Insights into Molecular Structure of Pterins Suitable for Biomedical Applications
Abstract
:1. Introduction
2. Different Oxidation States Relate to Different Biological Roles: Biochemistry In-Brief
2.1. Reducded Pterins
2.2. Semireduced Pterins
2.3. Oxidized Pterins
2.4. Pterin Free Radical Species
3. Redox Chemistry of Pterins and Their Free Radical Species
4. Computational Studies
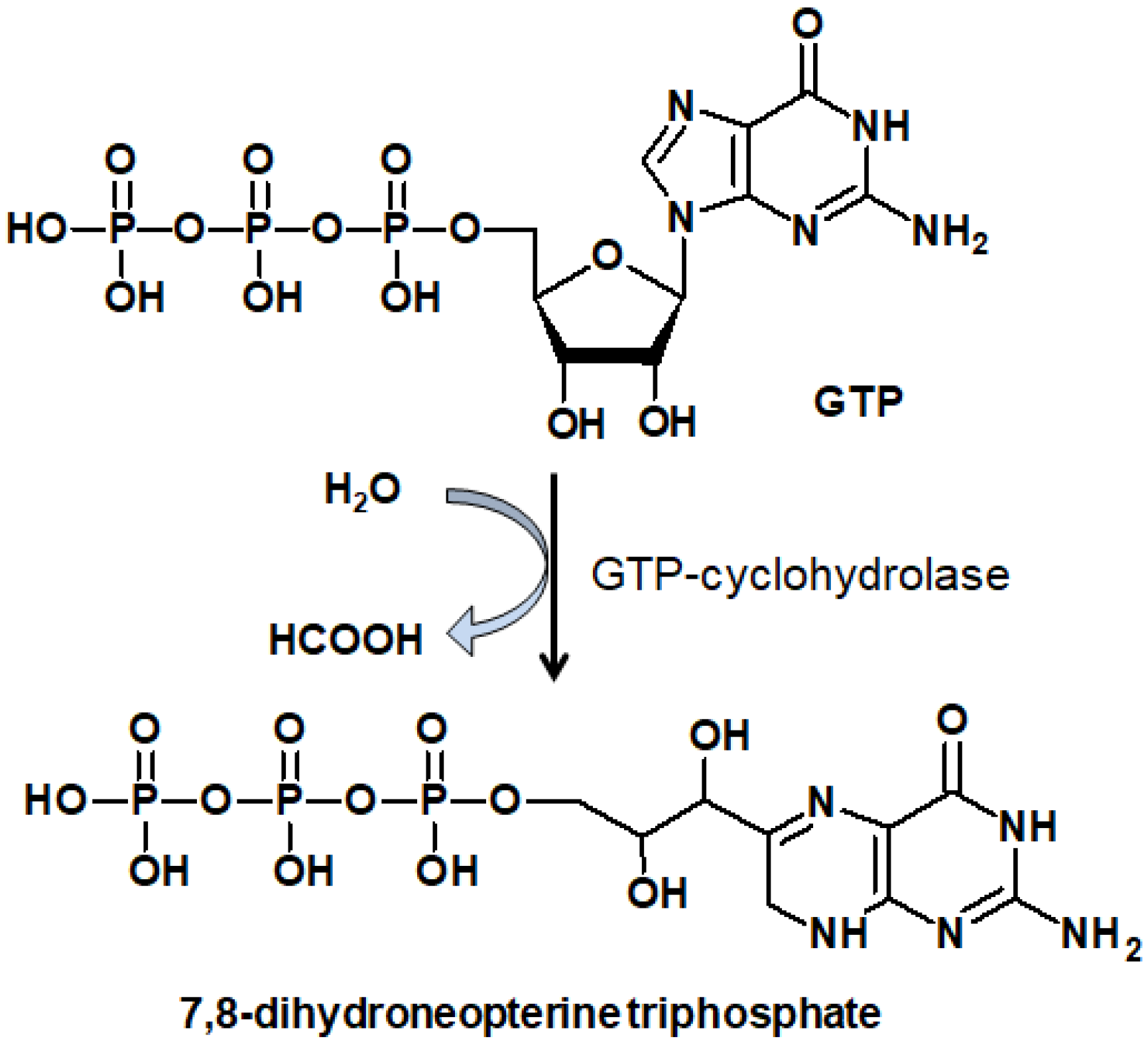
5. Interactions of Pterins with Metals
- Oxidized pterins possess a high fluorescence yield [173]. This gives a potential for their usage in bioimaging.
- Pterin triplets possess a high photosensitizing activity: they efficiently transfer energy to molecular oxygen and even biopolymer, inducing, for example, photoadducts and cyclobutane dimers of DNA [18,176]. The participation of oxidized pterins in photosensitized oxidation reactions will be discussed in detail in one of the next subsections.
5.1. Mutual Phototransformations of Pterins
5.2. Absorption Spectroscopy of Pterins
5.3. Luminescence
5.4. Photosensitization Reactions
5.5. Photogeneration of Singlet Oxygen
5.6. 1O2 Quenching by Pterins
5.7. H4pterins as Photoprotectors
6. Biochemical and Physiological Application of Pterin Photochemistry
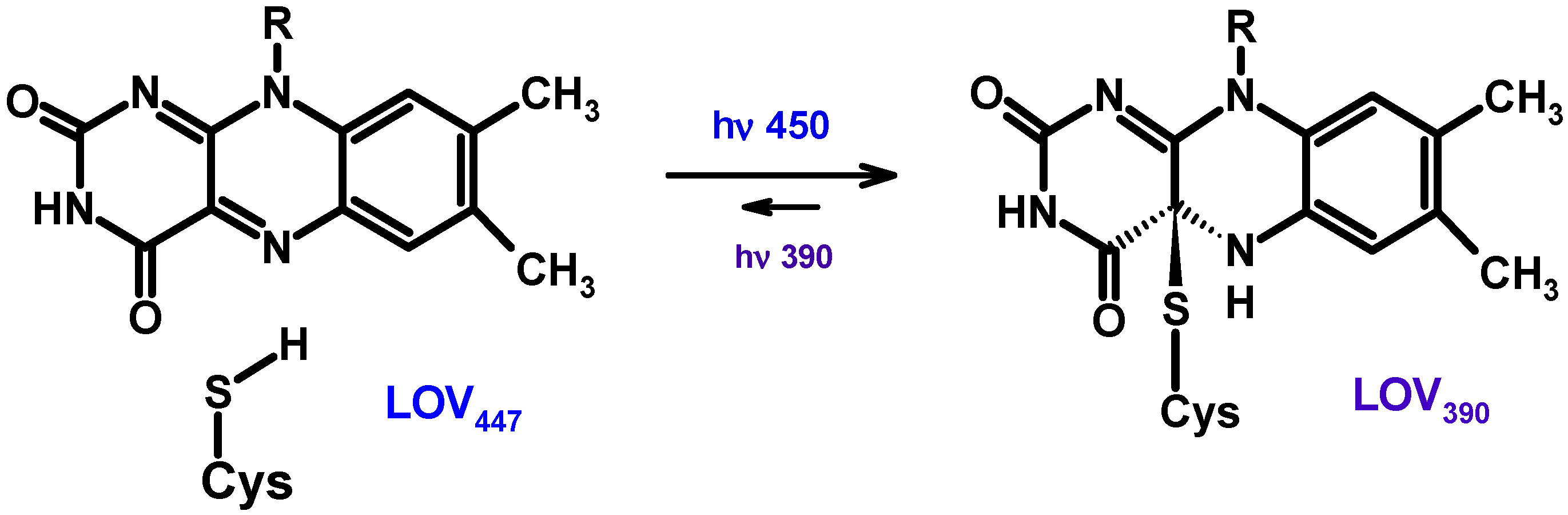
6.1. Evidence of Pteridine Participation in Photoreception
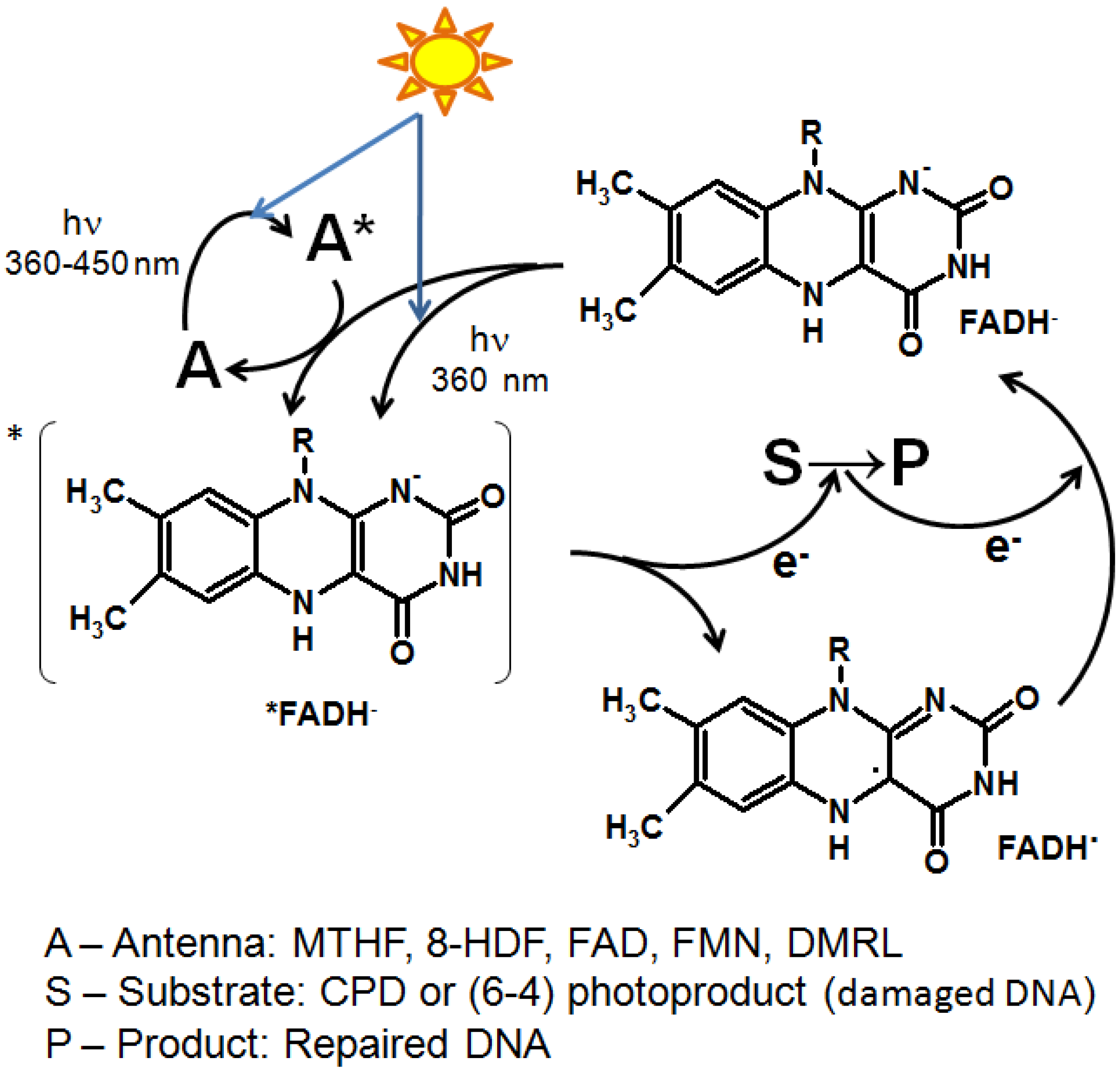
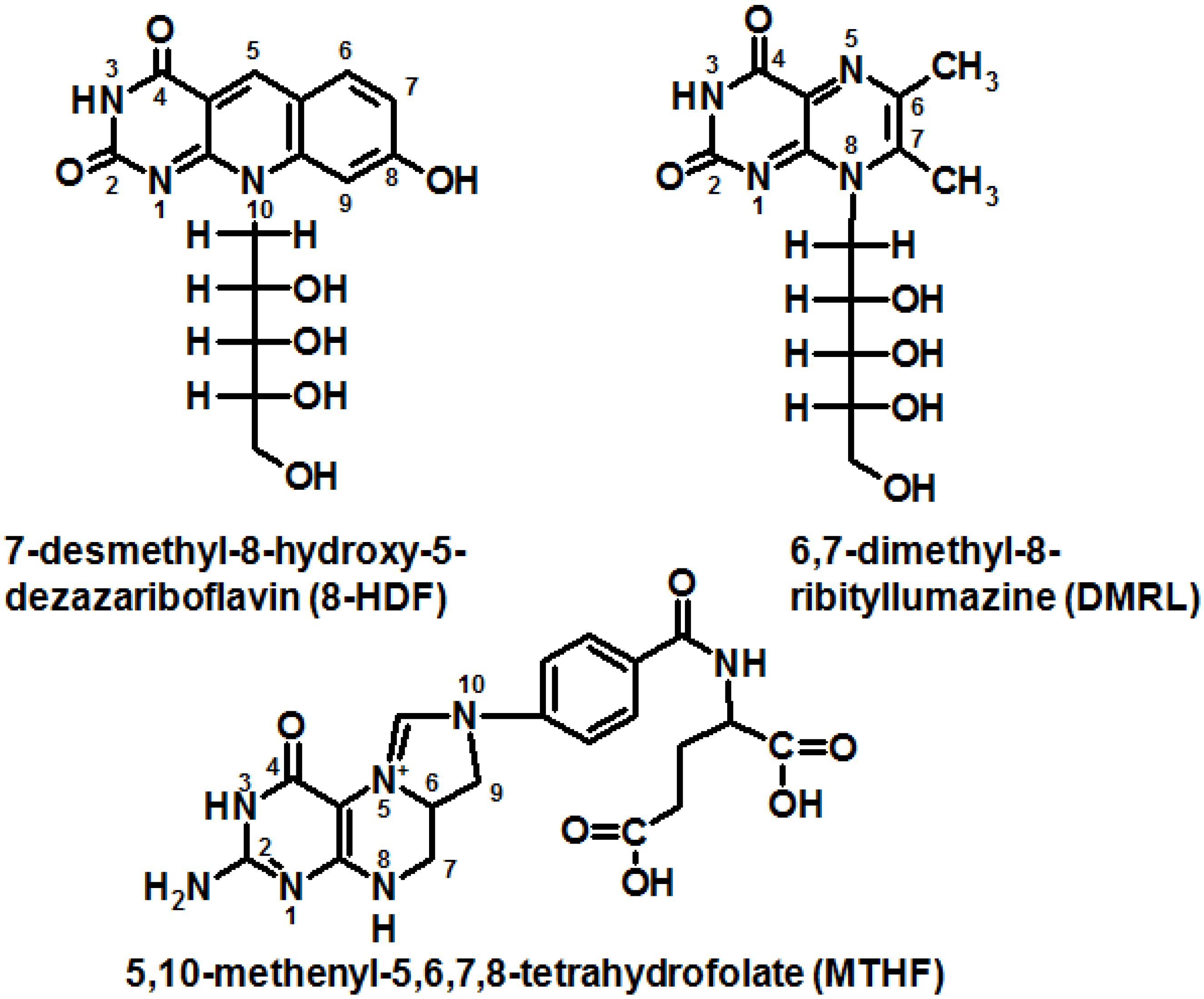
6.2. The Role of Pterin Coenzymes in the Photoregulation of Metabolism
6.3. Evolutionary Aspects of Pterin Photochemistry
7. Future Prospects
7.1. Oxidized Pterins
7.2. H2pterins
7.3. H4pterins
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schöpf, C.; Wieland, H. Über das Leukopterin, das weiße Flügelpigment der Kohlweißlinge (Pieris brassicae und P.napi). Berichte der Dtsch. Chem. Ges. 1926, 59, 2067–2072. [Google Scholar] [CrossRef]
- Kaufman, S. A new cofactor required for the enzymatic conversion of phenylalanine to tyrosine. J. Biol. Chem. 1958, 230, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.D.; Kaufman, S. Studies on the partially uncoupled oxidation of tetrahydropterins by phenylalanine hydroxylase. Neurochem. Res. 1991, 16, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Han, J. Tetrahydrobiopterin in energy metabolism and metabolic diseases. Pharmacol. Res. 2020, 157, 104827. [Google Scholar] [CrossRef]
- Momzikoff, A.; Gaill, F. Mise en évidence d’une émission d’isoxanthoptérine et de riboflavine par différentes espèces d’Ascidies. Experientia 1973, 29, 1438–1439. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Susic, M. Determination of pteridines and flavins in seawater by reverse-phase, high-performance liquid chromatography with fluorometric detection. Mar. Chem. 1985, 17, 185–198. [Google Scholar] [CrossRef]
- Dunlap, W.C.; Susic, M. Photochemical decomposition rates of pteridines and flavins in seawater exposed to surface solar radiation. Mar. Chem. 1986, 19, 99–107. [Google Scholar] [CrossRef]
- Moorthy, P.N.; Hayon, E. One-electron redox reactions of water-soluble vitamins. II. Pterin and folic acid. J. Org. Chem. 1976, 41, 1607–1613. [Google Scholar] [CrossRef]
- Chahidi, C.; Aubailly, M.; Momzikoff, A.; Bazin, M.; Santus, R. Photophysical and Photosensitizing Properties of 2-Amino-4-Pteridinone: A Natural Pigment. Photochem. Photobiol. 1981, 33, 641–649. [Google Scholar] [CrossRef]
- Malhotra, K.; Kim, S.-T.; Batschauer, A.; Dawut, L.; Sancar, A. Putative Blue-Light Photoreceptors from Arabidopsis thaliana and Sinapis alba with a High Degree of Sequence Homology to DNA Photolyase Contain the Two Photolyase Cofactors but Lack DNA Repair Activity. Biochemistry 1995, 34, 6892–6899. [Google Scholar] [CrossRef]
- Sancar, A. Cryptochrome: The second photoactive pigment in the eye and its role in circadian photoreception. Annu. Rev. Biochem. 2000, 69, 31–67. [Google Scholar] [CrossRef] [Green Version]
- Kritsky, M.S.; Lyudnikova, T.A.; Mironov, E.A.; Moskaleva, I. V The UV radiation-driven reduction of pterins in aqueous solution. J. Photochem. Photobiol. B Biol. 1997, 39, 43–48. [Google Scholar] [CrossRef]
- Kritsky, M.S.; Telegina, T.A.; Lyudnikova, T.A.; Umrikhina, A.V.; Zemskova, Y. Participation of free radicals in photoreduction of pterins and folic acid. Dokl. Biochem. Biophys. 2001, 380, 336–338. [Google Scholar] [CrossRef]
- Egorov, S.Y.; Krasnovsky, A.A.J.; Bashtanov, M.Y.; Mironov, E.A.; Ludnikova, T.A.; Kritsky, M.S. Photosensitization of singlet oxygen formation by pterins and flavins. Time-resolved studies of oxygen phosphorescence under laser excitation. Biochemistry 1999, 64, 1117–1121. [Google Scholar]
- Kritsky, M.S.; Lyudnikova, T.A.; Slutsky, E.S.; Filimonenkov, A.A.; Tikhonova, T.V.; Popov, V.O. Photoexcited flavins and pterins as electron injectors for multiheme cytochrome. Dokl. Biochem. Biophys. 2009, 424, 16–19. [Google Scholar] [CrossRef]
- Dántola, M.L.; Vignoni, M.; González, C.; Lorente, C.; Vicendo, P.; Oliveros, E.; Thomas, A.H. Electron-transfer processes induced by the triplet state of pterins in aqueous solutions. Free Radic. Biol. Med. 2010, 49, 1014–1022. [Google Scholar] [CrossRef]
- Lorente, C.; Petroselli, G.; Dántola, M.L.; Oliveros, E.; Thomas, A.H. Electron Transfer Initiated Reactions Photoinduced by Pterins. Pteridines 2011, 22, 111–119. [Google Scholar] [CrossRef]
- Estébanez, S.; Thomas, A.H.; Lorente, C. Deoxythymidine-Pterin Fluorescent Adduct Formation through a Photosensitized Process. Chemphyschem 2018, 19, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Castaño, C.; Serrano, M.P.; Lorente, C.; Borsarelli, C.D.; Thomas, A.H. Quenching of the Singlet and Triplet Excited States of Pterin by Amino Acids. Photochem. Photobiol. 2019, 95, 220–226. [Google Scholar] [CrossRef] [Green Version]
- Lorente, C.; Serrano, M.P.; Vignoni, M.; Dántola, M.L.; Thomas, A.H. A model to understand type I oxidations of biomolecules photosensitized by pterins. J. Photochem. Photobiol. 2021, 7, 100045. [Google Scholar] [CrossRef]
- Moon, Y.-J.; Kim, S.I.; Chung, Y.-H. Sensing and responding to UV-A in cyanobacteria. Int. J. Mol. Sci. 2012, 13, 16303–16332. [Google Scholar] [CrossRef] [PubMed]
- Takeda, J.; Nakata, R.; Ueno, H.; Murakami, A.; Iseki, M.; Watanabe, M. Possible involvement of a tetrahydrobiopterin in photoreception for UV-B-induced anthocyanin synthesis in carrot. Photochem. Photobiol. 2014, 90, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Vignoni, M.; Cabrerizo, F.M.; Lorente, C.; Claparols, C.; Oliveros, E.; Thomas, A.H. Photochemistry of dihydrobiopterin in aqueous solution. Org. Biomol. Chem. 2010, 8, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Vignoni, M.; Serrano, M.P.; Oliveros, E.; Thomas, A.H. Photodimerization of 7,8-dihydroneopterin in aqueous solution under UV-A irradiation. Photochem. Photobiol. 2011, 87, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Vignoni, M.; Lorente, C.; Cabrerizo, F.M.; Erra-Balsells, R.; Oliveros, E.; Thomas, A.H. Characterization and reactivity of photodimers of dihydroneopterin and dihydrobiopterin. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2012, 11, 979–987. [Google Scholar] [CrossRef]
- Buglak, A.A.; Telegina, T.A.; Lyudnikova, T.A.; Vechtomova, Y.L.; Kritsky, M.S. Photooxidation of tetrahydrobiopterin under UV irradiation: Possible pathways and mechanisms. Photochem. Photobiol. 2014, 90, 1017–1026. [Google Scholar] [CrossRef]
- Wood, J.M.; Schallreuter-Wood, K.U.; Lindsey, N.J.; Callaghan, S.; Gardner, M.L. A specific tetrahydrobiopterin binding domain on tyrosinase controls melanogenesis. Biochem. Biophys. Res. Commun. 1995, 206, 480–485. [Google Scholar] [CrossRef]
- Thöny, B.; Auerbach, G.; Blau, N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem. J. 2000, 347 Pt 1, 1–16. [Google Scholar] [CrossRef]
- Telegina, T.A.; Lyudnikova, T.A.; Buglak, A.A.; Vechtomova, Y.L.; Biryukov, M.V.; Demin, V.V.; Kritsky, M.S. Transformation of 6-tetrahydrobiopterin in aqueous solutions under UV-irradiation. J. Photochem. Photobiol. A Chem. 2018, 354, 155–162. [Google Scholar] [CrossRef]
- Buglak, A.A.; Telegina, T.A.; Vechtomova, Y.L.; Kritsky, M.S. Autoxidation and photooxidation of tetrahydrobiopterin: A theoretical study. Free Radic. Res. 2021, 55, 499–509. [Google Scholar] [CrossRef]
- Moon, Y.-J.; Kim, S.-J.; Park, Y.M.; Chung, Y.-H. Sensing UV/blue: Pterin as a UV-A absorbing chromophore of cryptochrome. Plant Signal. Behav. 2010, 5, 1127–1130. [Google Scholar] [CrossRef] [PubMed]
- Feirer, N.; Fuqua, C. Pterin function in bacteria. Pteridines 2017, 28, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Kirschning, A. Coenzymes and Their Role in the Evolution of Life. Angew. Chem. Int. Ed. 2021, 60, 6242–6269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorente, C.; Thomas, A.H. Photophysics and photochemistry of pterins in aqueous solution. Acc. Chem. Res. 2006, 39, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Stuehr, D.J.; Haque, M.M. Nitric oxide synthase enzymology in the 20 years after the Nobel Prize. Br. J. Pharmacol. 2019, 176, 177–188. [Google Scholar] [CrossRef] [Green Version]
- Watschinger, K.; Keller, M.A.; Golderer, G.; Hermann, M.; Maglione, M.; Sarg, B.; Lindner, H.H.; Hermetter, A.; Werner-Felmayer, G.; Konrat, R.; et al. Identification of the gene encoding alkylglycerol monooxygenase defines a third class of tetrahydrobiopterin-dependent enzymes. Proc. Natl. Acad. Sci. USA 2010, 107, 13672–13677. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, P.F. Mechanism of aromatic amino acid hydroxylation. Biochemistry 2003, 42, 14083–14091. [Google Scholar] [CrossRef] [Green Version]
- Flydal, M.I.; Alcorlo-Pagés, M.; Johannessen, F.G.; Martínez-Caballero, S.; Skjærven, L.; Fernandez-Leiro, R.; Martinez, A.; Hermoso, J.A. Structure of full-length human phenylalanine hydroxylase in complex with tetrahydrobiopterin. Proc. Natl. Acad. Sci. USA 2019, 116, 11229–11234. [Google Scholar] [CrossRef] [Green Version]
- Bendall, J.K.; Douglas, G.; McNeill, E.; Channon, K.M.; Crabtree, M.J. Tetrahydrobiopterin in Cardiovascular Health and Disease. Antioxid. Redox Signal. 2013, 20, 3040–3077. [Google Scholar] [CrossRef] [Green Version]
- Schallreuter, K.U.; Wood, J.M.; Ziegler, I.; Lemke, K.R.; Pittelkow, M.R.; Lindsey, N.J.; Gütlich, M. Defective tetrahydrobiopterin and catecholamine biosynthesis in the depigmentation disorder vitiligo. Biochim. Biophys. Acta 1994, 1226, 181–192. [Google Scholar] [CrossRef]
- Kisker, C.; Schindelin, H.; Baas, D.; Rétey, J.; Meckenstock, R.U.; Kroneck, P.M. A structural comparison of molybdenum cofactor-containing enzymes. FEMS Microbiol. Rev. 1998, 22, 503–521. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, A.C.; Bennett, M.J. Chapter 14—Biochemical genetic disorders. In Biochemical and Molecular Basis of Pediatric Disease, 5th ed.; Dietzen, D., Bennett, M., Wong, E., Haymond, S.B., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 439–476. ISBN 978-0-12-817962-8. [Google Scholar]
- Escalante-Semerena, J.C.; Leigh, J.A.; Rinehart, K.L.; Wolfe, R.S. Formaldehyde activation factor, tetrahydromethanopterin, a coenzyme of methanogenesis. Proc. Natl. Acad. Sci. USA 1984, 81, 1976–1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.W.; Oh, C.H.; Geyer, A.; Pfleiderer, W.; Park, Y.S. Characterization of a novel unconjugated pteridine glycoside, cyanopterin, in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta 1999, 1410, 61–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, Y.-J.; Lee, E.-M.; Park, Y.M.; Park, Y.S.; Chung, W.-I.; Chung, Y.-H. The role of cyanopterin in UV/blue light signal transduction of cyanobacterium Synechocystis sp. PCC 6803 phototaxis. Plant Cell Physiol. 2010, 51, 969–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keltjens, J.T.; Vogels, G.D. Methanopterin and methanogenic bacteria. Biofactors 1988, 1, 95–103. [Google Scholar] [PubMed]
- Mendel, R.R. The molybdenum cofactor. J. Biol. Chem. 2013, 288, 13165–13172. [Google Scholar] [CrossRef] [Green Version]
- Leimkühler, S. The biosynthesis of the molybdenum cofactors in Escherichia coli. Environ. Microbiol. 2020, 22, 2007–2026. [Google Scholar] [CrossRef] [Green Version]
- Basu, P.; Burgmayer, S.J.N. Pterin chemistry and its relationship to the molybdenum cofactor. Coord. Chem. Rev. 2011, 255, 1016–1038. [Google Scholar] [CrossRef] [Green Version]
- Lambert, S.R.; Miller, P.M.; Smith-Marshall, J.; Couser, N.L. Chapter 6—Genetic Abnormalities of the Crystalline Lens. In Ophthalmic Genetic Diseases; Couser, N.L., Ed.; Elsevier: Philadelphia, PA, USA, 2019; pp. 81–97. ISBN 978-0-323-65414-2. [Google Scholar]
- Salvail, H.; Balaji, A.; Yu, D.; Roth, A.; Breaker, R.R. Biochemical Validation of a Fourth Guanidine Riboswitch Class in Bacteria. Biochemistry 2020, 59, 4654–4662. [Google Scholar] [CrossRef]
- Hubert, L.; Sutton, V.R. Chapter 12—Disorders of purine and pyrimidine metabolism. In Clinical Aspects and Laboratory Determination; Garg, U., Smith, L., Eds.; Elsevier: San Diego, CA, USA, 2017; pp. 283–299. ISBN 978-0-12-802896-4. [Google Scholar]
- Pang, H.; Yokoyama, K. Chapter Eighteen—Lessons From the Studies of a CC Bond Forming Radical SAM Enzyme in Molybdenum Cofactor Biosynthesis. In Radical SAM Enzymes; Bandarian, V., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 606, pp. 485–522. ISBN 0076-6879. [Google Scholar]
- Zhu, W.; Winter, M.G.; Byndloss, M.X.; Spiga, L.; Duerkop, B.A.; Hughes, E.R.; Büttner, L.; de Lima Romão, E.; Behrendt, C.L.; Lopez, C.A.; et al. Precision editing of the gut microbiota ameliorates colitis. Nature 2018, 553, 208–211. [Google Scholar] [CrossRef]
- Naponelli, V.; Noiriel, A.; Ziemak, M.J.; Beverley, S.M.; Lye, L.-F.; Plume, A.M.; Botella, J.R.; Loizeau, K.; Ravanel, S.; Rébeillé, F.; et al. Phylogenomic and functional analysis of pterin-4a-carbinolamine dehydratase family (COG2154) proteins in plants and microorganisms. Plant Physiol. 2008, 146, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Varughese, K.I.; Skinner, M.M.; Whiteley, J.M.; Matthews, D.A.; Xuong, N.H. Crystal structure of rat liver dihydropteridine reductase. Proc. Natl. Acad. Sci. USA 1992, 89, 6080–6084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swoboda, K.J.; Walker, M.A. Neurotransmitter-Related Disorders. In Swaiman’s Pediatric Neurology, 6th ed.; Swaiman, K.F., Ashwal, S., Ferriero, D.M., Schor, N.F., Finkel, R.S., Gropman, A.L., Pearl, P.L., Shevell, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 355–361. ISBN 978-0-323-37101-8. [Google Scholar]
- Crabtree, M.J.; Channon, K.M. Synthesis and recycling of tetrahydrobiopterin in endothelial function and vascular disease. Nitric Oxide Biol. Chem. 2011, 25, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mhashal, A.R.; Vardi-Kilshtain, A.; Kohen, A.; Major, D.T. The role of the Met20 loop in the hydride transfer in Escherichia coli dihydrofolate reductase. J. Biol. Chem. 2017, 292, 14229–14239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cario, H.; Smith, D.E.C.; Blom, H.; Blau, N.; Bode, H.; Holzmann, K.; Pannicke, U.; Hopfner, K.-P.; Rump, E.-M.; Ayric, Z.; et al. Dihydrofolate reductase deficiency due to a homozygous DHFR mutation causes megaloblastic anemia and cerebral folate deficiency leading to severe neurologic disease. Am. J. Hum. Genet. 2011, 88, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Banka, S.; Blom, H.J.; Walter, J.; Aziz, M.; Urquhart, J.; Clouthier, C.M.; Rice, G.I.; de Brouwer, A.P.M.; Hilton, E.; Vassallo, G.; et al. Identification and characterization of an inborn error of metabolism caused by dihydrofolate reductase deficiency. Am. J. Hum. Genet. 2011, 88, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Raimondi, M.V.; Randazzo, O.; La Franca, M.; Barone, G.; Vignoni, E.; Rossi, D.; Collina, S. DHFR Inhibitors: Reading the Past for Discovering Novel Anticancer Agents. Molecules 2019, 24, 1140. [Google Scholar] [CrossRef] [Green Version]
- Wróbel, A.; Arciszewska, K.; Maliszewski, D.; Drozdowska, D. Trimethoprim and other nonclassical antifolates an excellent template for searching modifications of dihydrofolate reductase enzyme inhibitors. J. Antibiot. 2020, 73, 5–27. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Chen, P.; Sun, L.; Yuan, S.; Cheng, Z.; Lu, L.; Du, H.; Zhan, M. Sepiapterin reductase: Characteristics and role in diseases. J. Cell. Mol. Med. 2020, 24, 9495–9506. [Google Scholar] [CrossRef]
- Arrabal, L.; Teresa, L.; Sánchez-Alcudia, R.; Castro, M.; Medrano, C.; Gutiérrez-Solana, L.; Roldán, S.; Ormazábal, A.; Pérez-Cerdá, C.; Merinero, B.; et al. Genotype-phenotype correlations in sepiapterin reductase deficiency. A splicing defect accounts for a new phenotypic variant. Neurogenetics 2011, 12, 183–191. [Google Scholar] [CrossRef]
- Chen, Y.; Bao, X.; Wen, Y.; Wang, J.; Zhang, Q.; Yan, J. Clinical and Genetic Heterogeneity in a Cohort of Chinese Children With Dopa-Responsive Dystonia. Front. Pediatr. 2020, 8, 83. [Google Scholar] [CrossRef]
- Friedman, J. Sepiapterin Reductase Deficiency; Adam, M.P., Ardinger, H.H., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Mirzaa, G.M., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Wakabayashi, I.; Nakanishi, M.; Ohki, M.; Suehiro, A.; Uchida, K. A simple and useful method for evaluation of oxidative stress in vivo by spectrofluorometric estimation of urinary pteridines. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Niederwieser, A.; Curtius, H.C.; Gitzelmann, R.; Otten, A.; Baerlocher, K.; Blehovà, B.; Berlow, S.; Gröbe, H.; Rey, F.; Schaub, J.; et al. Excretion of pterins in phenylketonuria and phenylketonuria variants. Helv. Paediatr. Acta 1980, 35, 335–342. [Google Scholar] [PubMed]
- Hausen, A.; Fuchs, D.; Reibnegger, G.; Wachter, H. Urinary pteridines on patients suffering from cancer. A comment on the method and results of Rao and associates and of Trehan and associates. Cancer 1984, 53, 1634–1636. [Google Scholar] [CrossRef] [PubMed]
- Reibnegger, G.J.; Bichler, A.H.; Dapunt, O.; Fuchs, D.N.; Fuith, L.C.; Hausen, A.; Hetzel, H.M.; Lutz, H.; Werner, E.R.; Wachter, H. Neopterin as a prognostic indicator in patients with carcinoma of the uterine cervix. Cancer Res. 1986, 46, 950–955. [Google Scholar] [PubMed]
- Murr, C.; Widner, B.; Wirleitner, B.; Fuchs, D. Neopterin as a marker for immune system activation. Curr. Drug Metab. 2002, 3, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Burton, C.; Ma, Y. The role of urinary pteridines as disease biomarkers. Pteridines 2017, 28, 1–21. [Google Scholar] [CrossRef]
- Lindsay, A.; Gieseg, S.P. Pterins as diagnostic markers of exercise-induced stress: A systematic review. J. Sci. Med. Sport 2020, 23, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kośliński, P.; Pluskota, R.; Mądra-Gackowska, K.; Gackowski, M.; Markuszewski, M.J.; Kędziora-Kornatowska, K.; Koba, M. Comparison of Pteridine Normalization Methods in Urine for Detection of Bladder Cancer. Diagnostics 2020, 10, 612. [Google Scholar] [CrossRef] [PubMed]
- Kośliński, P.; Bujak, R.; Daghir, E.; Markuszewski, M.J. Metabolic profiling of pteridines for determination of potential biomarkers in cancer diseases. Electrophoresis 2011, 32, 2044–2054. [Google Scholar] [CrossRef]
- Espinosa-Mansilla, A.; Durán-Merás, I. Pteridine determination in human serum with special emphasis on HPLC methods with fluorimetric detection. Pteridines 2017, 28, 67–81. [Google Scholar] [CrossRef]
- Guibal, P.; Lo, A.; Maitre, P.; Moussa, F. Pterin determination in cerebrospinal fluid: State of the art. Pteridines 2017, 28, 83–89. [Google Scholar] [CrossRef]
- Martín Tornero, E.; Durán Merás, I.; Espinosa-Mansilla, A. HPLC determination of serum pteridine pattern as biomarkers. Talanta 2014, 128, 319–326. [Google Scholar] [CrossRef]
- Eisenhut, M. Neopterin in Diagnosis and Monitoring of Infectious Diseases. J. Biomark. 2013, 2013, 196432. [Google Scholar] [CrossRef] [Green Version]
- Gieseg, S.P.; Baxter-Parker, G.; Lindsay, A. Neopterin, Inflammation, and Oxidative Stress: What Could We Be Missing? Antioxidants 2018, 7, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, J.; da Silva, R.A.; da Luz Scheffer, D.; Penteado, R.; Solano, A.; Barros, L.; Budde, H.; Trostchansky, A.; Latini, A. Physical-Exercise-Induced Antioxidant Effects on the Brain and Skeletal Muscle. Antioxidants 2022, 11, 826. [Google Scholar] [CrossRef] [PubMed]
- Baxter-Parker, G.; Roffe, L.; Moltchanova, E.; Jefferies, J.; Raajasekar, S.; Hooper, G.; Gieseg, S.P. Urinary neopterin and total neopterin measurements allow monitoring of oxidative stress and inflammation levels of knee and hip arthroplasty patients. PLoS ONE 2021, 16, e0256072. [Google Scholar] [CrossRef]
- Kaski, J.C. Neopterin for prediction of in-hospital atrial fibrillation—The ‘forgotten biomarker’ strikes again. J. Intern. Med. 2018, 283, 591–593. [Google Scholar] [CrossRef]
- Rubach, M.P.; Mukemba, J.P.; Florence, S.M.; Lopansri, B.K.; Hyland, K.; Simmons, R.A.; Langelier, C.; Nakielny, S.; DeRisi, J.L.; Yeo, T.W.; et al. Cerebrospinal Fluid Pterins, Pterin-Dependent Neurotransmitters, and Mortality in Pediatric Cerebral Malaria. J. Infect. Dis. 2021, 224, 1432–1441. [Google Scholar] [CrossRef]
- Kip, A.E.; Wasunna, M.; Alves, F.; Schellens, J.H.M.; Beijnen, J.H.; Musa, A.M.; Khalil, E.A.G.; Dorlo, T.P.C. Macrophage Activation Marker Neopterin: A Candidate Biomarker for Treatment Response and Relapse in Visceral Leishmaniasis. Front. Cell. Infect. Microbiol. 2018, 8, 181. [Google Scholar] [CrossRef] [Green Version]
- Khalifa, M.; Elsharkawy, A.; Nour, A.; Ashour, S.; Shehata, S. Serum Neopterin as A Biomarker For Silicosis Among Clay Brick Industry Workers. Egypt. J. Occup. Med. 2022, 46, 17–32. [Google Scholar] [CrossRef]
- Zhelyazkova, Y.; Tacheva, T.; Ivanova, D.; Dimov, D.; Prakova, G.; Vlaykova, T. Serum neopterin and IL-6 as biomarkers in patients with COPD. Eur. Respir. J. 2017, 50, PA380. [Google Scholar] [CrossRef]
- Smukowska-Gorynia, A.; Marcinkowska, J.; Chmara, E.; Malaczynska-Rajpold, K.; Slawek-Szmyt, S.; Cieslewicz, A.; Janus, M.; Araszkiewicz, A.; Jankiewicz, S.; Komosa, A.; et al. Neopterin as a Biomarker in Patients with Pulmonary Arterial Hypertension and Chronic Thromboembolic Pulmonary Hypertension. Respiration 2018, 96, 222–230. [Google Scholar] [CrossRef]
- Zembron-Lacny, A.; Dziubek, W.; Tylutka, A.; Wacka, E.; Morawin, B.; Bulinska, K.; Stefanska, M.; Wozniewski, M.; Szuba, A. Assessment of Serum Neopterin as a Biomarker in Peripheral Artery Disease. Diagnostics 2021, 11, 1911. [Google Scholar] [CrossRef]
- Watanabe, T. Neopterin derivatives—A novel therapeutic target rather than biomarker for atherosclerosis and related diseases. Vasa 2020, 50, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Burton, C. Pteridine detection in urine: The future of cancer diagnostics? Biomark. Med. 2013, 7, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Kośliński, P.; Daghir-Wojtkowiak, E.; Szatkowska-Wandas, P.; Markuszewski, M.; Markuszewski, M.J. The metabolic profiles of pterin compounds as potential biomarkers of bladder cancer—Integration of analytical-based approach with biostatistical methodology. J. Pharm. Biomed. Anal. 2016, 127, 256–262. [Google Scholar] [CrossRef]
- Volgger, B.M.; Windbichler, G.H.; Zeimet, A.G.; Graf, A.H.; Bogner, G.; Angleitner-Boubenizek, L.; Rohde, M.; Denison, U.; Sliutz, G.; Fuith, L.C.; et al. Long-term significance of urinary neopterin in ovarian cancer: A study by the Austrian Association for Gynecologic Oncology (AGO). Ann. Oncol. 2016, 27, 1740–1746. [Google Scholar] [CrossRef]
- Melichar, B.; Spisarová, M.; Bartoušková, M.; Krčmová, L.K.; Javorská, L.; Študentová, H. Neopterin as a biomarker of immune response in cancer patients. Ann. Transl. Med. 2017, 5, 280. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, Y.; Gunel, N.; Coskun, U.; Pasaoglu, H.; Aslan, S.; Cetin, A. Serum neopterin levels in patients with breast cancer. Med. Oncol. 2008, 25, 403–407. [Google Scholar] [CrossRef]
- Gohar, S.; Al-Hassanin, S.; Shehata, A.; Soliman, S. Clinical Value of Serum Neopterin in Breast Cancer. Res. Oncol. 2018, 14, 70–74. [Google Scholar] [CrossRef]
- Pichler, R.; Fritz, J.; Heidegger, I.; Steiner, E.; Culig, Z.; Klocker, H.; Fuchs, D. Predictive and prognostic role of serum neopterin and tryptophan breakdown in prostate cancer. Cancer Sci. 2017, 108, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Hacisevki, A.; Baba, B.; Aslan, S.; Ozkan, Y. Neopterin: A possible biomarker in gastrointestinal cancer. Ankara Univ. Eczac. Fak. Derg. 2018, 42, 32–41. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Ji, X.; Zou, S. Changes of serum neopterin and its significance as biomarker in prediction the prognosis of patients with acute pancreatitis. J. Lab. Med. 2020, 44, 205–209. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Q.; Ma, L.; Li, J.; Xu, L. The early diagnostic value of serum neopterin and cartilage oligomeric matrix protein for osteoarticular changes among brucellosis patients at an early period. J. Orthop. Surg. Res. 2018, 13, 222. [Google Scholar] [CrossRef]
- Kutluana, U.; Kilciler, A.G.; Mizrak, S.; Dilli, U. Can neopterin be a useful immune biomarker for differentiating gastric intestinal metaplasia and gastric atrophy from non-atrophic non-metaplastic chronic gastritis? Gastroenterol. Hepatol. 2019, 42, 289–295. [Google Scholar] [CrossRef]
- Grabherr, F.; Effenberger, M.; Pedrini, A.; Mayr, L.; Schwärzler, J.; Reider, S.; Enrich, B.; Fritsche, G.; Wildner, S.; Bellmann-Weiler, R.; et al. Increased Fecal Neopterin Parallels Gastrointestinal Symptoms in COVID-19. Clin. Transl. Gastroenterol. 2021, 12, e00293. [Google Scholar] [CrossRef]
- Rehák, S.; Malirová, E.; Cermanová, M.; Suba, P.; Cerman, J.; Plisek, S.; Melichar, B. Cerebrospinal Fluid Neopterin in Patients with Tumors and other Disorders. Pteridines 2008, 19, 86–91. [Google Scholar] [CrossRef]
- Geng, M.; Xiao, H.; Liu, J.; Song, Y.; Fu, P.; Cheng, X.; Zhang, J.; Wang, G. The diagnostic role and dynamic changes in cerebrospinal fluid neopterin during treatment of patients with primary central nervous system lymphoma. Cancer Med. 2018, 7, 3889–3898. [Google Scholar] [CrossRef] [Green Version]
- Hagberg, L.; Cinque, P.; Gisslen, M.; Brew, B.J.; Spudich, S.; Bestetti, A.; Price, R.W.; Fuchs, D. Cerebrospinal fluid neopterin: An informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res. Ther. 2010, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Fuchs, D.; Gisslen, M. Laboratory diagnostic value of neopterin measurements in patients with COVID-19 infection. Pteridines 2021, 32, 1–4. [Google Scholar] [CrossRef]
- Chauvin, M.; Larsen, M.; Quirant, B.; Quentric, P.; Dorgham, K.; Royer, L.; Vallet, H.; Guihot, A.; Combadière, B.; Combadière, C.; et al. Elevated Neopterin Levels Predict Fatal Outcome in SARS-CoV-2-Infected Patients. Front. Cell. Infect. Microbiol. 2021, 11, 709893. [Google Scholar] [CrossRef] [PubMed]
- Hailemichael, W.; Kiros, M.; Akelew, Y.; Getu, S.; Andualem, H. Neopterin: A Promising Candidate Biomarker for Severe COVID-19. J. Inflamm. Res. 2021, 14, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Edén, A.; Grahn, A.; Bremell, D.; Aghvanyan, A.; Bathala, P.; Fuchs, D.; Gostner, J.; Hagberg, L.; Kanberg, N.; Kanjananimmanont, S.; et al. Viral Antigen and Inflammatory Biomarkers in Cerebrospinal Fluid in Patients With COVID-19 Infection and Neurologic Symptoms Compared With Control Participants Without Infection or Neurologic Symptoms. JAMA Netw. Open 2022, 5, e2213253. [Google Scholar] [CrossRef]
- Oettl, K.; Greilberger, J.; Reibnegger, G. Modulation of Free Radical Formation by Pterin Derivatives. Pteridines 2004, 15, 97–101. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.-C.; Wang, Z.-Q.; Tejero, J.; Yang, Y.-P.; Hemann, C.; Hille, R.; Stuehr, D.J. Catalytic reduction of a tetrahydrobiopterin radical within nitric-oxide synthase. J. Biol. Chem. 2008, 283, 11734–11742. [Google Scholar] [CrossRef] [Green Version]
- Oettl, K.; Reibnegger, G. Pteridine Derivatives as Modulators of Oxidative Stress. Curr. Drug Metab. 2002, 3, 203–209. [Google Scholar] [CrossRef]
- Kirsch, M.; Korth, H.-G.; Stenert, V.; Sustmann, R.; de Groot, H. The autoxidation of tetrahydrobiopterin revisited. Proof of superoxide formation from reaction of tetrahydrobiopterin with molecular oxygen. J. Biol. Chem. 2003, 278, 24481–24490. [Google Scholar] [CrossRef] [Green Version]
- Rebrin, I.; Bailey, S.W.; Boerth, S.R.; Ardell, M.D.; Ayling, J.E. Catalytic characterization of 4a-hydroxytetrahydropterin dehydratase. Biochemistry 1995, 34, 5801–5810. [Google Scholar] [CrossRef]
- Alhajji, E.; Boulghobra, A.; Bonose, M.; Berthias, F.; Moussa, F.; Maître, P. Multianalytical Approach for Deciphering the Specific MS/MS Transition and Overcoming the Challenge of the Separation of a Transient Intermediate, Quinonoid Dihydrobiopterin. Anal. Chem. 2022, 94, 12578–12585. [Google Scholar] [CrossRef]
- Yao, F.; Abdel-Rahman, A.A. Tetrahydrobiopterin paradoxically mediates cardiac oxidative stress and mitigates ethanol-evoked cardiac dysfunction in conscious female rats. Eur. J. Pharmacol. 2021, 909, 174406. [Google Scholar] [CrossRef]
- Vásquez-Vivar, J. Tetrahydrobiopterin, superoxide, and vascular dysfunction. Free Radic. Biol. Med. 2009, 47, 1108–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter-Parker, G.; Prebble, H.M.; Cross, S.; Steyn, N.; Shchepetkina, A.; Hock, B.D.; Cousins, A.; Gieseg, S.P. Neopterin formation through radical scavenging of superoxide by the macrophage synthesised antioxidant 7,8-dihydroneopterin. Free Radic. Biol. Med. 2020, 152, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Daff, S. NO synthase: Structures and mechanisms. Nitric Oxide Biol. Chem. 2010, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tejero, J.; Shiva, S.; Gladwin, M.T. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiol. Rev. 2018, 99, 311–379. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, Y.; Gu, L.; Liu, P.; Cao, J.; Zhang, S. The Critical Role of Tetrahydrobiopterin (BH4) Metabolism in Modulating Radiosensitivity: BH4/NOS Axis as an Angel or a Devil. Front. Oncol. 2021, 11, 720632. [Google Scholar] [CrossRef] [PubMed]
- Vujacic-Mirski, K.; Oelze, M.; Kuntic, I.; Kuntic, M.; Kalinovic, S.; Li, H.; Zielonka, J.; Münzel, T.; Daiber, A. Measurement of Tetrahydrobiopterin in Animal Tissue Samples by HPLC with Electrochemical Detection—Protocol Optimization and Pitfalls. Antioxidants 2022, 11, 1182. [Google Scholar] [CrossRef] [PubMed]
- Estelberger, W.; Fuchs, D.; Murr, C.; Wachter, H.; Reibnegger, G. Conformational investigation of the cofactor (6R,1’R,2’S-)-5,6,7,8-tetrahydrobiopterin. Biochim. Biophys. Acta 1995, 1249, 23–28. [Google Scholar] [CrossRef]
- Martínez, A.; Dao, K.; McKinney, J.; Teigen, K.; Frøystein, N. The conformation of 5,6,7,8-tetrahydrobiopterin and 7,8-dihydrobiopterin in solution: A 1H NMR study. Pteridines 2000, 11, 32–33. [Google Scholar] [CrossRef]
- Gogonea, V.; Shy, J.; Biswas, P. Electronic Structure, Ionization Potential, and Electron Affinity of the Enzyme Cofactor (6 R)-5,6,7,8-Tetrahydrobiopterin in the Gas Phase, Solution, and Protein Environments. J. Phys. Chem. B 2006, 110, 22861–22871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estelberger, W.; Mlekusch, W.; Reibnegger, G. The conformational flexibility of 5,6,7,8-tetrahydrobiopterin and 5,6,7,8-tetrahydroneopterin: A molecular dynamical simulation. FEBS Lett. 1995, 357, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Reibnegger, G.; Pauschenwein, J.; Werner, E.R. Electronic Structure of Tetrahydropteridine Derivatives. Pteridines 1999, 10, 91–94. [Google Scholar] [CrossRef]
- Soniat, M.; Martin, C.B. Theoretical Study on the Relative Energies of Neutral Pterin Tautomers. Pteridines 2008, 19, 120–124. [Google Scholar] [CrossRef]
- Soniat, M.; Martin, C.B. Theoretical Study on the Relative Energies of Anionic Pterin Tautomers. Pteridines 2009, 20, 124–128. [Google Scholar] [CrossRef]
- Nekkanti, S.; Martin, C.B. Theoretical study on the relative energies of cationic pterin tautomers. Pteridines 2015, 26, 13–22. [Google Scholar] [CrossRef]
- Reibnegger, G. QT-AIM analysis of neutral pterin and its anionic and cationic forms. Pteridines 2014, 25, 41–48. [Google Scholar] [CrossRef]
- Reibnegger, G. An ab initio and density functional theory study on neutral pterin radicals. Pteridines 2015, 26, 135–142. [Google Scholar] [CrossRef]
- Reibnegger, G. A DFT Study on the One-Electron Reduction/Oxidation of Biologically Relevant Pteridine Derivatives. ChemistrySelect 2018, 3, 10925–10931. [Google Scholar] [CrossRef]
- Buglak, A.A.; Telegina, T.A.; Kritsky, M.S. A quantitative structure–property relationship (QSPR) study of singlet oxygen generation by pteridines. Photochem. Photobiol. Sci. 2016, 15, 801–811. [Google Scholar] [CrossRef]
- Oettl, K.; Pfleiderer, W.; Reibnegger, G. Formation of Oxygen Radicals in Solutions of Different 7,8-Dihydropterins: Quantitative Structure-Activity Relationships. Helv. Chim. Acta 2000, 83, 954–965. [Google Scholar] [CrossRef]
- Walalawela, N.; Vignoni, M.; Urrutia, M.N.; Belh, S.J.; Greer, E.M.; Thomas, A.H.; Greer, A. Kinetic Control in the Regioselective Alkylation of Pterin Sensitizers: A Synthetic, Photochemical, and Theoretical Study. Photochem. Photobiol. 2018, 94, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Vignoni, M.; Walalawela, N.; Bonesi, S.M.; Greer, A.; Thomas, A.H. Lipophilic Decyl Chain-Pterin Conjugates with Sensitizer Properties. Mol. Pharm. 2018, 15, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, X.; Cao, Z. Theoretical study on the singlet excited state of pterin and its deactivation pathway. J. Phys. Chem. A 2007, 111, 9255–9262. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.-F.; Shen, L. Mechanistic Study of ROS-photogeneration by Pterin. Pteridines 2011, 22, 73–76. [Google Scholar] [CrossRef]
- Wolcan, E. On the origins of the absorption spectroscopy of pterin and Re(CO) 3(pterin)(H2O) aqueous solutions. A combined theoretical and experimental study. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2014, 129, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Malcomson, T.; Paterson, M.J. Theoretical determination of two-photon absorption in biologically relevant pterin derivatives. Photochem. Photobiol. Sci. 2020, 19, 1538–1547. [Google Scholar] [CrossRef]
- Abelleira, A.; Galang, R.D.; Clarke, M.J. Synthesis and electrochemistry of pterins coordinated to tetraammineruthenium(II). Inorg. Chem. 1990, 29, 633–639. [Google Scholar] [CrossRef]
- Kojima, T. Study on Proton-Coupled Electron Transfer in Transition Metal Complexes. Bull. Chem. Soc. Jpn. 2020, 93, 1571–1582. [Google Scholar] [CrossRef]
- Kojima, T. Development of functionality of metal complexes based on proton-coupled electron transfer. Dalt. Trans. 2020, 49, 7284–7293. [Google Scholar] [CrossRef]
- Mitome, H.; Ishizuka, T.; Kotani, H.; Shiota, Y.; Yoshizawa, K.; Kojima, T. Mechanistic Insights into C–H Oxidations by Ruthenium(III)-Pterin Complexes: Impact of Basicity of the Pterin Ligand and Electron Acceptability of the Metal Center on the Transition States. J. Am. Chem. Soc. 2016, 138, 9508–9520. [Google Scholar] [CrossRef]
- Ragone, F.; Ruiz, G.T.; Piro, O.E.; Echeverría, G.A.; Cabrerizo, F.M.; Petroselli, G.; Erra-Balsells, R.; Hiraoka, K.; García Einschlag, F.S.; Wolcan, E. Water-Soluble (Pterin)rhenium(I) Complex: Synthesis, Structural Characterization, and Two Reversible Protonation–Deprotonation Behavior in Aqueous Solutions. Eur. J. Inorg. Chem. 2012, 2012, 4801–4810. [Google Scholar] [CrossRef]
- Ragone, F.; Gara, P.D.; García Einschlag, F.S.; Lappin, A.G.; Ferraudi, G.J.; Wolcan, E.; Ruiz, G.T. Photophysics, photochemistry and thermally-induced redox reactions of a (Pterin)rhenium(I) complex. J. Photochem. Photobiol. A Chem. 2018, 358, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Heilmann, O.; Hornung, F.M.; Fiedler, J.; Kaim, W. Organometallic iridium(III) and rhenium(I) complexes with lumazine, alloxazine and pterin derivatives. J. Organomet. Chem. 1999, 589, 2–10. [Google Scholar] [CrossRef]
- Kumar, C.H.V.; Jagadeesh, R.V.; Shivananda, K.N.; Sandhya, Y.S.; Raju, C.N. Catalysis and Mechanistic Studies of Ru(III), Os(VIII), Pd(II), and Pt(IV) Metal Ions on Oxidative Conversion of Folic Acid. Ind. Eng. Chem. Res. 2010, 49, 1550–1560. [Google Scholar] [CrossRef]
- Petsi, M.; Zografos, A.L. Advances in Catalytic Aerobic Oxidations by Activation of Dioxygen-Monooxygenase Enzymes and Biomimetics. Synthesis 2018, 50, 4715–4745. [Google Scholar]
- Iyer, S.R.; Tidemand, K.D.; Babicz, J.T.J.; Jacobs, A.B.; Gee, L.B.; Haahr, L.T.; Yoda, Y.; Kurokuzu, M.; Kitao, S.; Saito, M.; et al. Direct coordination of pterin to Fe(II) enables neurotransmitter biosynthesis in the pterin-dependent hydroxylases. Proc. Natl. Acad. Sci. USA 2021, 118, e2022379118. [Google Scholar] [CrossRef]
- Pember, S.O.; Villafranca, J.J.; Benkovic, S.J. Phenylalanine hydroxylase from Chromobacterium violaceum is a copper-containing monooxygenase. Kinetics of the reductive activation of the enzyme. Biochemistry 1986, 25, 6611–6619. [Google Scholar] [CrossRef]
- Carr, R.T.; Benkovic, S.J. An examination of the copper requirement of phenylalanine hydroxylase from Chromobacterium violaceum. Biochemistry 1993, 32, 14132–14138. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Frey, P.A. Phenylalanine hydroxylase from Chromobacterium violaceum. Uncoupled oxidation of tetrahydropterin and the role of iron in hyroxylation. J. Biol. Chem. 1998, 273, 25594–25601. [Google Scholar] [CrossRef] [Green Version]
- Kohzuma, T.; Odani, A.; Morita, Y.; Takani, M.; Yamauchi, O. Pteridine-containing ternary copper(II) complexes as pterin cofactor-metal binding models. Structures, solution equilibria, and redox activities. Inorg. Chem. 1988, 27, 3854–3858. [Google Scholar] [CrossRef]
- Yamauchi, O. Amino acid- and pterin-metal chemistry as an approach to biological functions. Pure Appl. Chem. 1995, 67, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Crispini, A.; Pucci, D.; Bellusci, A.; Barberio, G.; La Deda, M.; Cataldi, A.; Ghedini, M. Hydrogen-Bonding Network in Metal−Pterin Complexes: Synthesis and Characterization of Water-Soluble Octahedral Nickel and Cadmium Pterine Derivatives. Cryst. Growth Des. 2005, 5, 1597–1601. [Google Scholar] [CrossRef]
- Martínez, A.; Vargas, R. Electron donor–acceptor properties of metal atoms interacting with pterins. New J. Chem. 2010, 34, 2988–2995. [Google Scholar] [CrossRef]
- Vargas, R.; Martínez, A. Non-conventional hydrogen bonds: Pterins-metal anions. Phys. Chem. Chem. Phys. 2011, 13, 12775–12784. [Google Scholar] [CrossRef] [PubMed]
- Smyth, C.; Mehigan, S.; Rakovich, Y.P.; McCabe, E.M.; Bell, S.E.J. Pterin detection using surface-enhanced Raman spectroscopy incorporating a straightforward silver colloid-based synthesis technique. J. Biomed. Opt. 2011, 16, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Buglak, A.A.; Kononov, A.I. Silver cluster interactions with Pterin: Complex structure, binding energies and spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 279, 121467. [Google Scholar] [CrossRef]
- Castillo, J.; Rozo, C.; Bertel, L.; Rindzevicius, T.; Mendez, S.; Martíneza, F.; Boisen, A. Orientation of Pterin-6-Carboxylic Acid on Gold Capped Silicon Nanopillars Platforms: Surface Enhanced Raman Spectroscopy and Density Functional Theory Studies. J. Braz. Chem. Soc. 2015, 27, 971–977. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.R.; Wang, Z.L.; Qu, F.; Luo, H.Q.; Li, N.B. Polyethylenimine-capped silver nanoclusters as a fluorescence probe for highly sensitive detection of folic acid through a two-step electron-transfer process. J. Agric. Food Chem. 2014, 62, 6592–6599. [Google Scholar] [CrossRef]
- Li, H.; Cheng, Y.; Liu, Y.; Chen, B. Fabrication of folic acid-sensitive gold nanoclusters for turn-on fluorescent imaging of overexpression of folate receptor in tumor cells. Talanta 2016, 158, 118–124. [Google Scholar] [CrossRef]
- Li, X.; Qiao, J.; Sun, Y.; Li, Z.; Qi, L. Ligand-modulated synthesis of gold nanoclusters for sensitive and selective detection of folic acid. J. Anal. Sci. Technol. 2021, 12, 17. [Google Scholar] [CrossRef]
- Ungor, D.; Szilágyi, I.; Csapó, E. Yellow-emitting Au/Ag bimetallic nanoclusters with high photostability for detection of folic acid. J. Mol. Liq. 2021, 338, 116695. [Google Scholar] [CrossRef]
- Fereja, S.L.; Li, P.; Guo, J.; Fang, Z.; Zhang, Z.; Zhuang, Z.; Zhang, X.; Liu, K.; Chen, W. Silver-enhanced fluorescence of bimetallic Au/Ag nanoclusters as ultrasensitive sensing probe for the detection of folic acid. Talanta 2021, 233, 122469. [Google Scholar] [CrossRef] [PubMed]
- Blach, D.; Alves De Souza, C.E.; Méndez, S.C.; Martínez, F.O. Conjugated anisotropic gold nanoparticles through pterin derivatives for a selective plasmonic photothermal therapy: In vitro studies in HeLa and normal human endocervical cells. Gold Bull. 2021, 54, 9–23. [Google Scholar] [CrossRef]
- Wei, C.C.; Crane, B.R.; Stuehr, D.J. Tetrahydrobiopterin radical enzymology. Chem. Rev. 2003, 103, 2365–2383. [Google Scholar] [CrossRef] [PubMed]
- Cabrerizo, F.M.; Petroselli, G.; Lorente, C.; Capparelli, A.L.; Thomas, A.H.; Braun, A.M.; Oliveros, E. Substituent Effects on the Photophysical Properties of Pterin Derivatives in Acidic and Alkaline Aqueous Solutions. Photochem. Photobiol. 2005, 81, 1234–1240. [Google Scholar] [CrossRef]
- Oliveros, E.; Dántola, M.L.; Vignoni, M.; Thomas, A.H.; Lorente, C. Production and quenching of reactive oxygen species by pterin derivatives, an intriguing class of biomolecules. Pure Appl. Chem. 2010, 83, 801–811. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.H.; Lorente, C.; Capparelli, A.L.; Pokhrel, M.R.; Braun, A.M.; Oliveros, E. Fluorescence of pterin, 6-formylpterin, 6-carboxypterin and folic acid in aqueous solution: pH effects. Photochem. Photobiol. Sci. 2002, 1, 421–426. [Google Scholar] [CrossRef]
- Neverov, K.V.; Mironov, E.A.; Lyudnikova, T.A.; Krasnovsky Jr, A.; Kritsky, M. Phosphorescence analysis of triplet state of pterins in connection with their photoreceptor function in biochemical systems. Biochemistry 1996, 61, 1149–1155. [Google Scholar]
- Dántola, M.L.; Urrutia, M.N.; Thomas, A.H. Effect of pterin impurities on the fluorescence and photochemistry of commercial folic acid. J. Photochem. Photobiol. B. 2018, 181, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Estébanez, S.; Lorente, C.; Tosato, M.G.; Miranda, M.A.; Marín, M.L.; Lhiaubet-Vallet, V.; Thomas, A.H. Photochemical formation of a fluorescent thymidine-pterin adduct in DNA. Dye. Pigment. 2019, 160, 624–632. [Google Scholar] [CrossRef] [Green Version]
- Cabrerizo, F.M.; Thomas, A.H.; Lorente, C.; Dántola, M.L.; Petroselli, G.; Erra-Balsells, R.; Capparelli, A.L. Generation of Reactive Oxygen Species during the Photolysis of 6-(Hydroxymethyl)pterin in Alkaline Aqueous Solutions. Helv. Chim. Acta 2004, 87, 349–365. [Google Scholar] [CrossRef]
- Buglak, A.A.; Telegina, T.A. A theoretical study of 5,6,7,8-tetrahydro-6-hydroxymethylpterin: Insight into intrinsic photoreceptor properties of 6-substituted tetrahydropterins. Photochem. Photobiol. Sci. 2019, 18, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Buglak, A.A. Photobiochemistry of Pterin Coenzymes; Research Centre of Biotechnology RAS: Moscow, Russian, 2016. [Google Scholar]
- DiScipio, R.M.; Santiago, R.Y.; Taylor, D.; Crespo-Hernández, C.E. Electronic relaxation pathways of the biologically relevant pterin chromophore. Phys. Chem. Chem. Phys. 2017, 19, 12720–12729. [Google Scholar] [CrossRef]
- Plotkin, M.; Hod, I.; Zaban, A.; Boden, S.A.; Bagnall, D.M.; Galushko, D.; Bergman, D.J. Solar energy harvesting in the epicuticle of the oriental hornet (Vespa orientalis). Naturwissenschaften 2010, 97, 1067–1076. [Google Scholar] [CrossRef]
- Roca-Sanjuán, D.; Galván, I.F.; Giussani, A.; Lindh, R. A theoretical analysis of the intrinsic light-harvesting properties of xanthopterin. Comput. Theor. Chem. 2014, 1040–1041, 230–236. [Google Scholar] [CrossRef]
- Yamazaki, S.; Domcke, W. Ab Initio Studies on the Photophysics of Guanine Tautomers: Out-of-Plane Deformation and NH Dissociation Pathways to Conical Intersections. J. Phys. Chem. A 2008, 112, 7090–7097. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, S.; Domcke, W.; Sobolewski, A.L. Nonradiative Decay Mechanisms of the Biologically Relevant Tautomer of Guanine. J. Phys. Chem. A 2008, 112, 11965–11968. [Google Scholar] [CrossRef]
- Serrano, M.P.; Vignoni, M.; Dántola, M.L.; Oliveros, E.; Lorente, C.; Thomas, A.H. Emission properties of dihydropterins in aqueous solutions. Phys. Chem. Chem. Phys. 2011, 13, 7419–7425. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yang, D.; Li, P. pH-Related and Site-Specific Excited-State Proton Transfer from Pterin to Acetate. J. Phys. Chem. B 2014, 118, 11707–11714. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sun, B.Q. pH-related fluorescence quenching mechanism of pterin derivatives and the effects of 6-site substituents. Can. J. Chem. 2018, 96, 404–410. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, M.E. Fluorescent pteridine probes for nucleic acid analysis. Methods Enzymol. 2008, 450, 201–231. [Google Scholar] [CrossRef]
- Moreno, A.; Knee, J.L.; Mukerji, I. Photophysical Characterization of Enhanced 6-Methylisoxanthopterin Fluorescence in Duplex DNA. J. Phys. Chem. B 2016, 120, 12232–12248. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.T.; Freelander, R.S.; Schulman, E.M.; Dunlap, R.B. Room temperature phosphorescence of selected pteridines. Anal. Chem. 1979, 51, 1921–1926. [Google Scholar] [CrossRef]
- Serrano, M.P.; Lorente, C.; Vieyra, F.E.M.; Borsarelli, C.D.; Thomas, A.H. Photosensitizing properties of biopterin and its photoproducts using 2’-deoxyguanosine 5’-monophosphate as an oxidizable target. Phys. Chem. Chem. Phys. 2012, 14, 11657–11665. [Google Scholar] [CrossRef]
- Swarna, S.; Lorente, C.; Thomas, A.H.; Martin, C.B. Rate constants of quenching of the fluorescence of pterins by the iodide anion in aqueous solution. Chem. Phys. Lett. 2012, 542, 62–65. [Google Scholar] [CrossRef]
- Estébanez, S.; Lorente, C.; Kaufman, T.S.; Larghi, E.L.; Thomas, A.H.; Serrano, M.P. Photophysical and Photochemical Properties of 3-methylpterin as a New and More Stable Pterin-type Photosensitizer. Photochem. Photobiol. 2018, 94, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Foote, C.S. Definition of type I and type II photosensitized oxidation. Photochem. Photobiol. 1991, 54, 659. [Google Scholar] [CrossRef] [PubMed]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef] [Green Version]
- Buglak, A.A.; Telegina, T.A.; Vorotelyak, E.A.; Kononov, A.I. Theoretical study of photoreactions between oxidized pterins and molecular oxygen. J. Photochem. Photobiol. A Chem. 2019, 372, 254–259. [Google Scholar] [CrossRef]
- Thomas, A.H.; Serrano, M.P.; Rahal, V.; Vicendo, P.; Claparols, C.; Oliveros, E.; Lorente, C. Tryptophan oxidation photosensitized by pterin. Free Radic. Biol. Med. 2013, 63, 467–475. [Google Scholar] [CrossRef]
- Reid, L.O.; Roman, E.A.; Thomas, A.H.; Dántola, M.L. Photooxidation of Tryptophan and Tyrosine Residues in Human Serum Albumin Sensitized by Pterin: A Model for Globular Protein Photodamage in Skin. Biochemistry 2016, 55, 4777–4786. [Google Scholar] [CrossRef] [PubMed]
- Dantola, M.L.; Reid, L.O.; Castaño, C.; Lorente, C.; Oliveros, E.; Thomas, A.H. Photosensitization of peptides and proteins by pterin derivatives. Pteridines 2017, 28, 105–114. [Google Scholar] [CrossRef]
- Di Mascio, P.; Martinez, G.R.; Miyamoto, S.; Ronsein, G.E.; Medeiros, M.H.G.; Cadet, J. Singlet Molecular Oxygen Reactions with Nucleic Acids, Lipids, and Proteins. Chem. Rev. 2019, 119, 2043–2086. [Google Scholar] [CrossRef] [PubMed]
- Vignoni, M.; Urrutia, M.N.; Junqueira, H.C.; Greer, A.; Reis, A.; Baptista, M.S.; Itri, R.; Thomas, A.H. Photo-Oxidation of Unilamellar Vesicles by a Lipophilic Pterin: Deciphering Biomembrane Photodamage. Langmuir 2018, 34, 15578–15586. [Google Scholar] [CrossRef] [PubMed]
- Dántola, M.L.; Denofrio, M.P.; Zurbano, B.; Gimenez, C.S.; Ogilby, P.R.; Lorente, C.; Thomas, A.H. Mechanism of photooxidation of folic acid sensitized by unconjugated pterins. Photochem. Photobiol. Sci. Off. J. Eur. Photochem. Assoc. Eur. Soc. Photobiol. 2010, 9, 1604–1612. [Google Scholar] [CrossRef]
- Laura Dántola, M.; Gojanovich, A.D.; Thomas, A.H. Inactivation of tyrosinase photoinduced by pterin. Biochem. Biophys. Res. Commun. 2012, 424, 568–572. [Google Scholar] [CrossRef]
- Yamada, H.; Arai, T.; Endo, N.; Yamashita, K.; Nonogawa, M.; Makino, K.; Fukuda, K.; Sasada, M.; Uchiyama, T. Photodynamic effects of a novel pterin derivative on a pancreatic cancer cell line. Biochem. Biophys. Res. Commun. 2005, 333, 763–767. [Google Scholar] [CrossRef]
- Tosato, M.G.; Schilardi, P.; Lorenzo de Mele, M.F.; Thomas, A.H.; Lorente, C.; Miñán, A. Synergistic effect of carboxypterin and methylene blue applied to antimicrobial photodynamic therapy against mature biofilm of Klebsiella pneumoniae. Heliyon 2020, 6, e03522. [Google Scholar] [CrossRef]
- Thomas, A.H.; Lorente, C.; Capparelli, A.L.; Martínez, C.G.; Braun, A.M.; Oliveros, E. Singlet oxygen (1Δg) production by pterin derivatives in aqueous solutions. Photochem. Photobiol. Sci. 2003, 2, 245–250. [Google Scholar] [CrossRef]
- Paula Denofrio, M.; Lorente, C.; Breitenbach, T.; Hatz, S.; Cabrerizo, F.M.; Thomas, A.H.; Ogilby, P.R. Photodynamic Effects of Pterin on HeLa Cells. Photochem. Photobiol. 2011, 87, 862–866. [Google Scholar] [CrossRef]
- Miyoshi, T.; Arai, T.; Nonogawa, M.; Makino, K.; Mori, H.; Yamashita, K.; Sasada, M. Anticancer photodynamic and non-photodynamic effects of pterin derivatives on a pancreatic cancer cell line. J. Pharmacol. Sci. 2008, 107, 221–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walalawela, N.; Urrutia, M.N.; Thomas, A.H.; Greer, A.; Vignoni, M. Alkane Chain-extended Pterin Through a Pendent Carboxylic Acid Acts as Triple Functioning Fluorophore, 1O2 Sensitizer and Membrane Binder. Photochem. Photobiol. 2019, 95, 1160–1168. [Google Scholar] [CrossRef]
- Xu, X.; Luan, F.; Liu, H.; Cheng, J.; Zhang, X. Prediction of the maximum absorption wavelength of azobenzene dyes by QSPR tools. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 83, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Chaudret, R.; Kiss, C.F.; Subramanian, L. Prediction of absorption wavelengths using a combination of semi-empirical quantum mechanics simulations and quantitative structure–property relationship modeling approaches. J. Photochem. Photobiol. A Chem. 2015, 299, 183–188. [Google Scholar] [CrossRef]
- Gopala Krishna, J.; Roy, K. QSPR modeling of absorption maxima of dyes used in dye sensitized solar cells (DSSCs). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 265, 120387. [Google Scholar] [CrossRef] [PubMed]
- López-Malo, D.; Bueso-Bordils, J.I.; Duart, M.J.; Alemán-López, P.A.; Martín-Algarra, R.V.; Antón-Fos, G.M.; Lahuerta-Zamora, L.; Martínez-Calatayud, J. QSPR studies on the photoinduced-fluorescence behaviour of pharmaceuticals and pesticides. SAR QSAR Environ. Res. 2017, 28, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Tanaka, K.; Funatsu, K. Random Forest Approach to QSPR Study of Fluorescence Properties Combining Quantum Chemical Descriptors and Solvent Conditions. J. Fluoresc. 2018, 28, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. Predictive models on photolysis and photoinduced toxicity of persistent organic chemicals. Front. Environ. Sci. Eng. 2013, 7, 803–814. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, X.; Bing, S.; Xue, Z.; Li, H. QSPR study on the photolysis half-life of PCDD/Fs adsorbed on spruce (Picea abies (L.) Karst.) needle surfaces under sunlight irradiation by using a molecular distance-edge vector index. RSC Adv. 2015, 5, 6617–6624. [Google Scholar] [CrossRef]
- Jalili-Jahani, N.; Fatehi, A.; Zeraatkar, E. PLS and N-PLS based MIA-QSPR modeling of the photodegradation half-lives for polychlorinated biphenyl congeners. RSC Adv. 2020, 10, 33753–33761. [Google Scholar] [CrossRef]
- Denofrio, M.P.; Thomas, A.H.; Braun, A.M.; Oliveros, E.; Lorente, C. Photochemical and photophysical properties of lumazine in aqueous solutions. J. Photochem. Photobiol. A Chem. 2008, 200, 282–286. [Google Scholar] [CrossRef]
- Mercader, A.G.; Duchowicz, P.R.; Fernández, F.M.; Castro, E.A.; Cabrerizo, F.M.; Thomas, A.H. Predictive modeling of the total deactivation rate constant of singlet oxygen by heterocyclic compounds. J. Mol. Graph. Model. 2009, 28, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Gueymard, C.A. Solar Radiation Spectrum BT. In Encyclopedia of Sustainability Science and Technology; Meyers, R.A., Ed.; Springer: New York, NY, USA, 2015; pp. 1–32. ISBN 978-1-4939-2493-6. [Google Scholar]
- Kritsky, M.S.; Telegina, T.A.; Vechtomova, Y.L.; Buglak, A.A. Why flavins are not competitors of chlorophyll in the evolution of biological converters of solar energy. Int. J. Mol. Sci. 2013, 14, 575. [Google Scholar] [CrossRef] [PubMed]
- Kavakli, I.H.; Baris, I.; Tardu, M.; Gül, Ş.; Öner, H.; Çal, S.; Bulut, S.; Yarparvar, D.; Berkel, Ç.; Ustaoğlu, P.; et al. The Photolyase/Cryptochrome Family of Proteins as DNA Repair Enzymes and Transcriptional Repressors. Photochem. Photobiol. 2017, 93, 93–103. [Google Scholar] [CrossRef] [Green Version]
- Hosokawa, Y.; Müller, P.; Kitoh-Nishioka, H.; Iwai, S.; Yamamoto, J. Limited solvation of an electron donating tryptophan stabilizes a photoinduced charge-separated state in plant (6–4) photolyase. Sci. Rep. 2022, 12, 5084. [Google Scholar] [CrossRef]
- Cellini, A.; Shankar, M.K.; Wahlgren, W.Y.; Nimmrich, A.; Furrer, A.; James, D.; Wranik, M.; Aumonier, S.; Beale, E.V.; Dworkowski, F.; et al. Structural basis of the radical pair state in photolyases and cryptochromes. Chem. Commun. 2022, 58, 4889–4892. [Google Scholar] [CrossRef]
- Losi, A.; Mandalari, C.; Gärtner, W. From Plant Infectivity to Growth Patterns: The Role of Blue-Light Sensing in the Prokaryotic World. Plants 2014, 3, 70–94. [Google Scholar] [CrossRef]
- Park, S.-Y.; Tame, J.R.H. Seeing the light with BLUF proteins. Biophys. Rev. 2017, 9, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Gauden, M.; Yeremenko, S.; Laan, W.; van Stokkum, I.H.M.; Ihalainen, J.A.; van Grondelle, R.; Hellingwerf, K.J.; Kennis, J.T.M. Photocycle of the Flavin-Binding Photoreceptor AppA, a Bacterial Transcriptional Antirepressor of Photosynthesis Genes. Biochemistry 2005, 44, 3653–3662. [Google Scholar] [CrossRef]
- Goings, J.J.; Li, P.; Zhu, Q.; Hammes-Schiffer, S. Formation of an unusual glutamine tautomer in a blue light using flavin photocycle characterizes the light-adapted state. Proc. Natl. Acad. Sci. USA 2020, 117, 26626–26632. [Google Scholar] [CrossRef]
- Tokonami, S.; Onose, M.; Nakasone, Y.; Terazima, M. Slow Conformational Changes of Blue Light Sensor BLUF Proteins in Milliseconds. J. Am. Chem. Soc. 2022, 144, 4080–4090. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Nakasone, Y.; Terazima, M. Selective Photoinduced Dimerization and Slow Recovery of a BLUF Domain of EB1. J. Phys. Chem. B 2022, 126, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.-W.; Chen, Z.; Zhou, Z.; Zhou, Y.; Tang, S.; Zhang, Y.; Zhang, T.; Ding, B.; Zhong, D. Direct Observation of Ultrafast Proton Rocking in the BLUF Domain. Angew. Chem. Int. Ed. 2022, 61, e202114423. [Google Scholar] [CrossRef]
- Sancar, A. Mechanisms of DNA Repair by Photolyase and Excision Nuclease (Nobel Lecture). Angew. Chem. Int. Ed. 2016, 55, 8502–8527. [Google Scholar] [CrossRef] [PubMed]
- Kavakli, I.H.; Ozturk, N.; Gul, S. DNA repair by photolyases. Adv. Protein Chem. Struct. Biol. 2019, 115, 1–19. [Google Scholar] [CrossRef]
- Vechtomova, Y.L.; Telegina, T.A.; Kritsky, M.S. Evolution of Proteins of the DNA Photolyase/Cryptochrome Family. Biochemistry 2020, 85, 131–153. [Google Scholar] [CrossRef]
- Ponnu, J.; Hoecker, U. Signaling Mechanisms by Arabidopsis Cryptochromes. Front. Plant Sci. 2022, 13, 844714. [Google Scholar] [CrossRef]
- Pooam, M.; El-Esawi, M.A.; Aguida, B.; Ahmad, M. Arabidopsis cryptochrome and Quantum Biology: New insights for plant science and crop improvement. J. Plant Biochem. Biotechnol. 2020, 29, 636–651. [Google Scholar] [CrossRef]
- Brazard, J.; Usman, A.; Lacombat, F.; Ley, C.; Martin, M.M.; Plaza, P.; Mony, L.; Heijde, M.; Zabulon, G.; Bowler, C. Spectro−Temporal Characterization of the Photoactivation Mechanism of Two New Oxidized Cryptochrome/Photolyase Photoreceptors. J. Am. Chem. Soc. 2010, 132, 4935–4945. [Google Scholar] [CrossRef]
- Hense, A.; Herman, E.; Oldemeyer, S.; Kottke, T. Proton transfer to flavin stabilizes the signaling state of the blue light receptor plant cryptochrome. J. Biol. Chem. 2015, 290, 1743–1751. [Google Scholar] [CrossRef] [Green Version]
- Partch, C.L.; Sancar, A. Photochemistry and Photobiology of Cryptochrome Blue-light Photopigments: The Search for a Photocycle. Photochem. Photobiol. 2005, 81, 1291–1304. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Guo, L.; Ai, Y.; Li, J.; Wang, L.; Sancar, A.; Luo, Y.; Zhong, D. Direct Determination of Resonance Energy Transfer in Photolyase: Structural Alignment for the Functional State. J. Phys. Chem. A 2014, 118, 10522–10530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vechtomova, Y.; Telegina, T.; Buglak, A.; Kritsky, M. UV Radiation in DNA Damage and Repair Involving DNA-Photolyases and Cryptochromes. Biomedicines 2021, 9, 1564. [Google Scholar] [CrossRef] [PubMed]
- Kiontke, S.; Göbel, T.; Brych, A.; Batschauer, A. DASH-type cryptochromes—Solved and open questions. Biol. Chem. 2020, 401, 1487–1493. [Google Scholar] [CrossRef]
- Lin, C.; Schneps, C.M.; Chandrasekaran, S.; Ganguly, A.; Crane, B.R. Mechanistic insight into light-dependent recognition of Timeless by Drosophila Cryptochrome. Structure 2022, 30, 851–861.e5. [Google Scholar] [CrossRef]
- Palayam, M.; Ganapathy, J.; Guercio, A.M.; Tal, L.; Deck, S.L.; Shabek, N. Structural insights into photoactivation of plant Cryptochrome-2. Commun. Biol. 2021, 4, 28. [Google Scholar] [CrossRef]
- Scheerer, P.; Zhang, F.; Kalms, J.; von Stetten, D.; Krauß, N.; Oberpichler, I.; Lamparter, T. The Class III Cyclobutane Pyrimidine Dimer Photolyase Structure Reveals a New Antenna Chromophore Binding Site and Alternative Photoreduction Pathways. J. Biol. Chem. 2015, 290, 11504–11514. [Google Scholar] [CrossRef] [Green Version]
- Petersen, J.; Rredhi, A.; Szyttenholm, J.; Oldemeyer, S.; Kottke, T.; Mittag, M. The World of Algae Reveals a Broad Variety of Cryptochrome Properties and Functions. Front. Plant Sci. 2021, 12, 766509. [Google Scholar] [CrossRef]
- Chen, S.; Liu, C.; Zhou, C.; Wei, Z.; Li, Y.; Xiong, L.; Yan, L.; Lv, J.; Shen, L.; Xu, L. Identification and characterization of a prokaryotic 6-4 photolyase from Synechococcus elongatus with a deazariboflavin antenna chromophore. Nucleic Acids Res. 2022, 50, 5757–5771. [Google Scholar] [CrossRef]
- Terai, Y.; Sato, R.; Matsumura, R.; Iwai, S.; Yamamoto, J. Enhanced DNA repair by DNA photolyase bearing an artificial light-harvesting chromophore. Nucleic Acids Res. 2020, 48, 10076–10086. [Google Scholar] [CrossRef]
- Geisselbrecht, Y.; Frühwirth, S.; Schroeder, C.; Pierik, A.J.; Klug, G.; Essen, L.-O. CryB from Rhodobacter sphaeroides: A unique class of cryptochromes with new cofactors. EMBO Rep. 2012, 13, 223–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, D.; Hu, Q.; Yan, Z.; Chen, W.; Yan, C.; Huang, X.; Zhang, J.; Yang, P.; Deng, H.; Wang, J.; et al. Structural basis of ultraviolet-B perception by UVR8. Nature 2012, 484, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Schallreuter, K.U.; Chavan, B.; Rokos, H.; Hibberts, N.; Panske, A.; Wood, J.M. Decreased phenylalanine uptake and turnover in patients with vitiligo. Mol. Genet. Metab. 2005, 86 (Suppl. 1), S27–S33. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Aisa, H.A. Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo. Molecules 2017, 22, 1303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telegina, T.A.; Vechtomova, Y.L.; Kritsky, M.S.; Madirov, E.I.; Nizamutdinov, A.S.; Obuhov, Y.N.; Buglak, A.A. Tetrahydrobiopterin Photooxidation: A Key Process in Vitiligo Phototherapy. Appl. Biochem. Microbiol. 2021, 57, 571–578. [Google Scholar] [CrossRef]
- Castaño, C.; Lorente, C.; Martins-Froment, N.; Oliveros, E.; Thomas, A.H. Degradation of α-melanocyte-stimulating hormone photosensitized by pterin. Org. Biomol. Chem. 2014, 12, 3877–3886. [Google Scholar] [CrossRef]
- Dayrit, J.F. The Histopathology of Vitiligo in Brown Skin. In Melasma and Vitiligo in Brown Skin; Handog, E.B., Enriquez-Macarayo, M.J., Eds.; Springer: New Delhi, India, 2017; pp. 217–225. ISBN 978-81-322-3664-1. [Google Scholar]
- Davis, M.D.; Kaufman, S.; Milstien, S. Conversion of 6-substituted tetrahydropterins to 7-isomers via phenylalanine hydroxylase-generated intermediates. Proc. Natl. Acad. Sci. USA 1991, 88, 385–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichinger, A.; Danecka, M.K.; Möglich, T.; Borsch, J.; Woidy, M.; Büttner, L.; Muntau, A.C.; Gersting, S.W. Secondary BH4 deficiency links protein homeostasis to regulation of phenylalanine metabolism. Hum. Mol. Genet. 2018, 27, 1732–1742. [Google Scholar] [CrossRef] [Green Version]
- Schallreuter, K.U.; Wood, J.M.; Körner, C.; Harle, K.M.; Schulz-Douglas, V.; Werner, E.R. 6-Tetrahydrobiopterin functions as a UVB-light switch for de novo melanogenesis. Biochim. Biophys. Acta 1998, 1382, 339–344. [Google Scholar] [CrossRef]
- Jain, A.; Mal, J.; Mehndiratta, V.; Chander, R.; Patra, S.K. Study of oxidative stress in vitiligo. Indian J. Clin. Biochem. 2011, 26, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Li, S.; Li, C. Perspectives of New Advances in the Pathogenesis of Vitiligo: From Oxidative Stress to Autoimmunity. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Yang, Y.; Xiang, L.; Zhang, C. The Role of Oxidative Stress in the Pathogenesis of Vitiligo: A Culprit for Melanocyte Death. Oxid. Med. Cell. Longev. 2022, 2022, 8498472. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M.; Chavan, B.; Hafeez, I.; Schallreuter, K.U. Regulation of tyrosinase by tetrahydropteridines and H2O2. Biochem. Biophys. Res. Commun. 2004, 325, 1412–1417. [Google Scholar] [CrossRef] [PubMed]
- Hasse, S.; Gibbons, N.C.J.; Rokos, H.; Marles, L.K.; Schallreuter, K.U. Perturbed 6-Tetrahydrobiopterin Recycling via Decreased Dihydropteridine Reductase in Vitiligo: More Evidence for H2O2 Stress. J. Investig. Dermatol. 2004, 122, 307–313. [Google Scholar] [CrossRef] [Green Version]
- Haavik, J.; Døskeland, A.P.; Flatmark, T. Stereoselective effects in the interactions of pterin cofactors with rat-liver phenylalanine 4-monooxygenase. Eur. J. Biochem. 1986, 160, 1–8. [Google Scholar] [CrossRef]
- Davis, M.D.; Ribeiro, P.; Tipper, J.; Kaufman, S. “7-tetrahydrobiopterin,” a naturally occurring analogue of tetrahydrobiopterin, is a cofactor for and a potential inhibitor of the aromatic amino acid hydroxylases. Proc. Natl. Acad. Sci. USA 1992, 89, 10109–10113. [Google Scholar] [CrossRef] [Green Version]
- Schallreuter, K.U.; Wood, J.M.; Pittelkow, M.R.; Gütlich, M.; Lemke, K.R.; Rödl, W.; Swanson, N.N.; Hitzemann, K.; Ziegler, I. Regulation of melanin biosynthesis in the human epidermis by tetrahydrobiopterin. Science 1994, 263, 1444–1446. [Google Scholar] [CrossRef]
- Rokos, H.; Beazley, W.D.; Schallreuter, K.U. Oxidative stress in vitiligo: Photo-oxidation of pterins produces H(2)O(2) and pterin-6-carboxylic acid. Biochem. Biophys. Res. Commun. 2002, 292, 805–811. [Google Scholar] [CrossRef]
- Kritsky, M.S.; Telegina, T.A.; Vechtomova, Y.L.; Kolesnikov, M.P.; Lyudnikova, T.A.; Golub, O.A. Excited flavin and pterin coenzyme molecules in evolution. Biochemistry 2010, 75, 1200–1216. [Google Scholar] [CrossRef]
- Heinz, B.; Ried, W.; Dose, K. Thermal Generation of Pteridines and Flavines from Amino Acid Mixtures. Angew. Chemie Int. Ed. 1979, 18, 478–483. [Google Scholar] [CrossRef]
- Heinz, B.; Ried, W. The formation of chromophores through amino acid thermolysis and their possible role as prebiotic photoreceptors. Biosystems 1981, 14, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, W. S. W. Fox and K. Dose, Molecular Evolution and the Origin of Life. Z. Allg. Mikrobiol. 1973, 13, 732. [Google Scholar] [CrossRef]
- Kritsky, M.S.; Telegina, T.A.; Lyudnikova, T.A.; Zemskova, Y.L. Coenzymes in Evolution of the RNA World. In Life in the Universe; Seckbach, J., Chela-Flores, J., Owen, T., Raulin, F., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 115–118. ISBN 978-94-007-1003-0. [Google Scholar]
- Schmidt, W.; Butler, W.L. Flavin-mediated photoreactions in artificial systems: A possible model for the blue-light photoreceptor pigment in living systems. Photochem. Photobiol. 1976, 24, 71–75. [Google Scholar] [CrossRef]
- Schirrmeister, B.E.; Sanchez-Baracaldo, P.; Wacey, D. Cyanobacterial evolution during the Precambrian. Int. J. Astrobiol. 2016, 15, 187–204. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.-C.; Sun, G.-X.; Yan, Y.; Konstantinidis, K.T.; Zhang, S.-Y.; Deng, Y.; Li, X.-M.; Cui, H.-L.; Musat, F.; Popp, D.; et al. The Great Oxidation Event expanded the genetic repertoire of arsenic metabolism and cycling. Proc. Natl. Acad. Sci. USA 2020, 117, 10414–10421. [Google Scholar] [CrossRef]
- Kalashnikov, M.; Choi, W.; Yu, C.-C.; Sung, Y.; Dasari, R.R.; Badizadegan, K.; Feld, M.S. Assessing light scattering of intracellular organelles in single intact living cells. Opt. Express 2009, 17, 19674–19681. [Google Scholar] [CrossRef] [Green Version]
- Kalashnikov, M.; Choi, W.; Hunter, M.; Yu, C.-C.; Dasari, R.R.; Feld, M.S. Assessing the contribution of cell body and intracellular organelles to the backward light scattering. Opt. Express 2012, 20, 816–826. [Google Scholar] [CrossRef]
- Stolik, S.; Delgado, J.A.; Pérez, A.; Anasagasti, L. Measurement of the penetration depths of red and near infrared light in human “ex vivo” tissues. J. Photochem. Photobiol. B 2000, 57, 90–93. [Google Scholar] [CrossRef]
- Liu, W.; Liu, X.Y.; Qian, Y.T.; Zhou, D.D.; Liu, J.W.; Chen, T.; Sun, W.; Ma, D.L. Urinary metabolomic investigations in vitiligo patients. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Bao, P.Z.; Ye, J.; Han, L.S.; Qiu, W.J.; Zhang, H.W.; Yu, Y.G.; Wang, J.G.; Gu, X.F. Application of isoxanthopterin as a new pterin marker in the differential diagnosis of hyperphenylalaninemia. World J. Pediatr. 2019, 15, 66–71. [Google Scholar] [CrossRef]
- Buglak, A.; Kononov, A. Computational study of interactions between silver nanoclusters and pterin. FEBS Open Bio 2021, 11, 103–507. [Google Scholar]
- Mirgorodskaya, A.B.; Kuznetsova, D.A.; Kushnazarova, R.A.; Gabdrakhmanov, D.R.; Zhukova, N.A.; Lukashenko, S.S.; Sapunova, A.S.; Voloshina, A.D.; Sinyashin, O.G.; Mamedov, V.A.; et al. Soft nanocarriers for new poorly soluble conjugate of pteridine and benzimidazole: Synthesis and cytotoxic activity against tumor cells. J. Mol. Liq. 2020, 317, 114007. [Google Scholar] [CrossRef]
- Escudero, A.; Carrillo-Carrión, C.; Castillejos, M.C.; Romero-Ben, E.; Rosales-Barrios, C.; Khiar, N. Photodynamic therapy: Photosensitizers and nanostructures. Mater. Chem. Front. 2021, 5, 3788–3812. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, S.; Wu, H.; Lou, X. A novel folic acid-conjugated TiO2-SiO2 photosensitizer for cancer targeting in photodynamic therapy. Colloids Surf. B Biointerfaces 2015, 125, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, M.N.; Sosa, M.J.; Pissinis, D.E.; Cánneva, A.; Miñán, A.G.; Vignoni, M.; Calvo, A.; Thomas, A.H.; Schilardi, P.L. Immobilization of alkyl-pterin photosensitizer on silicon surfaces through in situ SN2 reaction as suitable approach for photodynamic inactivation of Staphylococcus aureus. Colloids Surf. B Biointerfaces 2021, 198, 111456. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Yamashita, T.; Shinohara, M.; Sasaki, N.; Takaya, T.; Nakajima, K.; Inoue, N.; Masano, T.; Tawa, H.; Satomi-Kobayashi, S.; et al. Plasma tetrahydrobiopterin/dihydrobiopterin ratio—A possible marker of endothelial dysfunction. Circ. J. 2009, 73, 955–962. [Google Scholar] [CrossRef] [Green Version]
- Yogavel, M.; Nettleship, J.E.; Sharma, A.; Harlos, K.; Jamwal, A.; Chaturvedi, R.; Sharma, M.; Jain, V.; Chhibber-Goel, J.; Sharma, A. Structure of 6-hydroxymethyl-7,8-dihydropterin pyrophosphokinase-dihydropteroate synthase from Plasmodium vivax sheds light on drug resistance. J. Biol. Chem. 2018, 293, 14962–14972. [Google Scholar] [CrossRef] [Green Version]
- Coppen, A.; Swade, C.; Jones, S.A.; Armstrong, R.A.; Blair, J.A.; Leeming, R.J. Depression and tetrahydrobiopterin: The folate connection. J. Affect. Disord. 1989, 16, 103–107. [Google Scholar] [CrossRef]
- Haavik, J.; Flatmak, T. Isolation and characterization of tetrahydropterin oxidation products generated in the tyrosine 3-monooxygenase (tyrosine hydroxylase) reaction. Eur. J. Biochem. 1987, 168, 21–26. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Lombard, J.; Soule, T.; Dunaj, S.; Wu, S.H.; Wojciechowski, M.F. Timing the Evolutionary Advent of Cyanobacteria and the Later Great Oxidation Event Using Gene Phylogenies of a Sunscreen. MBio 2022, 10, e00561-19. [Google Scholar] [CrossRef] [Green Version]
- Strähle, J.; Fischer, B. Metal complexes of tetrahydroneopterin. In 9 Zurich, Switzerland, September 3–8, 1989; Curtius, H.-C., Ghisla, S., Blau, N., Eds.; De Gruyter: Berlin, Germany, 2019; pp. 110–113. [Google Scholar]
- Yasumura, M.; Morimoto, T.; Takagi, M.; Hirose, H.; Taguchi, O. Protective effects of 5,6,7,8-tetrahydroneopterin against X-ray radiation injury in mice. Biochim. Biophys. Acta 1999, 1453, 378–384. [Google Scholar] [CrossRef] [PubMed]






















| Compound | λF, nm | ΦF | τF |
|---|---|---|---|
| Ptr0 Ptr−1 | 439 456 | 0.33 0.27 | 7.6 5.0 |
| Mep0 Mep−1 | 448 460 | 0.61 0.61 | 13.3 11.2 |
| Hmp0 Hmp−1 | 449 457 | 0.53 0.46 | 11.0 8.4 |
| Fop0 | 446 | 0.12 | 7.9 |
| Fop−1 | 454 | 0.07 | 2.2 |
| Cap0 | 439 | 0.28 | 5.8 |
| Cap−1 | 451 | 0.18 | 4.1 |
| Dmp0 | 433 | 0.85 | 13.5 |
| Dmp−1 | 445 | 0.84 | 11.6 |
| Bip0 | 441 | 0.36 | 9.1 |
| Bip−1 | 455 | 0.29 | 7.6 |
| Nep0 | 440 | 0.31 | 7.4 |
| Nep−1 | 454 | 0.47 | 10.7 |
| Rap0 | 441 | 0.47 | 10.7 |
| Rap−1 | 455 | 0.40 | 7.5 |
| H2Fop | 528 | 8.7 × 10−3 | 0.34 |
| Sep | 533 | 7.0 × 10−3 | 0.28 |
| H2Bip H2Nep | 425 425 | 9 × 10−3 5 × 10−3 | 0.30 0.31 |
| H2Hmp | 425 | 3 × 10−3 | 0.21 |
| H2Mep | 410 | 3 × 10−3 | 0.18 |
| Compound | ΦΔ | |
|---|---|---|
| Ptr0 Ptr−1 | 0.18 0.30 | - 2.9 |
| Cap0 Cap−1 | 0.27 0.37 | - 1.4 |
| Fop0 Fop−1 | 0.45 0.47 | - 1.4 |
| Bip0 Bip−1 | 0.34 0.40 | - 2.4 |
| Nep0 Nep−1 | 0.23 0.34 | - 2.3 |
| Mep0 Mep−1 | 0.10 0.14 | - 8.0 |
| Dmp0 Dmp−1 | 0.04 0.10 | - 31 |
| Rap0 Rap−1 | 0.13 0.16 | - 3.6 |
| Hmp0 Hmp−1 | 0.15 0.21 | - 3.1 |
| H2Fop0 | 0.001 | 210 |
| H2Bip0 | 0.001 | 370 |
| H2Nep0 | 0.001 | 460 |
| H2Xap0 | 0.001 | 190 |
| Sep0 | 0.001 | 550 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buglak, A.A.; Kapitonova, M.A.; Vechtomova, Y.L.; Telegina, T.A. Insights into Molecular Structure of Pterins Suitable for Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 15222. https://doi.org/10.3390/ijms232315222
Buglak AA, Kapitonova MA, Vechtomova YL, Telegina TA. Insights into Molecular Structure of Pterins Suitable for Biomedical Applications. International Journal of Molecular Sciences. 2022; 23(23):15222. https://doi.org/10.3390/ijms232315222
Chicago/Turabian StyleBuglak, Andrey A., Marina A. Kapitonova, Yulia L. Vechtomova, and Taisiya A. Telegina. 2022. "Insights into Molecular Structure of Pterins Suitable for Biomedical Applications" International Journal of Molecular Sciences 23, no. 23: 15222. https://doi.org/10.3390/ijms232315222






