Use of CGF in Oral and Implant Surgery: From Laboratory Evidence to Clinical Evaluation
Abstract
:1. Introduction
2. Results
2.1. Biological Characterization of the CGF-Permeated Implants
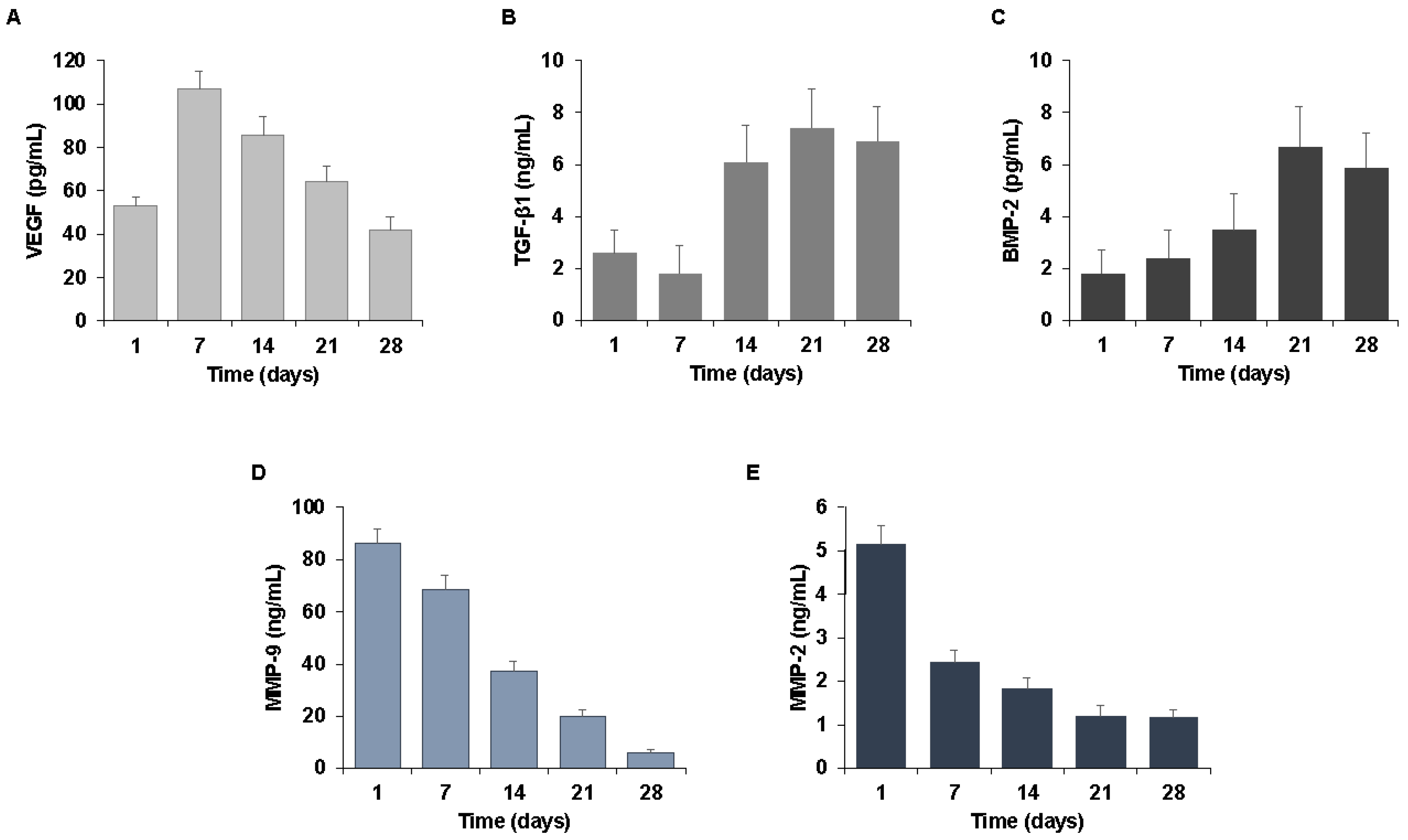
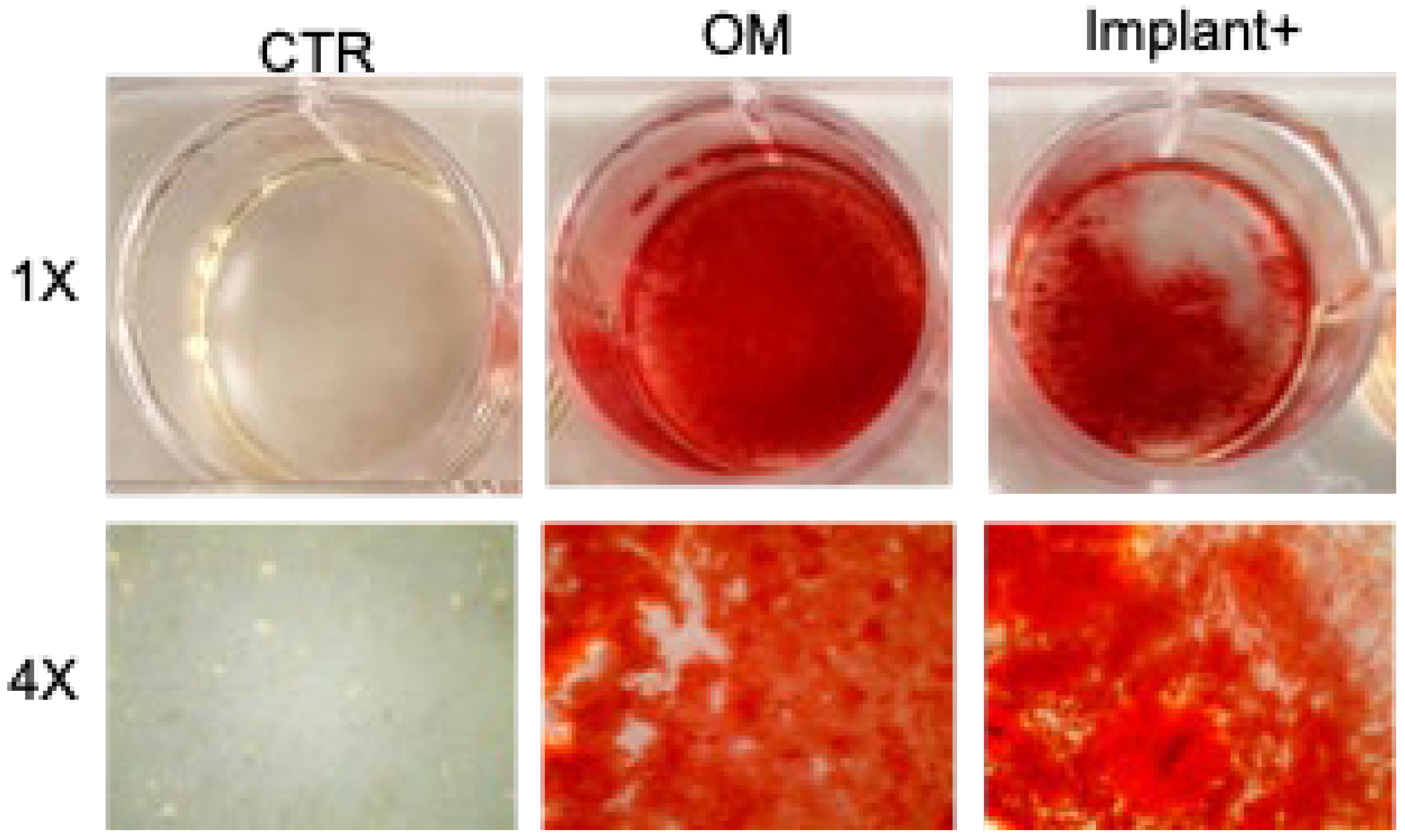
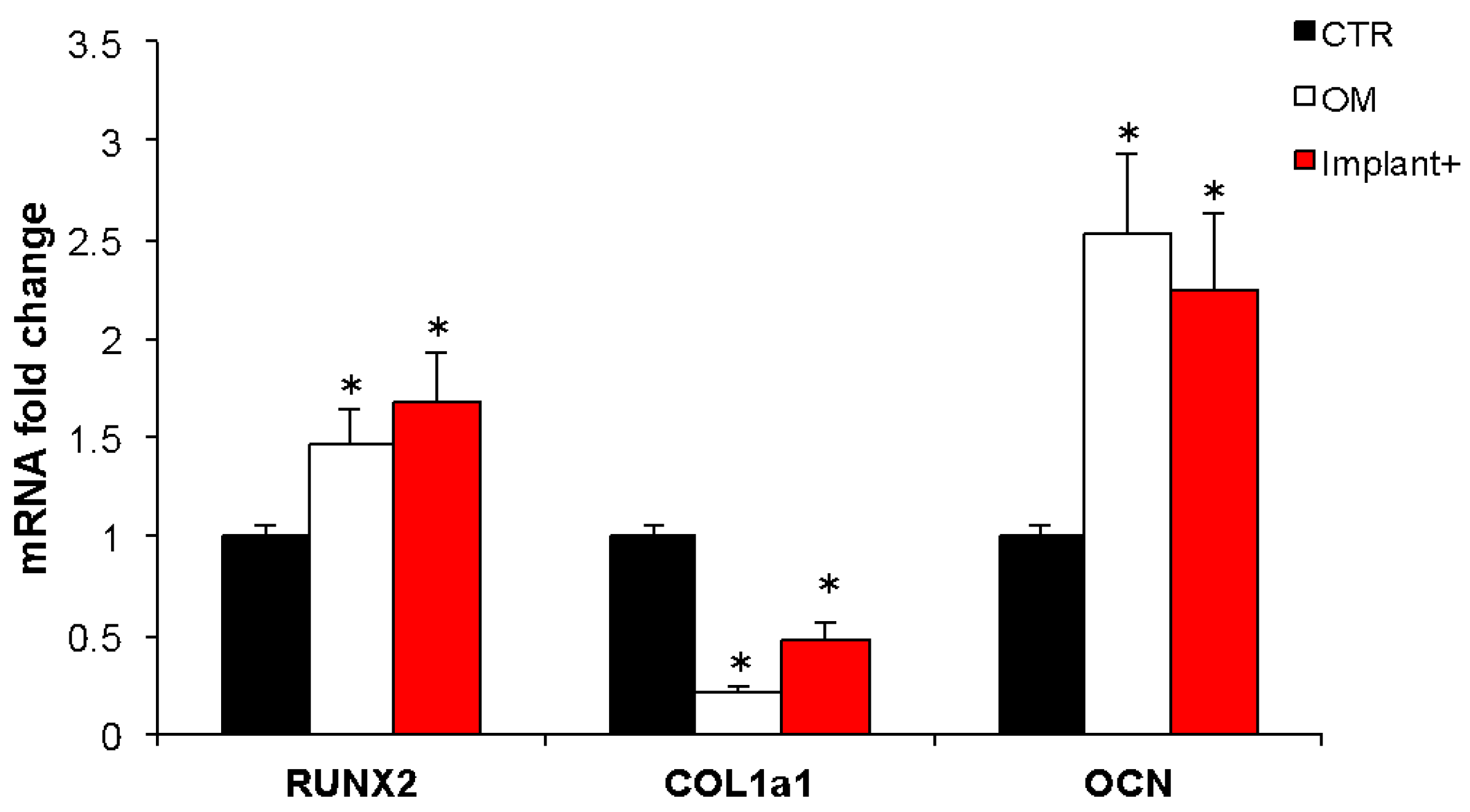
2.2. Osteogenic Properties of the CGF-Permeated Implants
2.3. Clinical Evaluation of CGF-Permeated Implants
3. Discussion
4. Materials and Methods
4.1. Preparation of CGF-Coated Dental Implants
4.2. Scanning Electron Microscopy Analysis
4.3. Growth Factors Release
4.4. Endothelial Cell Adhesion on CGF-Permeated Implants
4.5. Cell Culture and Osteogenic Differentiation
4.6. Alizarin Red Staining
4.7. Real-Time PCR
4.8. Clinical Applications of CGF-Permeated Implants
4.9. Limitations of the Study
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weber, H.P.; Crohin, C.C.; Fiorellini, J.P. A five-year clinical and radiographic study of non submerged dental implants. Clin. Oral. Impl. Res. 2000, 11, 144–153. [Google Scholar] [CrossRef]
- Leonhardt, A.; Grondahl, K.; Bergstrom, C.; Lekholm, U. Long-term followup of osseointegrated titanium implants using clinical, radiographic and microbiological parameters. Clin. Oral. Impl. Res. 2002, 13, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Felice, P.; Cannizzaro, G.; Checchi, V.; Marchetti, C.; Pellegrino, G.; Censi, P.; Esposito, M. Vertical bone augmentation versus 7 mm long implants in posterior atrophic mandibles. Results of a randomized controlled clinical trial of up to 4 months after loading. Eur. J. Oral. Implantol. 2009, 2, 7–20. [Google Scholar] [PubMed]
- Lee, D.J.; Saponaro, P.C. Management of Edentulous Patients. Dent. Clin. N. Am. 2019, 63, 249–261. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.A. Determinants of correct clinical reporting. Int. J. Prosthodont. 1998, 11, 517–521. [Google Scholar]
- Albrektsson, T.; Wennerberg, A. The impact of oral implants—Past and future, 1966–2042. J. Can. Dent. Assoc. 2005, 71, 327. [Google Scholar]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [Green Version]
- Ananth, H.; Kundapur, V.; Mohammed, H.S.; Anand, M.; Amarnath, G.S.; Mankar, S. A Review on Biomaterials in Dental Implantology. Int. J. Biomed. Sci. 2015, 11, 113–120. [Google Scholar]
- Pietruszka, P.; Chruścicka, I.; Duś-Ilnicka, I.; Paradowska-Stolarz, A. PRP and PRF-Subgroups and Divisions When Used in Dentistry. J. Pers. Med. 2021, 11, 944. [Google Scholar] [CrossRef]
- Rochira, A.; Siculella, L.; Damiano, F.; Palermo, A.; Ferrante, F.; Carluccio, M.A.; Calabriso, N.; Giannotti, L.; Stanca, E. Concentrated Growth Factors (CGF) Induce Osteogenic Differentiation in Human Bone Marrow Stem Cells. Biology 2020, 9, 370. [Google Scholar] [CrossRef]
- Rodella, L.F.; Favero, G.; Boninsegna, R.; Buffoli, B.; Labanca, M.; Scari, G.; Sacco, L.; Batani, T.; Rezzani, R. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc. Res. Tech. 2011, 74, 772–777. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, S.H.; Sandor, G.K.; Kim, Y.D. Comparison of platelet-rich plasma (PRP), platelet-rich fibrin (PRF), and concentrated growth factor (CGF) in rabbit-skull defect healing. Arch. Oral Biol. 2014, 59, 550–558. [Google Scholar] [CrossRef]
- Masuki, H.; Okudera, T.; Watanebe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant Dent. 2016, 2, 19. [Google Scholar] [CrossRef]
- Qiao, J.; An, N.; Ouyang, X. Quantification of growth factors in different platelet concentrates. Platelets 2017, 28, 774–778. [Google Scholar] [CrossRef]
- Sohn, D.S.; Heo, J.U.; Kwak, D.H.; Kim, D.E.; Kim, J.M.; Moon, J.W.; Lee, J.H.; Park, I.S. Bone regeneration in the maxillary sinus using an autologous fibrin-rich block with concentrated growth factors alone. Implant Dent. 2011, 20, 389–395. [Google Scholar] [CrossRef] [Green Version]
- Tabatabaei, F.; Aghamohammadi, Z.; Tayebi, L. In vitro and in vivo effects of concentrated growth factor on cells and tissues. J. Biomed. Mater. Res. A 2020, 108, 1338–1350. [Google Scholar] [CrossRef]
- Stanca, E.; Calabriso, N.; Giannotti, L.; Nitti, P.; Damiano, F.; Stanca, B.D.C.; Carluccio, M.A.; De Benedetto, G.E.; Demitri, C.; Palermo, A.; et al. Analysis of CGF Biomolecules, Structure and Cell Population: Characterization of the Stemness Features of CGF Cells and Osteogenic Potential. Int. J. Mol. Sci. 2021, 22, 8867. [Google Scholar] [CrossRef]
- Calabriso, N.; Stanca, E.; Rochira, A.; Damiano, F.; Giannotti, L.; Di Chiara Stanca, B.; Massaro, M.; Scoditti, E.; Demitri, C.; Nitti, P.; et al. Angiogenic Properties of Concentrated Growth Factors (CGFs): The Role of Soluble Factors and Cellular Components. Pharmaceutics 2021, 13, 635. [Google Scholar] [CrossRef]
- Palermo, A.; Ferrante, F.; Stanca, E.; Damiano, F.; Gnoni, A.; Batani, T.; Carluccio, M.A.; Demitri, C.; Siculella, L. Release of VEGF from Dental Implant Surface (IML® Implant) Coated with Concentrated Growth Factors (CGF) and the Liquid Phase of CGF (LPCGF): In Vitro Results and Future Expectations. Appl. Sci. 2019, 9, 2114. [Google Scholar] [CrossRef] [Green Version]
- Ding, Z.Y.; Tan, Y.; Peng, Q.; Zuo, J.; Li, N. Novel applications of platelet concentrates in tissue regeneration. Exp. Ther. Med. 2021, 21, 226–238. [Google Scholar] [CrossRef]
- Di Liddo, R.; Bertalot, T.; Borean, A.; Pirola, I.; Argentoni, A.; Schrenk, S.; Cenzi, C.; Capelli, S.; Conconi, M.T.; Parnigotto, P.P. Leucocyte and Platelet-rich Fibrin: A carrier of autologous multipotent cells for regenerative medicine. J. Cell. Mol. Med. 2018, 22, 1840–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef]
- Ansari, M. Bone tissue regeneration: Biology, strategies and interface studies. Prog. Biomater. 2019, 8, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Logan, N.J.; Camman, M.; Williams, G.; Higgins, C.A. Demethylation of ITGAV accelerates osteogenic differentiation in a blast-induced heterotopic ossification in vitro cell culture model. Bone 2018, 117, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Hanna, H.; Mir, L.M.; Andre, F.M. In vitro osteoblastic differentiation of mesenchymal stem cells generates cell layers with distinct properties. Stem Cell Res. Ther. 2018, 9, 203–213. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Zhao, H.; Han, X.; Zhao, T.; Qu, P.; Li, G.; Wang, W. Extracellular vesicle-encapsulated miR-22-3p from bone marrow mesenchymal stem cell promotes osteogenic differentiation via FTO inhibition. Stem Cell Res. Ther. 2020, 11, 227–240. [Google Scholar] [CrossRef]
- Fang, D.; Li, D.; Li, C.; Yang, W.; Xiao, F.; Long, Z. Efficacy and Safety of Concentrated Growth Factor Fibrin on the Extraction of Mandibular Third Molars: A Prospective, Randomized, Double-Blind Controlled Clinical Study. J. Oral Maxillofac. Surg. 2022, 80, 700–708. [Google Scholar] [CrossRef]
- Jin, R.; Song, G.; Chai, J.; Gou, X.; Yuan, G.; Chen, Z. Effects of concentrated growth factor on proliferation, migration, and differentiation of human dental pulp stem cells in vitro. J. Tissue Eng. 2018, 9, 2041731418817505. [Google Scholar] [CrossRef]
- Zhang, L.; Ai, H. Concentrated growth factor promotes proliferation, osteogenic differentiation, and angiogenic potential of rabbit periosteum-derived cells in vitro. J. Orthop. Surg. Res. 2019, 14, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Jiang, H. A Comprehensive Review of Concentrated Growth Factors and Their Novel Applications in Facial Reconstructive and Regenerative Medicine. Aesthetic Plast. Surg. 2020, 44, 1047–1057. [Google Scholar] [CrossRef]
- Manole, E.; Niculite, C.; Lambrescu, I.M.; Gaina, G.; Ioghen, O.; Ceafalan, L.C.; Hinescu, M.E. Macrophages and Stem Cells-Two to Tango for Tissue Repair? Biomolecules 2021, 11, 697. [Google Scholar] [CrossRef]
- Everts, P.A.; Knape, J.T.; Weibrich, G.; Schönberger, J.P.; Hoffmann, J.; Overdevest, E.P.; Box, H.A.; van Zundert, A. Platelet-rich plasma and platelet gel: A review. J. Extra Corpor. Technol. 2006, 38, 174–187. [Google Scholar]
- Parithimarkalaignan, S.; Padmanabhan, T.V. Osseointegration: An update. J. Indian. Prosthodont. Soc. 2013, 13, 2–6. [Google Scholar] [CrossRef]
- Lee, J.W.Y.; Bance, M.L. Physiology of Osseointegration. Otolaryngol. Clin. N. Am. 2019, 52, 231–242. [Google Scholar] [CrossRef]
- Hadzik, J.; Kubasiewicz-Ross, P.; Simka, W.; Gębarowski, T.; Barg, E.; Cieśla-Niechwiadowicz, A.; Trzcionka Szajna, A.; Szajna, E.; Gedrange, T.; Kozakiewicz, M.; et al. Fractal Dimension and Texture Analysis in the Assessment of Experimental Laser-Induced Periodic Surface Structures (LIPSS) Dental Implant Surface-In Vitro Study Preliminary Report. Materials 2022, 15, 2713. [Google Scholar] [CrossRef]
- Kubasiewicz-Ross, P.; Hadzik, J.; Gedrange, T.; Dominiak, M.; Jurczyszyn, K.; Pitułaj, A.; Nawrot-Hadzik, I.; Bortkiewicz, O.; Fleischer, M. Antimicrobial Efficacy of Different Decontamination Methods as Tested on Dental Implants with Various Types of Surfaces. Med. Sci. Monit. 2020, 26, e920513-1–e920513-8. [Google Scholar] [CrossRef]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef]
- Ferrara, N.; Gerber, H.P. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol. 2001, 106, 148–156. [Google Scholar] [CrossRef]
- Niu, G.; Chen, X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr. Drug Targets 2010, 11, 1000–1017. [Google Scholar] [CrossRef]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef] [Green Version]
- Xue, T.; Wei, L.; Qiao, L.; Qiu, J.; Zha, D. Does bone morphogenetic proteins play an important role in chronic rhinosinusitis? Med. Hypotheses 2009, 72, 228. [Google Scholar] [CrossRef] [PubMed]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Koolwijk, P.; van Erck, M.G.; de Vree, W.J.; Vermeer, M.A.; Weich, H.A.; Hanemaaijer, R.; van Hinsbergh, V.W. Cooperative effect of TNFalpha, bFGF, and VEGF on the formation of tubular structures of human microvascular endothelial cells in a fibrin matrix. Role of urokinase activity. J. Cell Biol. 1996, 132, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Gorodetsky, R.; Clark, R.A.; An, J.; Gailit, J.; Levdansky, L.; Vexler, A.; Berman, E.; Marx, G. Fibrin microbeads (FMB) as biodegradable carriers for culturing cells and for accelerating wound healing. J. Invest. Dermatol. 1999, 112, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ren, H.; Shang, Q.; Shen, G.; Tang, K.; Yu, F.; Chen, G.; Zhang, Z.; Zhao, W.; Zhang, P.; et al. Effects of concentrated growth factor on the proliferation, migration, and osteogenic differentiation of rat bone marrow mesenchymal stem cells: An in vitro study. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Hou, Y.; Song, P.; Zhou, M.; Nie, M.; Liu, X. Modulation of proliferation and differentiation of gingiva-derived mesenchymal stem cells by concentrated growth factors: Potential implications in tissue engineering for dental regeneration and repair. Int. J. Mol. Med. 2019, 44, 37–46. [Google Scholar] [CrossRef] [Green Version]
- Taschieri, S.; Khijmatgar, S.; Corbella, S.; Francetti, L.; Parrini, M.; Corradini, C.; Del Fabbro, M. Effect of concentrated growth factors on quality of life of patients undergoing implant therapy: A cohort study. J. Biol. Regul. Homeost. Agents 2021, 35, 147–154. [Google Scholar] [CrossRef]
- Shetye, A.G.; Rathee, M.; Jain, P.; Agarkar, V.; Kaushik, S.; Alam, M. Effect of advanced platelet-rich fibrin and concentrated growth factor on tissues around implants in maxillary anterior region. J. Indian Prosthodont. Soc. 2022, 22, 169–178. [Google Scholar] [CrossRef]
- Özveri Koyuncu, B.; İçpınar Çelik, K.; Özden Yüce, M.; Günbay, T.; Çömlekoğlu, M.E. The role of concentrated growth factor on implant stability: A preliminary study. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 363–367. [Google Scholar] [CrossRef]
- Krawiec, M.; Olchowy, C.; Kubasiewicz-Ross, P.; Hadzik, J.; Dominiak, M. Role of implant loading time in the prevention of marginal bone loss after implant-supported restorations: A targeted review. Dent. Med. Probl. 2022, 59, 475–481. [Google Scholar] [CrossRef]
- Skośkiewicz-Malinowska, K.; Noack, B.; Kaderali, L.; Malicka, B.; Lorenz, K.; Walczak, K.; Weber, M.T.; Mendak-Ziółko, M.; Hoffmann, T.; Ziętek, M.; et al. Oral Health and Quality of Life in Old Age: A Cross-Sectional Pilot Project in Germany and Poland. Adv. Clin. Exp. Med. 2016, 25, 951–959. [Google Scholar] [CrossRef] [Green Version]
- Calabriso, N.; Gnoni, A.; Stanca, E.; Cavallo, A.; Damiano, F.; Siculella, L.; Carluccio, M.A. Hydroxytyrosol Ameliorates Endothelial Function under Inflammatory Conditions by Preventing Mitochondrial Dysfunction. Oxid. Med. Cell. Longev. 2018, 2018, 9086947. [Google Scholar] [CrossRef]
- Wang, L.; Wan, M.; Li, Z.; Zhong, N.; Liang, D.; Ge, L. A comparative study of the effects of concentrated growth factors in two different forms on osteogenesis in vitro. Mol. Med. Rep. 2019, 20, 1039–1048. [Google Scholar] [CrossRef]





| Traditional Implants | CGF-Permeated Implants | p Value (1) | |
|---|---|---|---|
| N (%) | 10 (50.0) | 10 (50.0) | |
| Bleeding on probing | 0.10 | ||
| No | 5 (50.0) | 8 (80.0) | |
| Yes | 5 (50.0) | 2 (20.0) | |
| Successful implantation | 0.35 | ||
| No | 2 (20.0) | 1 (10.0) | |
| Yes | 8 (80.0) | 9 (90.0) |
| Gene Name | Accession Number | Sequences | pb |
|---|---|---|---|
| RunX2 | NM_001278478.2 | F: gacaaccgcaccatggtgg | 160 |
| R: tctggtacctctccgaggg | |||
| Col1a1 | NM_000088.3 | F: agggaatgcctggtgaacg | 90 |
| R: gagagccatcagcacctttg | |||
| Ocn | NM_199173.6 | F: gctacctgtatcaatggct | 111 |
| R: cgatgtggtcagccaactc | |||
| Gapdh | AJ005371.1 | F: atggccttccgtgtccccac | 245 |
| R: acgcctgcttcaccaccttc |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palermo, A.; Giannotti, L.; Di Chiara Stanca, B.; Ferrante, F.; Gnoni, A.; Nitti, P.; Calabriso, N.; Demitri, C.; Damiano, F.; Batani, T.; et al. Use of CGF in Oral and Implant Surgery: From Laboratory Evidence to Clinical Evaluation. Int. J. Mol. Sci. 2022, 23, 15164. https://doi.org/10.3390/ijms232315164
Palermo A, Giannotti L, Di Chiara Stanca B, Ferrante F, Gnoni A, Nitti P, Calabriso N, Demitri C, Damiano F, Batani T, et al. Use of CGF in Oral and Implant Surgery: From Laboratory Evidence to Clinical Evaluation. International Journal of Molecular Sciences. 2022; 23(23):15164. https://doi.org/10.3390/ijms232315164
Chicago/Turabian StylePalermo, Andrea, Laura Giannotti, Benedetta Di Chiara Stanca, Franco Ferrante, Antonio Gnoni, Paola Nitti, Nadia Calabriso, Christian Demitri, Fabrizio Damiano, Tiziano Batani, and et al. 2022. "Use of CGF in Oral and Implant Surgery: From Laboratory Evidence to Clinical Evaluation" International Journal of Molecular Sciences 23, no. 23: 15164. https://doi.org/10.3390/ijms232315164







