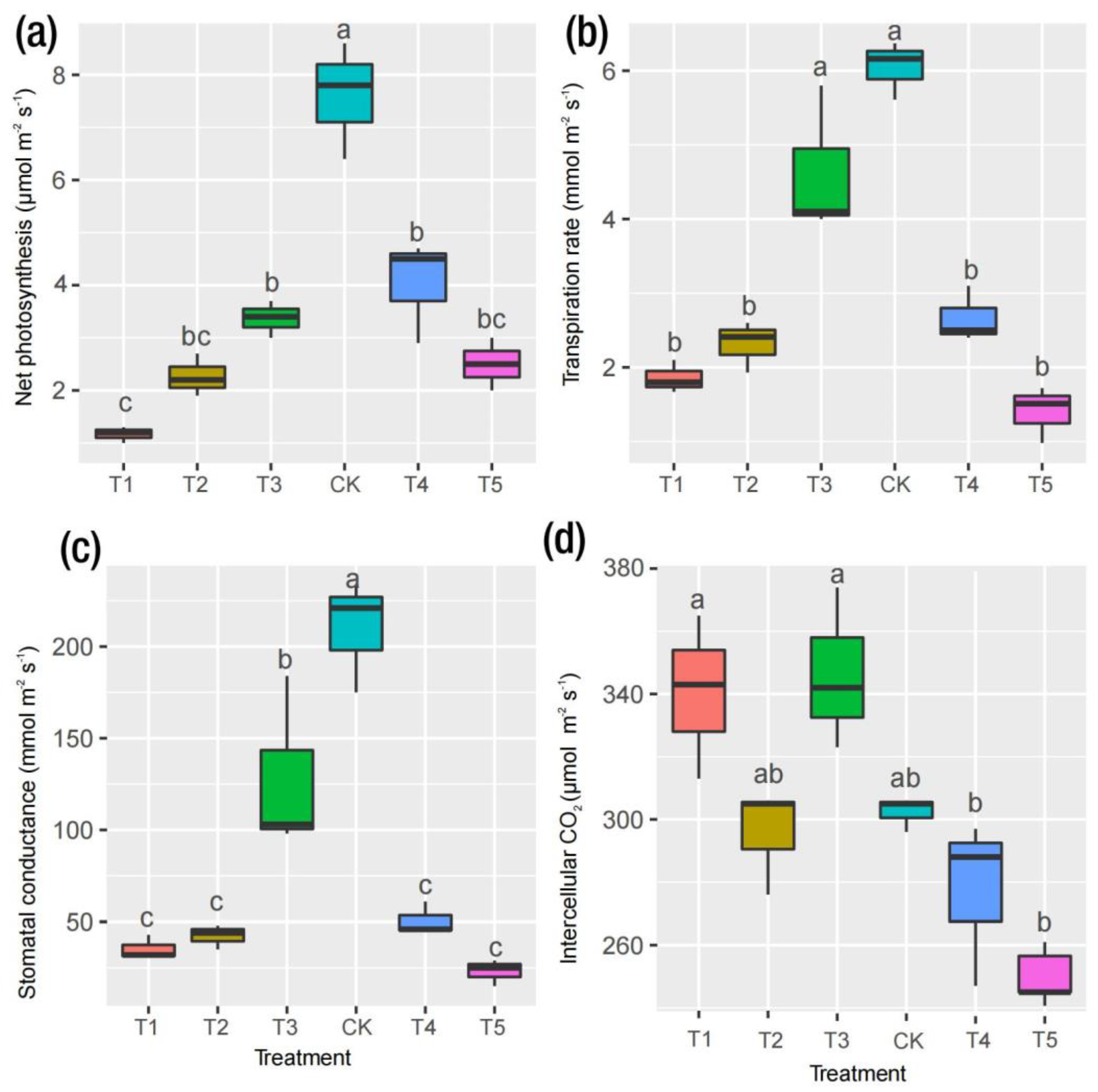
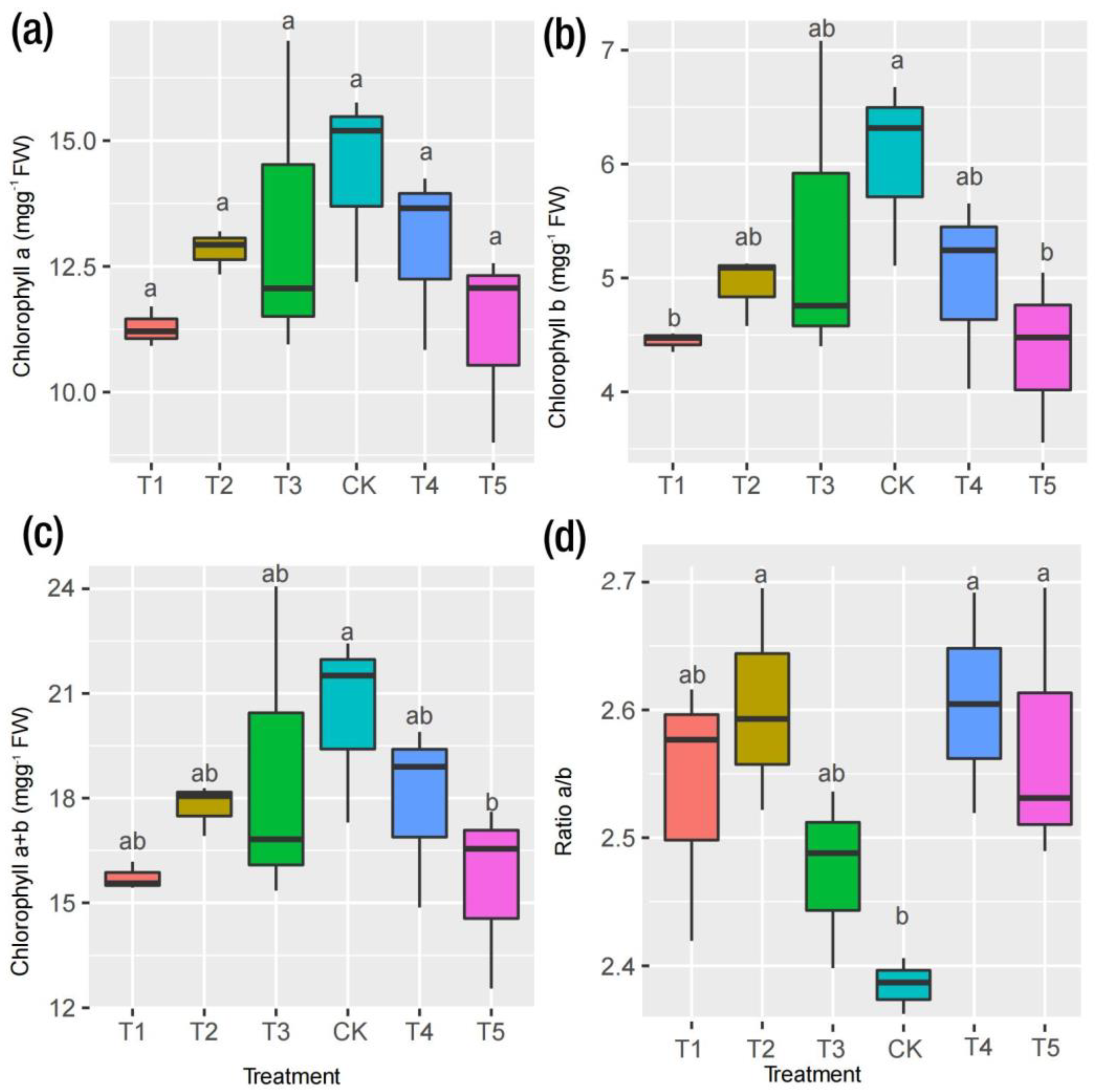
2.2. Physiological Responses to Mg and Leaf Pigments
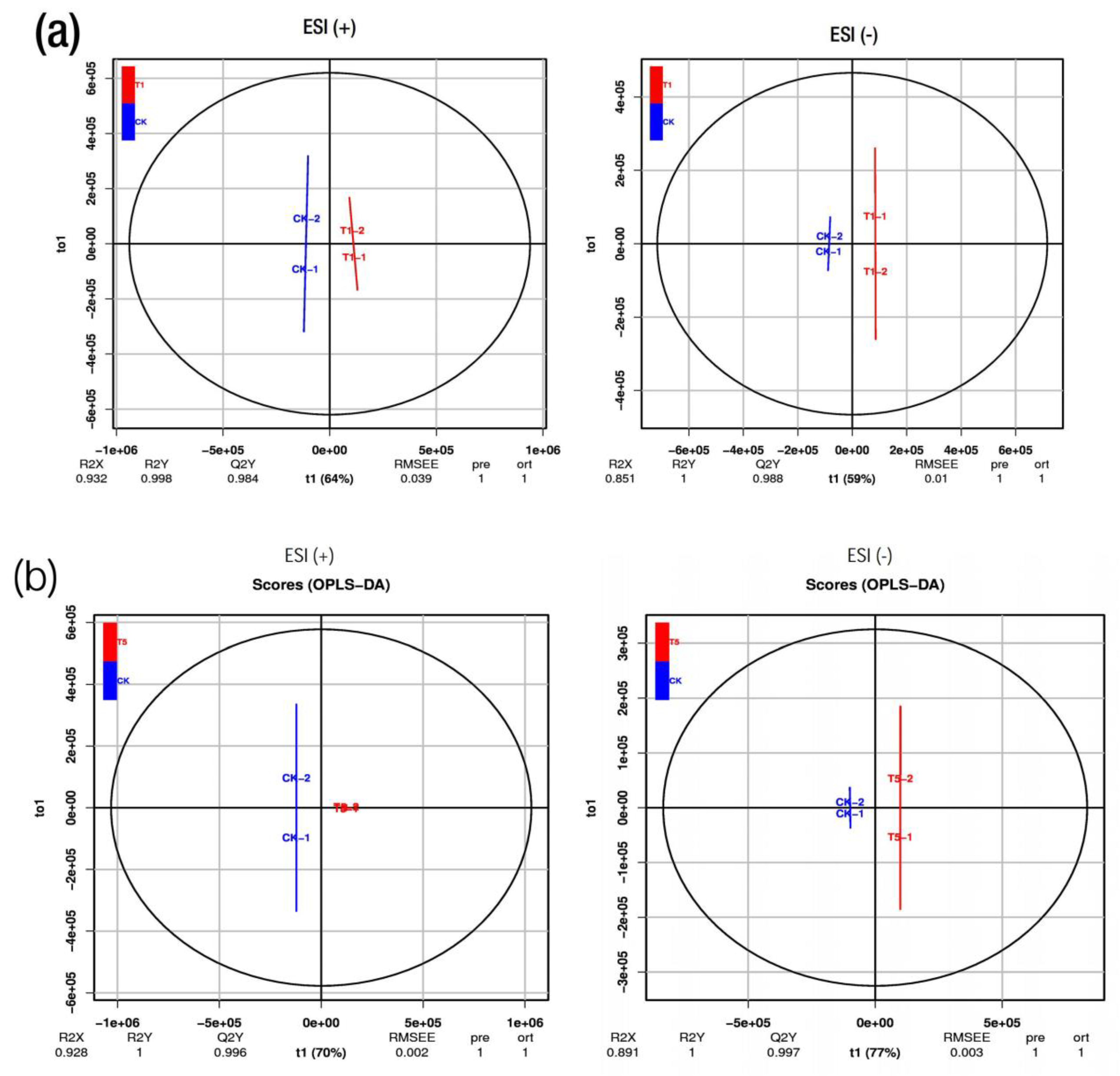
2.3. Metabolic Profiles in M. alba Leaves: Responses to Various Applications of Mg
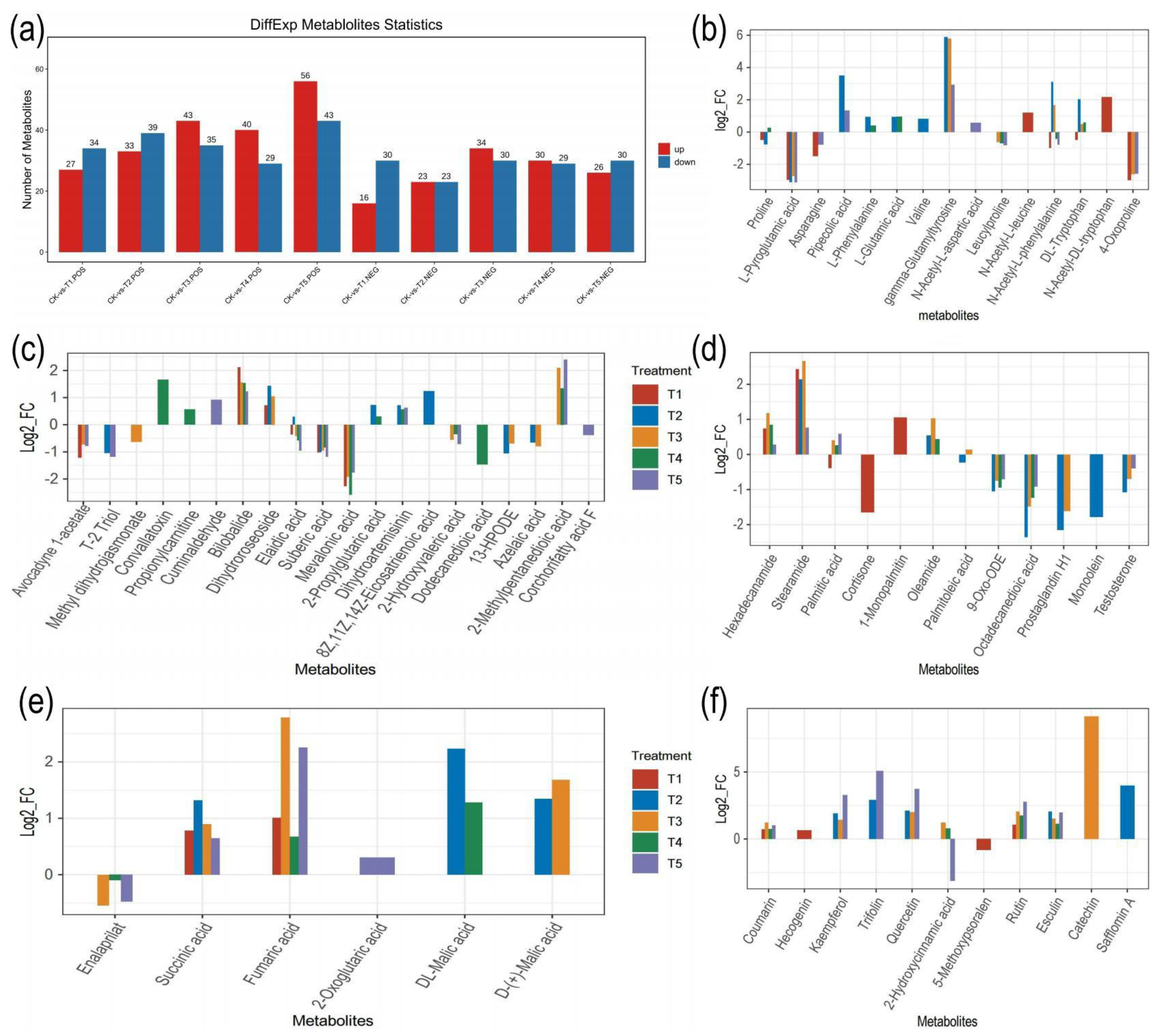
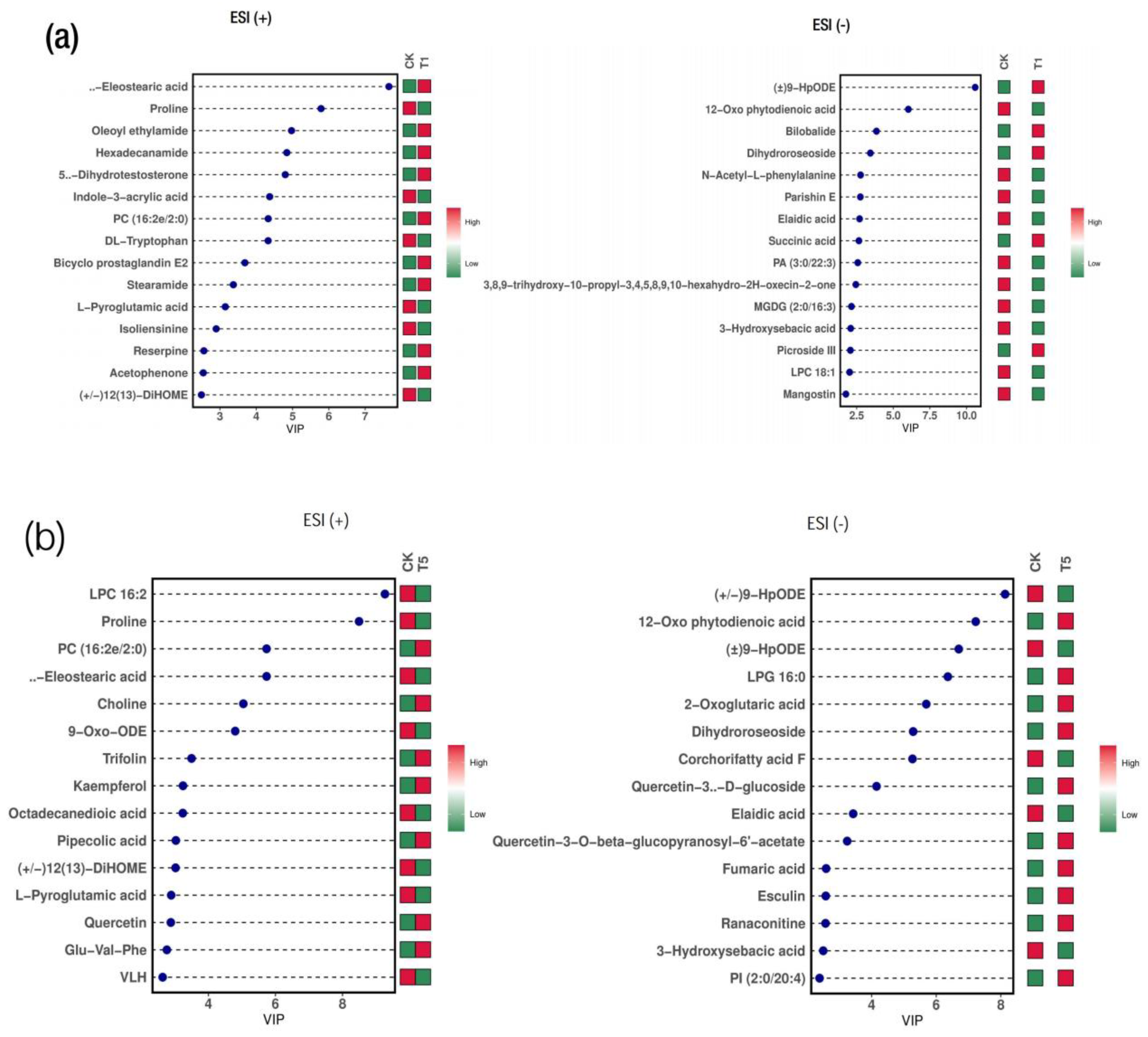
2.4. Analysis of the Differential Metabolites in M. alba Leaves: Responses to Various Mg Applications and Deficiency
2.5. Correlation and Interaction Networks Analysis between the Differential Metabolites’ Profiles
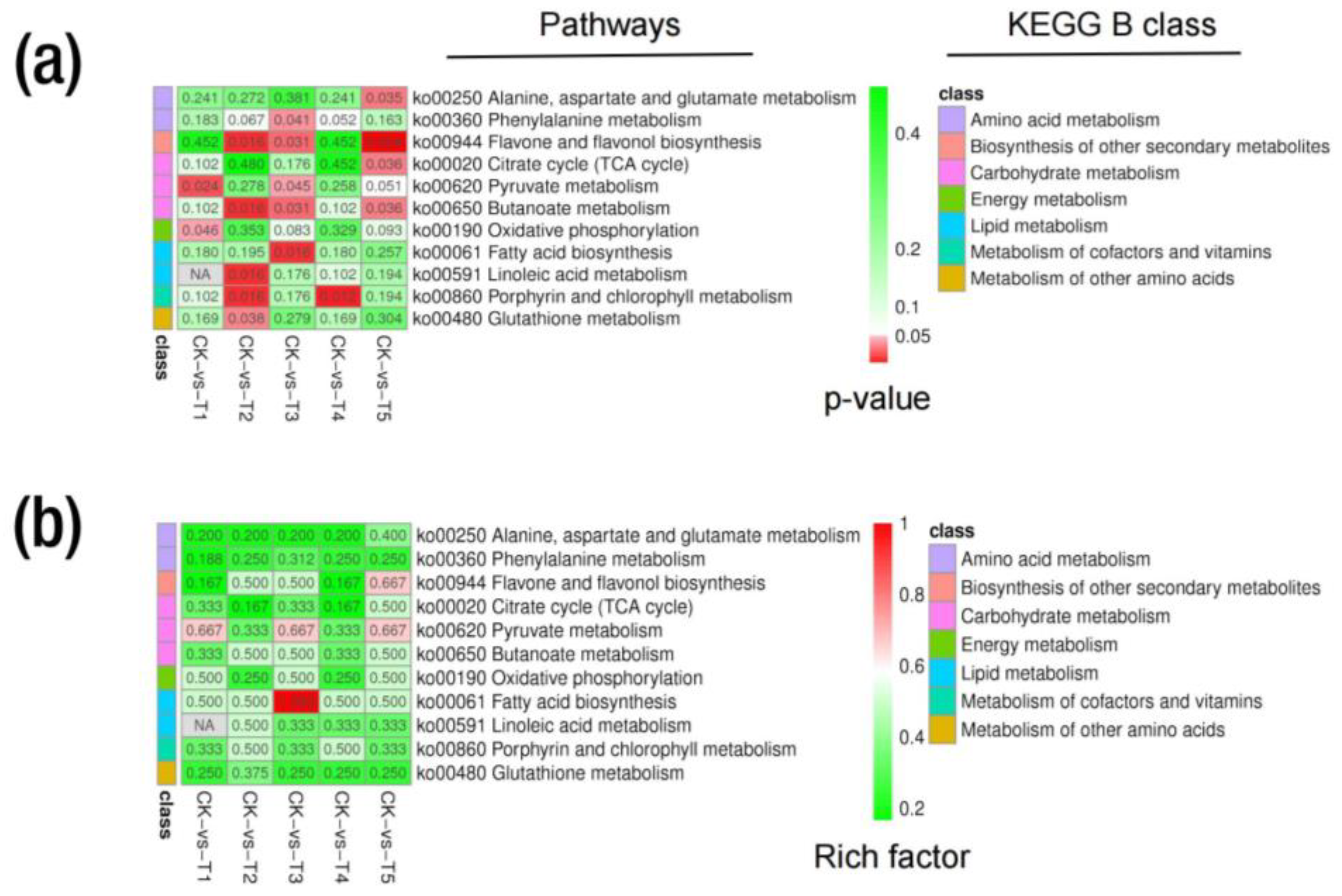
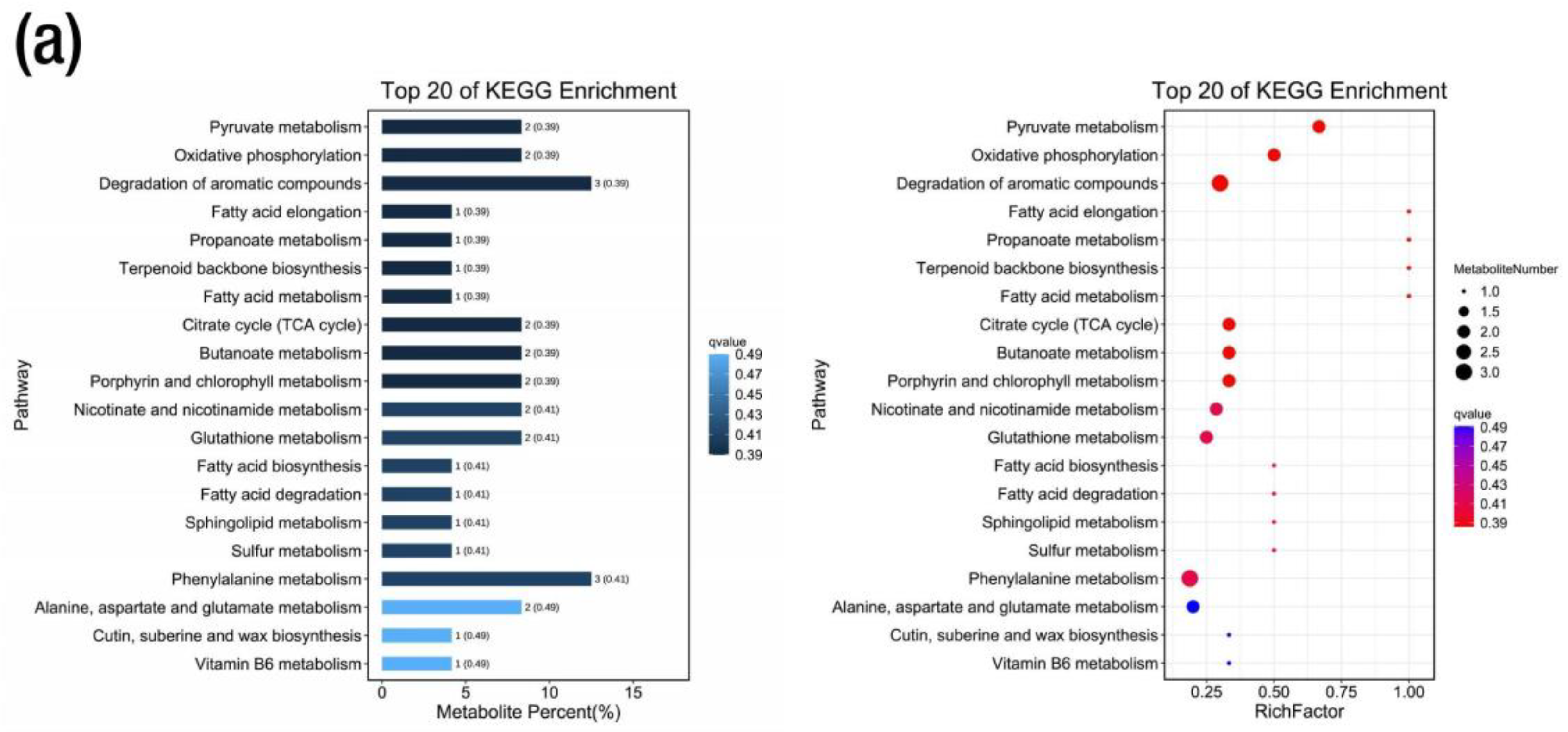
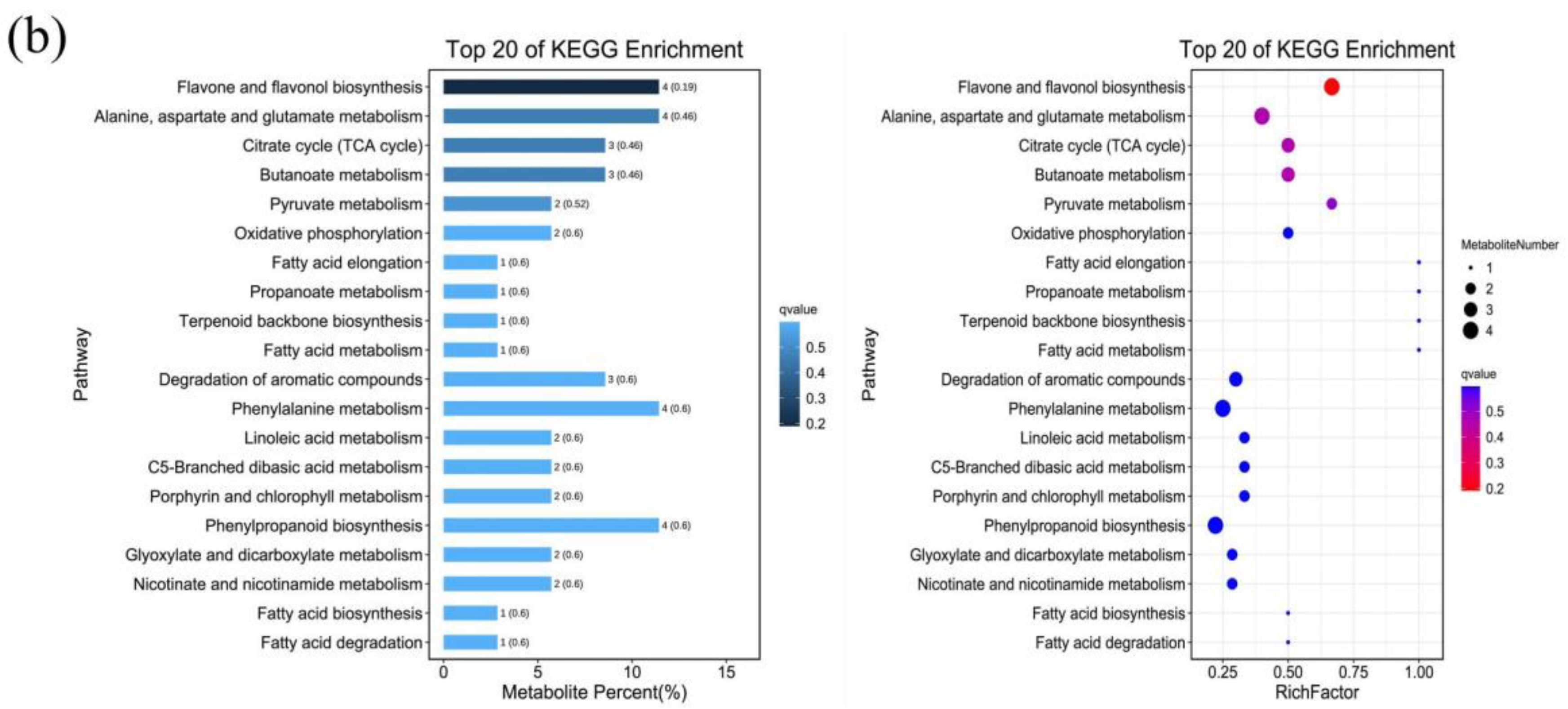
2.6. Metabolic KEGG Pathway and Enrichment Analysis
2.7. Correlation Analysis between the Differential Metabolites’ Profiles and Mulberry Physiological Parameters Measured
3. Discussion
3.1. Physiological Responses to Mg Supply and Deficiency in M. alba
3.2. Differential Metabolites in M. alba Leaves: Response to Various Mg Treatments and Deficiency
3.3. Mulberry Plants’ Response to Mg Supply and Deficiency Induces Amino Acids and Organic Acids
3.4. Lipid Metabolites’ Reponses to Mg Supply and Deficiency in Mulberry Plants
3.5. Mulberry Plants Induce Secondary Metabolites’ Response to Mg Deficiency and Supply
3.6. Correlations between the Differential Metabolite Profiles and Mulberry Physiological Parameters
4. Materials and Methods
4.1. Source of Mulberry Plants and Growth Conditions
4.2. Mineral Analysis
4.3. Physiological Parameters Measurement
4.4. Determination of Chlorophyll Content
4.5. Sample Preparation for Metabolite Extraction
4.6. UHPLC-MS/MS Analysis
4.7. Data Processing and Metabolite Identification
4.8. Data Quality Control
4.9. Hierarchical Cluster Analysis
4.10. PCA and OPLS-DA Analysis
4.11. Differential Metabolite Identification and KEGG Annotations and Enrichment Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ackah, M.; Shi, Y.; Wu, M.; Wang, L.; Guo, P.; Guo, L.; Jin, X.; Li, S.; Zhang, Q.; Qiu, C.; et al. Metabolomics Response to Drought Stress in Morus alba L. Variety Yu-711. Plants 2021, 10, 1636. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T.; Mogili, T.; Sivaprasad, V. Improvement of abiotic stress adaptive traits in mulberry (Morus spp.): An update on biotechnological interventions. 3 Biotech 2017, 7, 214. [Google Scholar] [CrossRef]
- Lu, L.; Tang, Y.; Xie, J.-S.; Yuan, Y.-L. The role of marginal agricultural land-based mulberry planting in biomass energy production. Renew. Energy 2009, 34, 1789–1794. [Google Scholar] [CrossRef]
- Attaribo, T.; Huang, G.; Xin, X.; Zeng, Q.; Zhang, Y.; Zhang, N.; Tang, L.; Sedjoah, R.-C.A.-A.; Zhang, R.; Lee, K.S.; et al. Effect of the silkworm pupa protein–glucose conjugate on the thermal stability and antioxidant activity of anthocyanins. Food Funct. 2021, 12, 4132–4141. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Mun, H.I.; Kim, Y.X.; Suh, D.H.; Lee, S.; Singh, D.; Jung, E.S.; Lee, C.H.; Sung, J. Metabolomic response of Perilla frutescens leaves, an edible-medicinal herb, to acclimatize magnesium oversupply. PLoS ONE 2020, 15, e0236813. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wei, C.; Xu, W.; Yang, J.; Wang, X.; Qiu, Y. Characteristics of salt contents in soils under greenhouse conditions in China. Environ. Sci. Pollut. Res. 2018, 26, 3882–3892. [Google Scholar] [CrossRef]
- Niu, Y.; Chen, P.; Zhang, Y.; Wang, Z.; Hu, S.; Jin, G.; Tang, C.; Guo, L. Natural variation among Arabidopsis thaliana accessions in tolerance to high magnesium supply. Sci. Rep. 2018, 8, 13640. [Google Scholar] [CrossRef]
- Huang, Z.-R.; Zhang, H.; Ye, X.; Lai, N.-W.; Yang, L.-T.; Guo, J.-X.; Chen, L.-S. UHPLC-Q-TOF/MS-based metabolomics reveals altered metabolic profiles in magnesium deficient leaves of Citrus sinensis. Sci. Hortic. 2020, 278, 109870. [Google Scholar] [CrossRef]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2013, 368, 5–21. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I.; Yazici, A.M. Magnesium: A forgotten element in crop production. Better Crops 2010, 94, 23–25. [Google Scholar]
- Guo, W.; Chen, S.; Hussain, N.; Cong, Y.; Liang, Z.; Chen, K. Magnesium stress signaling in plant: Just a beginning. Plant Signal. Behav. 2015, 10, e992287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbona, V.; Manzi, M.; De Ollas, C.; Gómez-Cadenas, A. Metabolomics as a Tool to Investigate Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Kuzhiumparambil, U.; Pernice, M.; Jiang, Z.; Ralph, P.J. Metabolomics: An emerging frontier of systems biology in marine macrophytes. Algal Res. 2016, 16, 76–92. [Google Scholar] [CrossRef]
- dos Santos, V.S.; Macedo, F.A.; Vale, J.S.D.; Silva, D.B.; Carollo, C.A. Metabolomics as a tool for understanding the evolution of Tabebuia sensu lato. Metabolomics 2017, 13, 72. [Google Scholar] [CrossRef]
- Tewari, R.K.; Kumar, P.; Sharma, P.N. Magnesium deficiency induced oxidative stress and antioxidant responses in mulberry plants. Sci. Hortic. 2006, 108, 7–14. [Google Scholar] [CrossRef]
- Ye, X.; Chen, X.-F.; Deng, C.-L.; Yang, L.-T.; Lai, N.-W.; Guo, J.-X. Magnesium-Deficiency Effects on Pigments, Photosynthesis and Photosynthetic Electron Transport of Leaves, and Nutrients of Leaf Blades and Veins in Citrus sinensis Seedlings. Plants 2019, 8, 389. [Google Scholar] [CrossRef] [Green Version]
- Warnes, G.R.; Bolker, B.; Lumley, T.; Johnson, R.C. gmodels: Various R programming tools for model fitting. R Package Version 2015, 2, 2. [Google Scholar]
- Kwon, M.C.; Kim, Y.X.; Lee, S.; Jung, E.S.; Singh, D.; Sung, J.; Lee, C.H. Comparative Metabolomics Unravel the Effect of Magnesium Oversupply on Tomato Fruit Quality and Associated Plant Metabolism. Metabolites 2019, 9, 231. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, L.A.; Batista, B.L.; Lobato, A.K.D.S. Lobato, 24-Epibrasinolide delays chlorophyll degradation and stimulates the pho-tosynthetic machinery in magnesium-stressed soybean plants. J. Plant Growth Regul. 2021, 42, 183–198. [Google Scholar] [CrossRef]
- Hauer-Jákli, M.; Tränkner, M. Critical Leaf Magnesium Thresholds and the Impact of Magnesium on Plant Growth and Photo-Oxidative Defense: A Systematic Review and Meta-Analysis From 70 Years of Research. Front. Plant Sci. 2019, 10, 766. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Khan, S.; Deng, Y.; Jin, X.; Ma, H.; Li, X.; Yin, L.; Huang, J. Physiological Response to Short-Term Magnesium Deficiency in Banana Cultivars. J. Soil Sci. Plant Nutr. 2021, 21, 2826–2836. [Google Scholar] [CrossRef]
- Cakmak, I. Magnesium in Crop Production, Food Quality and Human Health; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–4. [Google Scholar]
- Zhou, M.; Gong, X.; Ying, W.; Chao, L.; Hong, M.; Wang, L.; Fashui, H. Cerium Relieves the Inhibition of Chlorophyll Biosynthesis of Maize Caused by Magnesium Deficiency. Biol. Trace Element Res. 2010, 143, 468–477. [Google Scholar] [CrossRef]
- Li, C.-P.; Qi, Y.-P.; Zhang, J.; Yang, L.-T.; Wang, D.-H.; Ye, X.; Lai, N.-W.; Tan, L.-L.; Lin, D.; Chen, L.-S. Magnesium-deficiency-induced alterations of gas exchange, major metabolites and key enzymes differ among roots, and lower and upper leaves of Citrus sinensis seedlings. Tree Physiol. 2017, 37, 1564–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant 2018, 163, 414–431. [Google Scholar] [CrossRef] [Green Version]
- Verma, K.K.; Song, X.-P.; Zeng, Y.; Li, D.-M.; Guo, D.-J.; Rajput, V.D.; Chen, G.-L.; Barakhov, A.; Minkina, T.M.; Li, Y.-R. Characteristics of Leaf Stomata and Their Relationship with Photosynthesis in Saccharum officinarum Under Drought and Silicon Application. ACS Omega 2020, 5, 24145–24153. [Google Scholar] [CrossRef]
- dos Santos, L.A.D.; da Silva, B.R.S.D.; da Silva Lobato, A.K. Foliar-applied 24-epibrassinolide systemically triggers tolerance to magnesium stress in soybean plants: Plausible responses focused on root and leaf structures. Bot. Lett. 2021, 168, 558–569. [Google Scholar] [CrossRef]
- Zhang, Q.; Ackah, M.; Wang, M.; Amoako, F.K.; Shi, Y.; Wang, L.; Dari, L.; Li, J.; Jin, X.; Jiang, Z.; et al. The impact of boron nutrient supply in mulberry (Morus alba) response to metabolomics, enzyme activities, and physiological parameters. Plant Physiol. Biochem. 2023, 200, 107649. [Google Scholar] [CrossRef]
- Yang, N.; Jiang, J.; Xie, H.; Bai, M.; Xu, Q.; Wang, X.; Yu, X.; Chen, Z.; Guan, Y. Metabolomics Reveals Distinct Carbon and Nitrogen Metabolic Responses to Magnesium Deficiency in Leaves and Roots of Soybean [Glycine max (Linn.) Merr.]. Front. Plant Sci. 2017, 8, 2091. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.-Y.; Qi, Y.-P.; Lee, J.; Yang, L.-T.; Guo, P.; Jiang, H.-X.; Chen, L.-S. Proteomic analysis of Citrus sinensis roots and leaves in response to long-term magnesium-deficiency. BMC Genom. 2015, 16, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, S.; Ze, Y.; Liu, C.; Li, N.; Zhou, M.; Duan, Y.; Hong, F. Cerium Relieves the Inhibition of Nitrogen Metabolism of Spinach Caused by Magnesium Deficiency. Biol. Trace Element Res. 2009, 132, 247–258. [Google Scholar] [CrossRef]
- Verma, D.; Jalmi, S.; Bhagat, P.K.; Verma, N.; Sinha, A.K. A bHLH transcription factor, MYC2, imparts salt intolerance by regulating proline biosynthesis in Arabidopsis. FEBS J. 2019, 287, 2560–2576. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, R.; Barchi, B.; Turlapati, S.A.; Gagne, M.; Minocha, R.; Long, S.; Minocha, S.C. Glutamate, Ornithine, Arginine, Proline, and Polyamine Metabolic Interactions: The Pathway Is Regulated at the Post-Transcriptional Level. Front. Plant Sci. 2016, 7, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [Green Version]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Skrumsager Møller, I.; White, P. Chapter 6—Functions of macronutrients. In Marschner’s Mineral Nutrition of Plants, 4th ed.; Rengel, Z., Cakmak, I., White, P.J., Eds.; Academic Press: San Diego, CA, USA, 2023; pp. 201–281. [Google Scholar]
- Ruan, J.; Ma, L.; Yang, Y. Magnesium nutrition on accumulation and transport of amino acids in tea plants. J. Sci. Food Agric. 2011, 92, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.H.; Newbigin, E.D.; Tester, M.; Bacic, A.; Roessner, U. Metabolic responses to salt stress of barley (Hordeum vulgare L.) cultivars, Sahara and Clipper, which differ in salinity tolerance. J. Exp. Bot. 2009, 60, 4089–4103. [Google Scholar]
- Igamberdiev, A.U.; Eprintsev, A.T. Organic Acids: The Pools of Fixed Carbon Involved in Redox Regulation and Energy Balance in Higher Plants. Front. Plant Sci. 2016, 7, 1042. [Google Scholar] [CrossRef] [Green Version]
- Bose, J.; Babourina, O.; Rengel, Z. Role of magnesium in alleviation of aluminium toxicity in plants. J. Exp. Bot. 2011, 62, 2251–2264. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Yang, G.; You, X.; Zhou, C.; Lu, Y.; Chen, L. Magnesium deficiency-induced changes in organic acid metabolism of Citrus sinensis roots and leaves. Biol. Plant. 2013, 57, 481–486. [Google Scholar] [CrossRef]
- Araújo, W.L.; Tohge, T.; Ishizaki, K.; Leaver, C.J.; Fernie, A.R. Protein degradation—An alternative respiratory substrate for stressed plants. Trends Plant Sci. 2011, 16, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Araújo, W.L.; Martins, A.O.; Fernie, A.R.; Tohge, T. 2-Oxoglutarate: Linking TCA cycle function with amino acid, glucosinolate, flavonoid, alkaloid, and gib-berellin biosynthesis. Front. Plant Sci. 2014, 5, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Martín, J.; Canales, F.J.; Tweed, J.K.S.; Lee, M.R.F.; Rubiales, D.; Gómez-Cadenas, A.; Arbona, V.; Mur, L.A.J.; Prats, E. Fatty Acid Profile Changes During Gradual Soil Water Depletion in Oats Suggests a Role for Jasmonates in Coping with Drought. Front. Plant Sci. 2018, 9, 1077. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.C.; Peng, W.T.; Li, J.; Liao, H. Functional dissection and transport mechanism of magnesium in plants. Semin. Cell Dev. Biol. 2018, 74, 142–152. [Google Scholar] [CrossRef]
- Ciscomani-Larios, J.P.; Sánchez-Chávez, E.; Jacobo-Cuellar, J.L.; Sáenz-Hidalgo, H.K.; Orduño-Cruz, N.; Cruz-Alvarez, O.; Ávila-Quezada, G.D. Biofortification efficiency with magnesium salts on the increase of bioactive compounds and antioxidant capacity in snap beans. Ciência Rural. 2021, 51, 6. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2013, 77, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.A.; Deng, X.; Adeel, M.; Rizwan, M.; Shakoor, N.; Yang, Y.; Javed, R. Influence of calcium and magnesium elimination on plant biomass and secondary metabolites of Stevia rebaudiana Bertoni. Biotechnol. Appl. Biochem. 2021, 69, 2008–2016. [Google Scholar] [CrossRef]
- Antunes, A.C.; Acunha, T.D.S.; Perin, E.C.; Rombaldi, C.V.; Galli, V.; Chaves, F.C. Untargeted metabolomics of strawberry (Fragaria x ananassa ‘Camarosa’) fruit from plants grown under osmotic stress conditions. J. Sci. Food Agric. 2019, 99, 6973–6980. [Google Scholar] [CrossRef]
- López-Bucio, J.; Nieto-Jacobo, M.F.; Ramírez-Rodríguez, V.; Herrera-Estrella, L. Organic acid metabolism in plants: From adaptive physiology to transgenic varieties for cultivation in extreme soils. Plant Sci. 2000, 160, 1–13. [Google Scholar] [CrossRef]
- Chen, Y.-D.; Zhu, Y.; Xin, J.-P.; Zhao, C.; Tian, R.-N. Succinic acid inhibits photosynthesis of Microcystis aeruginosa via damaging PSII oxygen-evolving complex and reaction center. Environ. Sci. Pollut. Res. 2021, 28, 58470–58479. [Google Scholar] [CrossRef]
- Buer, C.S.; Imin, N.; Djordjevic, M. Flavonoids: New Roles for Old Molecules. J. Integr. Plant Biol. 2010, 52, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Jezek, M.; Allan, A.C.; Jones, J.J.; Geilfus, C.M. Why do plants blush when they are hungry? New Phytol. 2023, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.G.; Swinny, E.; Markham, K.R.; Winefield, C. Flavonoid gene expression and UV photoprotection in transgenic and mutant Petunia leaves. Phytochemistry 2002, 59, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, Q.; Wang, L.; Du, Q.; Ackah, M.; Guo, P.; Zheng, D.; Wu, M.; Zhao, W. Functional Characterization of MaZIP4, a Gene Regulating Copper Stress Tolerance in Mulberry (Morus atro-purpurea R.). Life 2022, 12, 1311. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Kan, B.; He, H.; Li, T.; Ding, Y.; Du, P.; Lai, W.; Hu, H.; Huang, J. Transcriptome analysis reveals MYB and WRKY transcription factors involved in banana (Musa paradisiaca AA) magnesium deficiency. Planta 2021, 254, 115. [Google Scholar] [CrossRef]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef]
- Deng, Z.-M.; Dai, F.-F.; Zhou, Q.; Cheng, Y.-X. Hsa_circ_0000301 facilitates the progression of cervical cancer by targeting miR-1228-3p/IRF4 Axis. BMC Cancer 2021, 21, 583. [Google Scholar] [CrossRef]
- Thevenot, E.A. ropls: PCA, PLS (-DA) and OPLS (-DA) for multivariate analysis and feature selection of omics data. R Package Version 2016, 1. [Google Scholar]
- Bylesjö, M.; Rantalainen, M.; Cloarec, O.; Nicholson, J.K.; Holmes, E.; Trygg, J. OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. J. Chemom. 2006, 20, 341–351. [Google Scholar] [CrossRef]
- Saccenti, E.; Hoefsloot, H.C.J.; Smilde, A.K.; Westerhuis, J.A.; Hendriks, M.M.W.B. Reflections on univariate and multivariate analysis of metabolomics data. Metabolomics 2013, 10, 361–374. [Google Scholar] [CrossRef]
- Rao; Sui, J.; Zhang, J. Metabolomics reveals significant variations in metabolites and correlations regarding the matu-ration of walnuts (Juglans regia L.). Biol. Open 2016, 5, 829–836. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package ‘corrplot’. Statistician 2017, 56, e24. [Google Scholar]
- Chen, X.; Xie, C.; Sun, L.; Ding, J.; Cai, H. Longitudinal Metabolomics Profiling of Parkinson’s Disease-Related α-Synuclein A53T Transgenic Mice. PLoS ONE 2015, 10, e0136612. [Google Scholar] [CrossRef]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 29–34. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Ackah, M.; Wang, L.; Amoako, F.K.; Shi, Y.; Essoh, L.G.; Li, J.; Zhang, Q.; Li, H.; Zhao, W. Magnesium Nutrient Application Induces Metabolomics and Physiological Responses in Mulberry (Morus alba) Plants. Int. J. Mol. Sci. 2023, 24, 9650. https://doi.org/10.3390/ijms24119650
Jin X, Ackah M, Wang L, Amoako FK, Shi Y, Essoh LG, Li J, Zhang Q, Li H, Zhao W. Magnesium Nutrient Application Induces Metabolomics and Physiological Responses in Mulberry (Morus alba) Plants. International Journal of Molecular Sciences. 2023; 24(11):9650. https://doi.org/10.3390/ijms24119650
Chicago/Turabian StyleJin, Xin, Michael Ackah, Lei Wang, Frank Kwarteng Amoako, Yisu Shi, Lionnelle Gyllye Essoh, Jianbin Li, Qiaonan Zhang, Haonan Li, and Weiguo Zhao. 2023. "Magnesium Nutrient Application Induces Metabolomics and Physiological Responses in Mulberry (Morus alba) Plants" International Journal of Molecular Sciences 24, no. 11: 9650. https://doi.org/10.3390/ijms24119650




