Fetuin-A Promotes 3-Dimensional Growth in LNCaP Prostate Cancer Cells by Sequestering Extracellular Vesicles to Their Surfaces to Act as Signaling Platforms
Abstract
:1. Introduction
2. Results
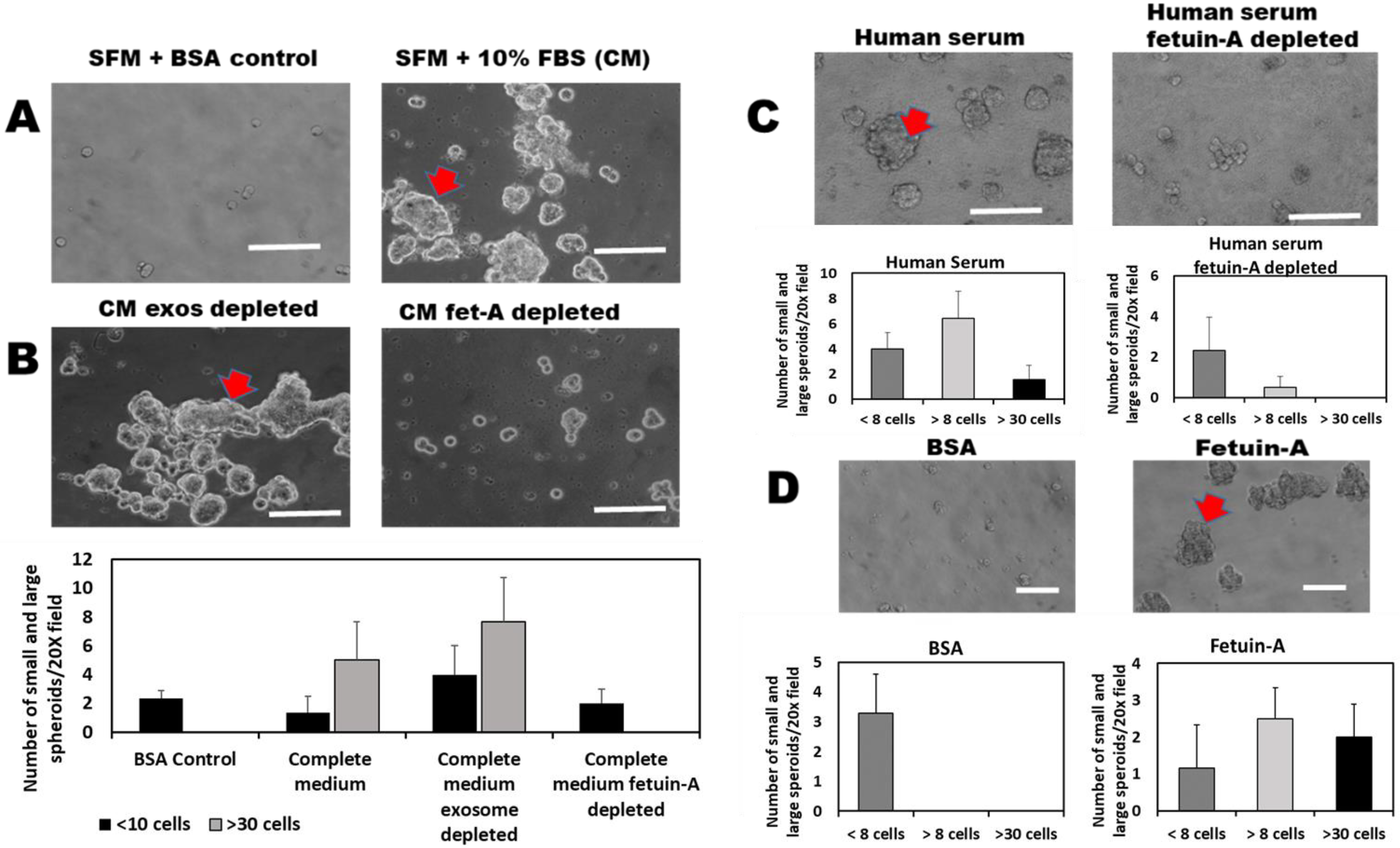
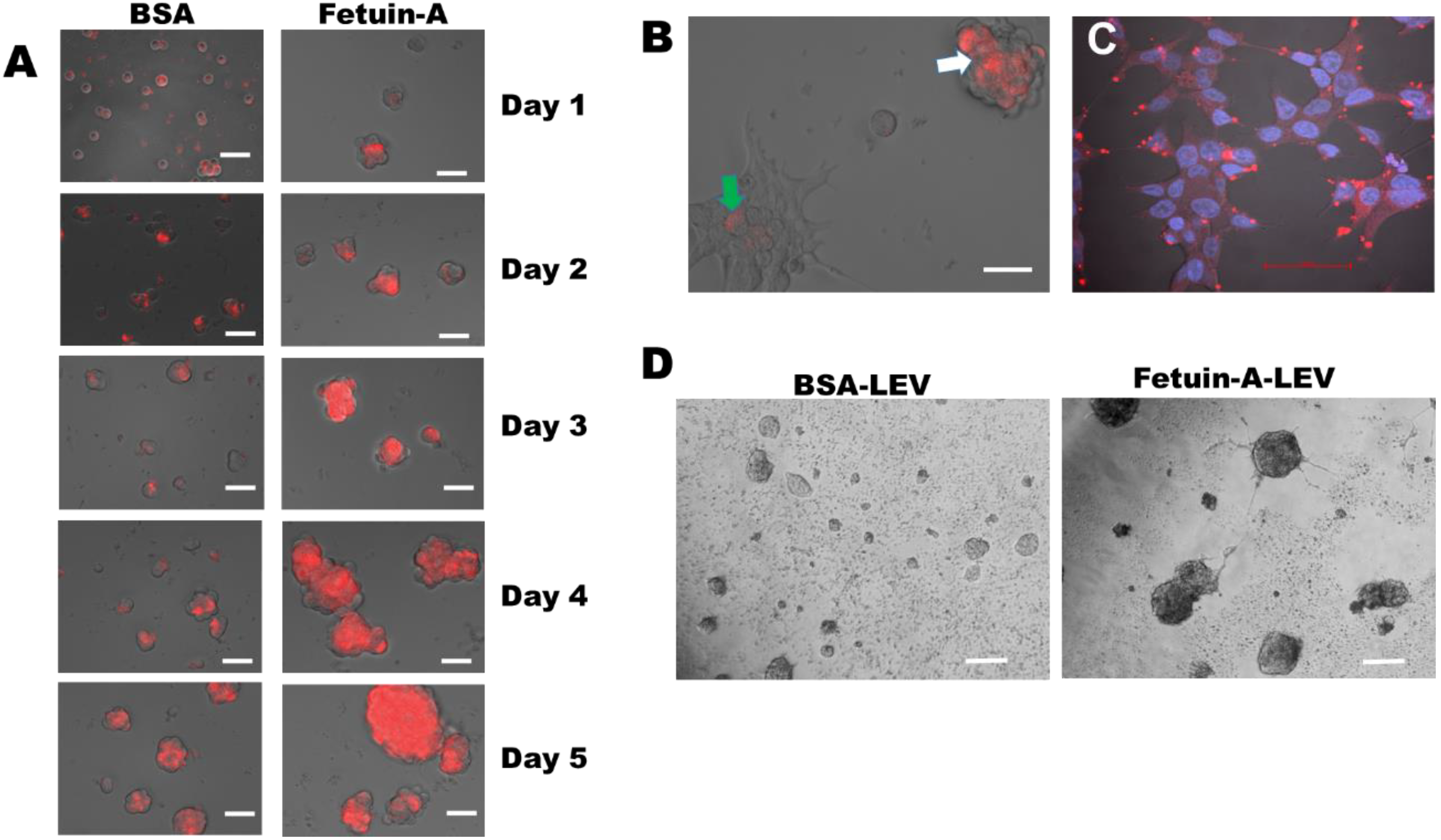
2.1. Fetuin-A Is Indispensable in the 3-D Growth of LNCaP Cells
2.2. 3-D Growth of LNCaP Cells Is Mediated by Exosomes and Other Extracellular Vesicles Isolated from the Cells in the Presence of Fetuin-A
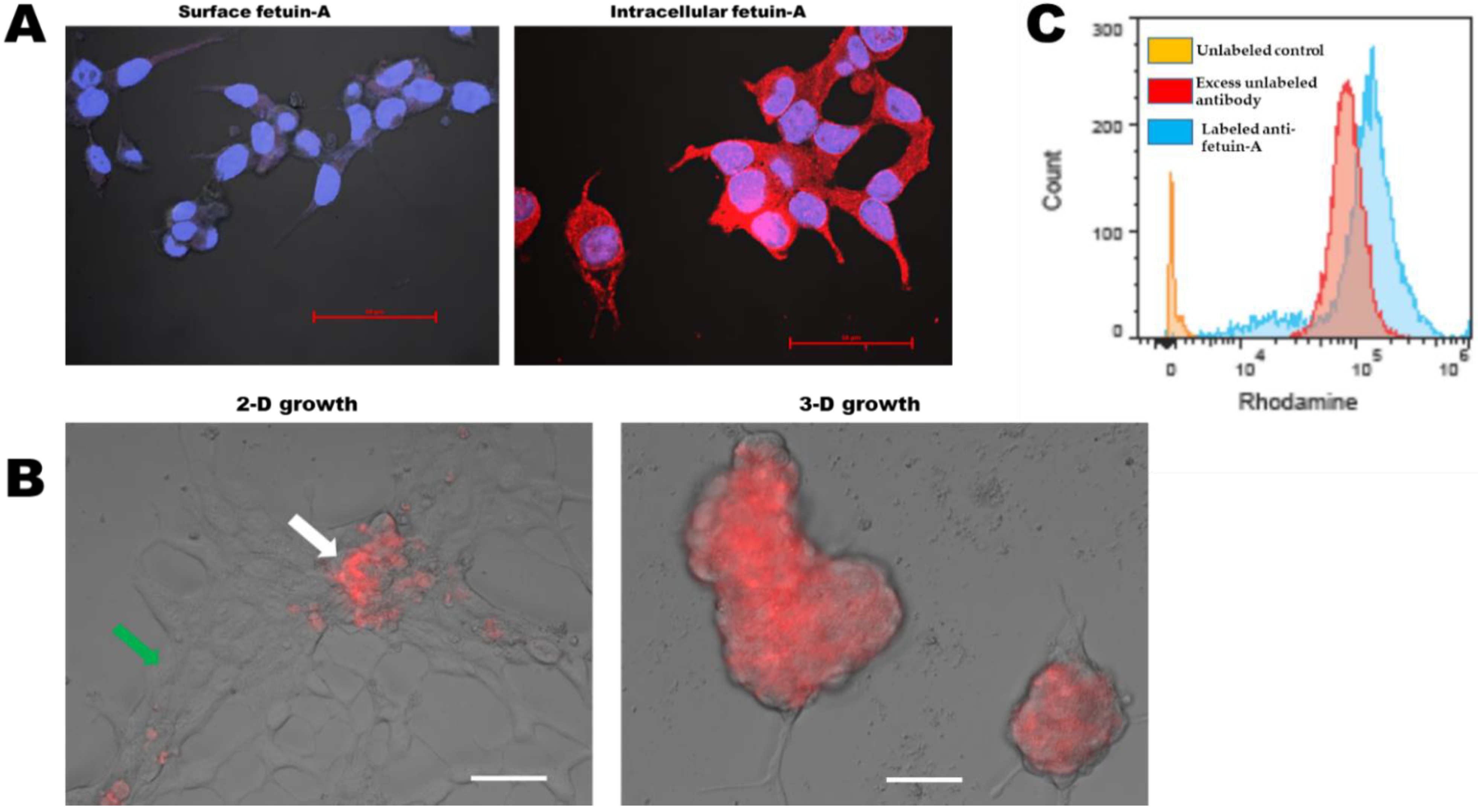
2.3. Fetuin-A Sequesters Rhodamine Isothiocyanate Labeled Large Extracellular Vesicles (LEV) on the Surfaces of Cells Growing in 3-D but Internalizes These Vesicles in Adhered and Spread LNCaP Cells
2.4. Large Extracellular Vesicles Associated with Labeled EGF Promote Rapid 3-D Growth of LNCaP Cells while Sequestered on the Cell Surface
2.5. Fetuin-A Is Rapidly Internalized by Attached and Adhered Cells while in Detached or Cells Growing as Spheroids, It Remains on the Cell Surface
2.6. Growth Signaling in LNCaP Cells
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Materials
4.3. Isolation of Extracellular Vesicles
4.4. Three-Dimensional Growth Assays (3-D)
4.5. Labeling of Proteins and Extracellular Vesicles with Rhodamine Isothiocyanate Isothiocyanate
4.6. Cell Surface vs. Intracellular Disposition of Labeled Large Extracellular Vesicles
4.7. Cell Surface vs. Intracellular Disposition of Labeled EGF Associated with LEV
4.8. Cell Surface vs. Intracellular Disposition of Fetuin-A
4.8.1. Cell Surface Fetuin-A on Attached and Spread Cells
4.8.2. Intracellular Fetuin-A in Attached and Spread Cells
4.8.3. Cell Surface Expression of Fetuin-A on Detached Spherical Cells
4.9. Western Blot Analysis
4.10. Experimental Rigor and Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Watson, K.; Koumangoye, R.; Thompson, P.; Sakwe, A.M.; Patel, T.; Pratap, S.; Ochieng, J. Fetuin-A triggers the secretion of a novel set of exosomes in detached tumor cells that mediate their adhesion and spreading. FEBS Lett. 2012, 586, 3458–3463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Guo, J.; Gu, J.; Wang, Z.; Wang, G.; Li, H.; Wang, J. Identifying the key genes and microRNAs in colorectal cancer liver metastasis by bioinformatics analysis and in vitro experiments. Oncol. Rep. 2019, 41, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Akutekwe, A.; Seker, H.; Yang, S. In silico discovery of significant pathways in colorectal cancer metastasis using a two-stage optimisation approach. IET Syst. Biol. 2015, 9, 294–302. [Google Scholar] [CrossRef]
- Terkelsen, O.B.; Jahnen-Dechent, W.; Nielsen, H.; Moos, T.; Fink, E.; Nawratil, P.; Muller-Esterl, W.; Mollgard, K. Rat fetuin: Distribution of protein and mRNA in embryonic and neonatal rat tissues. Anat. Embryol. 1998, 197, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Hausler, M.; Schafer, C.; Osterwinter, C.; Jahnen-Dechent, W. The physiologic development of fetuin-a serum concentrations in children. Pediatr. Res. 2009, 66, 660–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mintz, P.J.; Rietz, A.C.; Cardo-Vila, M.; Ozawa, M.G.; Dondossola, E.; Do, K.A.; Kim, J.; Troncoso, P.; Logothetis, C.J.; Sidman, R.L.; et al. Discovery and horizontal follow-up of an autoantibody signature in human prostate cancer. Proc. Natl. Acad. Sci. USA 2015, 112, 2515–2520. [Google Scholar] [CrossRef] [Green Version]
- Nangami, G.N.; Sakwe, A.M.; Izban, M.G.; Rana, T.; Lammers, P.E.; Thomas, P.; Chen, Z.; Ochieng, J. Fetuin-A (alpha 2HS glycoprotein) modulates growth, motility, invasion, and senescence in high-grade astrocytomas. Cancer Med. 2016, 5, 3532–3543. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.X.; O’Neill, K.D.; Chen, X.; Duan, D.; Wang, E.; Sturek, M.S.; Edwards, J.M.; Moe, S.M. Fetuin-A uptake in bovine vascular smooth muscle cells is calcium dependent and mediated by annexins. Am. J. Physiol. Ren. Physiol. 2007, 292, F599–F606. [Google Scholar] [CrossRef] [Green Version]
- Sakwe, A.M.; Koumangoye, R.; Goodwin, S.J.; Ochieng, J. Fetuin-A ({alpha}2HS-glycoprotein) is a major serum adhesive protein that mediates growth signaling in breast tumor cells. J. Biol. Chem. 2010, 285, 41827–41835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nangami, G.; Koumangoye, R.; Goodwin, J.S.; Sakwe, A.M.; Marshall, D.; Higginbotham, J.; Ochieng, J. Fetuin-A associates with histones intracellularly and shuttles them to exosomes to promote focal adhesion assembly resulting in rapid adhesion and spreading in breast carcinoma cells. Exp. Cell Res. 2014, 328, 388–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, J.; Escara-Wilke, J.; Keller, J.M.; Jung, Y.; Taichman, R.S.; Pienta, K.J.; Keller, E.T. Primary prostate cancer educates bone stroma through exosomal pyruvate kinase M2 to promote bone metastasis. J. Exp. Med. 2019, 216, 2883–2899. [Google Scholar] [CrossRef] [PubMed]
- Suchorska, W.M.; Lach, M.S. The role of exosomes in tumor progression and metastasis (Review). Oncol. Rep. 2016, 35, 1237–1244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Vizio, D.; Morello, M.; Dudley, A.C.; Schow, P.W.; Adam, R.M.; Morley, S.; Mulholland, D.; Rotinen, M.; Hager, M.H.; Insabato, L.; et al. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am. J. Pathol. 2012, 181, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Ballangrud, A.M.; Yang, W.H.; Dnistrian, A.; Lampen, N.M.; Sgouros, G. Growth and characterization of LNCaP prostate cancer cell spheroids. Clin. Cancer Res. 1999, 5, 3171s–3176s. [Google Scholar] [PubMed]
- Xu, Y.; Chen, S.Y.; Ross, K.N.; Balk, S.P. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006, 66, 7783–7792. [Google Scholar] [CrossRef] [Green Version]
- Ochieng, J.; Pratap, S.; Khatua, A.K.; Sakwe, A.M. Anchorage-independent growth of breast carcinoma cells is mediated by serum exosomes. Exp. Cell Res. 2009, 315, 1875–1888. [Google Scholar] [CrossRef] [Green Version]
- Ramteke, A.; Ting, H.; Agarwal, C.; Mateen, S.; Somasagara, R.; Hussain, A.; Graner, M.; Frederick, B.; Agarwal, R.; Deep, G. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol. Carcinog. 2015, 54, 554–565. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Zhao, D.; Spring, D.J.; De Pinho, R.A. Genetics and biology of prostate cancer. Genes Dev. 2018, 32, 1105–1140. [Google Scholar] [CrossRef] [Green Version]
- Wadosky, K.M.; Koochekpour, S. Molecular mechanisms underlying resistance to androgen deprivation therapy in prostate cancer. Oncotarget 2016, 7, 64447–64470. [Google Scholar] [CrossRef] [Green Version]
- Cao, B.; Qi, Y.; Zhang, G.; Xu, D.; Zhan, Y.; Alvarez, X.; Guo, Z.; Fu, X.; Plymate, S.R.; Sartor, O.; et al. Androgen receptor splice variants activating the full-length receptor in mediating resistance to androgen-directed therapy. Oncotarget 2014, 5, 1646–1656. [Google Scholar] [CrossRef] [Green Version]
- Koumangoye, P.B.; Sakwe, A.M.; Goodwin, J.S.; Patel, T.; Ochieng, J. Detachment of breast tumor cells induces rapid secretion of exosomes which subsequently mediate cellular adhesion and spreading. PLoS ONE 2011, 6, e24234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, P.D.; Sakwe, A.; Koumangoye, R.; Yarbrough, W.G.; Ochieng, J.; Marshall, D.R. Alpha-2 Heremans Schmid Glycoprotein (AHSG) modulates signaling pathways in head and neck squamous cell carcinoma cell line SQ20B. Exp. Cell Res. 2014, 321, 123–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccin, A.; Murphy, W.G.; Smith, O.P. Circulating microparticles: Pathophysiology and clinical implications. Blood Rev. 2007, 21, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.M.; Saunders, N.R.; Mollgard, K.; Dziegielewska, K.M. Fetuin—An old friend revisited. Bioessays 1992, 14, 749–755. [Google Scholar] [CrossRef]
- Mori, K.; Emoto, M.; Inaba, M. Fetuin-A: A multifunctional protein. Recent Pat. Endocr. Metab. Immune Drug Discov. 2011, 5, 124–146. [Google Scholar] [CrossRef]
- Lajoie, P.; Goetz, J.G.; Dennis, J.W.; Nabi, I.R. Lattices, rafts, and scaffolds: Domain regulation of receptor signaling at the plasma membrane. J. Cell Biol. 2009, 185, 381–385. [Google Scholar] [CrossRef] [Green Version]
- Campbell, I.D. Modular proteins at the cell surface. Biochem. Soc. Trans. 2003, 31, 1107–1114. [Google Scholar] [CrossRef] [Green Version]
- Al-Nedawi, K.; Meehan, B.; Rak, J. Microvesicles: Messengers and mediators of tumor progression. Cell Cycle 2009, 8, 2014–2018. [Google Scholar] [CrossRef]
- De Rita, R.M.; Sayeed, A.; Garcia, V.; Krishn, S.R.; Shields, C.D.; Sarker, S.; Friedman, A.; McCue, P.; Molugu, S.K.; Rodeck, U.; et al. Tumor-Derived Extracellular Vesicles Require beta1 Integrins to Promote Anchorage-Independent Growth. iScience 2019, 14, 199–209. [Google Scholar] [CrossRef] [Green Version]
- Iempridee, T. Long non-coding RNA H19 enhances cell proliferation and anchorage-independent growth of cervical cancer cell lines. Exp. Biol. Med. 2017, 242, 184–193. [Google Scholar] [CrossRef] [Green Version]
- Mulvey, H.E.; Chang, A.; Adler, J.; del Tatto, M.; Perez, K.; Quesenberry, P.J.; Chatterjee, D. Extracellular vesicle-mediated phenotype switching in malignant and non-malignant colon cells. BMC Cancer 2015, 15, 571. [Google Scholar] [CrossRef] [PubMed]
- Al-Nedawi, K.; Meehan, B.; Micallef, J.; Lhotak, V.; May, L.; Guha, A.; Rak, J. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 2008, 10, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Rak, J.; Guha, A. Extracellular vesicles–vehicles that spread cancer genes. Bioessays 2012, 34, 489–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, D.; Lee, T.H.; Spinelli, C.; Chennakrishnaiah, S.; D’Asti, E.; Rak, J. Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation. Semin. Cell Dev. Biol. 2017, 67, 11–22. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochieng, J.; Korolkova, O.Y.; Li, G.; Jin, R.; Chen, Z.; Matusik, R.J.; Adunyah, S.; Sakwe, A.M.; Ogunkua, O. Fetuin-A Promotes 3-Dimensional Growth in LNCaP Prostate Cancer Cells by Sequestering Extracellular Vesicles to Their Surfaces to Act as Signaling Platforms. Int. J. Mol. Sci. 2022, 23, 4031. https://doi.org/10.3390/ijms23074031
Ochieng J, Korolkova OY, Li G, Jin R, Chen Z, Matusik RJ, Adunyah S, Sakwe AM, Ogunkua O. Fetuin-A Promotes 3-Dimensional Growth in LNCaP Prostate Cancer Cells by Sequestering Extracellular Vesicles to Their Surfaces to Act as Signaling Platforms. International Journal of Molecular Sciences. 2022; 23(7):4031. https://doi.org/10.3390/ijms23074031
Chicago/Turabian StyleOchieng, Josiah, Olga Y. Korolkova, Guoliang Li, Renjie Jin, Zhenbang Chen, Robert J. Matusik, Samuel Adunyah, Amos M. Sakwe, and Olugbemiga Ogunkua. 2022. "Fetuin-A Promotes 3-Dimensional Growth in LNCaP Prostate Cancer Cells by Sequestering Extracellular Vesicles to Their Surfaces to Act as Signaling Platforms" International Journal of Molecular Sciences 23, no. 7: 4031. https://doi.org/10.3390/ijms23074031






