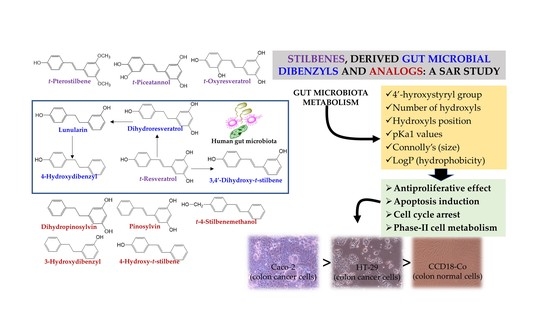
Main Determinants Affecting the Antiproliferative Activity of Stilbenes and Their Gut Microbiota Metabolites in Colon Cancer Cells: A Structure–Activity Relationship Study
Abstract
:1. Introduction
2. Results
2.1. Antiproliferative Activity of Stilbenes and Dibenzyls
2.2. Cell Metabolism of Stilbenes and Dibenzyls
2.3. Structure–Antiproliferative Activity Analysis of Stilbenes and Dibenzyls in Caco-2 and HT-29 Cells
2.4. Effect of Stilbenes and Dibenzyls on Cell Cycle and Apoptosis Induction in Caco-2 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Lines, Cell Culture Conditions, and Treatments
4.3. Cell Cycle Distribution and Apoptosis Analysis
4.4. Metabolism of Stilbenes and Dibenzyls in Cancer Cell Lines
4.4.1. UPLC-QTOF-MS Analyses
4.4.2. Identification and Quantification of Metabolites
4.5. Analysis of the Molecular Structure of Stilbenes and Dibenzyls
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomé-Carneiro, J.; Larrosa, M.; González-Sarrías, A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Resveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.H.; Nealon, R.S.; Scholey, A.; Howe, P.R. Low dose resveratrol improves cerebrovascular function in type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 393–399. [Google Scholar] [CrossRef]
- Thaung-Zaw, J.J.; Howe, P.R.; Wong, R.H. Long-term effects of resveratrol on cognition, cerebrovascular function and cardio-metabolic markers in postmenopausal women: A 24-month randomised, double-blind, placebo-controlled, crossover study. Clin. Nutr. 2021, 40, 820–829. [Google Scholar] [CrossRef]
- Teimouri, M.; Homayouni-Tabrizi, M.; Rajabian, A.; Amiri, H.; Hosseini, H. Anti-inflammatory effects of resveratrol in patients with cardiovascular disease: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2022, 70, 102863. [Google Scholar] [CrossRef]
- Patel, K.R.; Brown, V.A.; Jones, D.J.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [Google Scholar] [CrossRef] [Green Version]
- Núñez-Sánchez, M.A.; González-Sarrías, A.; Romo-Vaquero, M.; García-Villalba, R.; Selma, M.V.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Dietary phenolics against colorectal cancer—From promising preclinical results to poor translation into clinical trials: Pitfalls and future needs. Mol. Nutr. Food Res. 2015, 59, 1274–1291. [Google Scholar] [CrossRef]
- de Ligt, M.; Bergman, M.; Fuentes, R.M.; Essers, H.; Moonen-Kornips, E.; Havekes, B.; Schrauwen-Hinderling, V.B.; Schrauwen, P. No effect of resveratrol supplementation after 6 months on insulin sensitivity in overweight adults: A randomized trial. Am. J. Clin. Nutr. 2020, 112, 1029–1038. [Google Scholar] [CrossRef]
- Zeraattalab-Motlagh, S.; Jayedi, A.; Shab-Bidar, S. The effects of resveratrol supplementation in patients with type 2 diabetes, metabolic syndrome, and nonalcoholic fatty liver disease: An umbrella review of meta-analyses of randomized controlled trials. Am. J. Clin. Nutr. 2021, 114, 1675–1685. [Google Scholar] [CrossRef]
- Cai, H.; Scott, E.; Kholghi, A.; Andreadi, C.; Rufini, A.; Karmokar, A.; Britton, R.G.; Horner-Glister, E.; Greaves, P.; Jawad, D.; et al. Cancer chemoprevention: Evidence of a nonlinear dose response for the protective effects of resveratrol in humans and mice. Sci. Transl. Med. 2015, 7, 298ra117. [Google Scholar] [CrossRef] [Green Version]
- Mankowski, R.T.; You, L.; Buford, T.W.; Leeuwenburgh, C.; Manini, T.M.; Schneider, S.; Qiu, P.; Anton, S.D. Higher dose of resveratrol elevated cardiovascular disease risk biomarker levels in overweight older adults—A pilot study. Exp. Gerontol. 2020, 131, 110821. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Giménez-Bastida, J.A.; Selma, M.V.; González-Sarrías, A.; Espín, J.C. Main drivers of (poly)phenol effects on human health: Metabolite production and/or gut microbiota-associated metabotypes? Food Funct. 2021, 12, 10324–10355. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, e1900952. [Google Scholar] [CrossRef] [PubMed]
- Frankenfeld, C.L. Cardiometabolic risk and gut microbial phytoestrogen metabolite phenotypes. Mol. Nutr. Food Res. 2017, 61, 1500900. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Giménez-Bastida, J.A.; Cortés-Martín, A.; Ávila-Gálvez, M.Á.; Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C.; González-Sarrías, A. Urolithins: A Comprehensive Update on their Metabolism, Bioactivity, and Associated Gut Microbiota. Mol. Nutr. Food Res. 2022, 66, e2101019. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Aguirre, C.E.; Vallejo, F.; Beltrán, D.; Aguilar-Aguilar, E.; Puigcerver, J.; Alajarín, M.; Berná, J.; Selma, M.V.; Espín, J.C. Lunularin Producers versus Non-producers: Novel Human Metabotypes Associated with the Metabolism of Resveratrol by the Gut Microbiota. J. Agric. Food Chem. 2022, 70, 10521–10531. [Google Scholar] [CrossRef]
- Iglesias-Aguirre, C.E.; Vallejo, F.; Beltrán, D.; Berná, J.; Puigcerver, J.; Alajarín, M.; Selma, M.V.; Espín, J.C. 4-Hydroxydibenzyl: A novel metabolite from the human gut microbiota after consuming resveratrol. Food Funct. 2022, 13, 7487–7493. [Google Scholar] [CrossRef] [PubMed]
- Günther, I.; Rimbach, G.; Mack, C.I.; Weinert, C.H.; Danylec, N.; Lüersen, K.; Birringer, M.; Bracher, F.; Soukup, S.T.; Kulling, S.E.; et al. The Putative Caloric Restriction Mimetic Resveratrol has Moderate Impact on Insulin Sensitivity, Body Composition, and the Metabolome in Mice. Mol. Nutr. Food Res. 2020, 64, e1901116. [Google Scholar] [CrossRef]
- Li, F.; Han, Y.; Wu, X.; Cao, X.; Gao, Z.; Sun, Y.; Wang, M.; Xiao, H. Gut Microbiota-Derived Resveratrol Metabolites, Dihydroresveratrol and Lunularin, Significantly Contribute to the Biological Activities of Resveratrol. Front. Nutr. 2022, 9, 912591. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; González-Sarrías, A.; Espín, J.C. In Vitro Research on Dietary Polyphenols and Health: A Call of Caution and a Guide on How To Proceed. J. Agric. Food Chem. 2018, 66, 7857–7858. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador-Palmer, R.; Jihad-Jebbar, A.; López-Blanch, R.; Dellinger, T.H.; Dellinger, R.W.; Estrela, J.M. Pterostilbene in Cancer Therapy. Antioxidants 2021, 10, 492. [Google Scholar] [CrossRef]
- Likhitwitayawuid, K. Oxyresveratrol: Sources, Productions, Biological Activities, Pharmacokinetics, and Delivery Systems. Molecules 2021, 26, 4212. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Tomás-Barberán, F.A.; Espín, J.C. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J. Nutr. Biochem. 2006, 17, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.A.; Ávila-Gálvez, M.Á.; Espín, J.C.; González-Sarrías, A. Conjugated Physiological Resveratrol Metabolites Induce Senescence in Breast Cancer Cells: Role of p53/p21 and p16/Rb Pathways, and ABC Transporters. Mol. Nutr. Food Res. 2019, 63, e1900629. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Tomás-Barberán, F.A.; Espín, J.C. Grape polyphenol resveratrol and the related molecule 4-hydroxystilbene induce growth inhibition, apoptosis, S-phase arrest, and upregulation of cyclins A, E, and B1 in human SK-Mel-28 melanoma cells. J. Agric. Food Chem. 2003, 51, 4576–4584. [Google Scholar] [CrossRef]
- Matsuoka, A.; Takeshita, K.; Furuta, A.; Ozaki, M.; Fukuhara, K.; Miyata, N. The 4’-hydroxy group is responsible for the in vitro cytogenetic activity of resveratrol. Mutat. Res. 2002, 521, 29–35. [Google Scholar] [CrossRef]
- Stivala, L.A.; Savio, M.; Carafoli, F.; Perucca, P.; Bianchi, L.; Maga, G.; Forti, L.; Pagnoni, U.M.; Albini, A.; Prosperi, E.; et al. Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J. Biol. Chem. 2001, 276, 22586–22594. [Google Scholar] [CrossRef] [Green Version]
- González-Sarrías, A.; Giménez-Bastida, J.A.; Núñez-Sánchez, M.Á.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F.A.; Espín, J.C. Phase-II metabolism limits the antiproliferative activity of urolithins in human colon cancer cells. Eur. J. Nutr. 2014, 53, 853–864. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Núñez-Sánchez, M.Á.; García-Villalba, R.; Tomás-Barberán, F.A.; Espín, J.C. Antiproliferative activity of the ellagic acid-derived gut microbiota isourolithin A and comparison with its urolithin A isomer: The role of cell metabolism. Eur. J. Nutr. 2017, 56, 831–841. [Google Scholar] [CrossRef]
- Aires, V.; Limagne, E.; Cotte, A.K.; Latruffe, N.; Ghiringhelli, F.; Delmas, D. Resveratrol metabolites inhibit human metastatic colon cancer cells progression and synergize with chemotherapeutic drugs to induce cell death. Mol. Nutr. Food Res. 2013, 57, 1170–1181. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; Espín, J.C.; González-Sarrías, A. Physiological Relevance of the Antiproliferative and Estrogenic Effects of Dietary Polyphenol Aglycones versus Their Phase-II Metabolites on Breast Cancer Cells: A Call of Caution. J. Agric. Food Chem. 2018, 66, 8547–8555. [Google Scholar] [CrossRef]
- Nutakul, W.; Sobers, H.S.; Qiu, P.; Dong, P.; Decker, E.A.; McClements, D.J.; Xiao, H. Inhibitory effects of resveratrol and pterostilbene on human colon cancer cells: A side-by-side comparison. J. Agric. Food Chem. 2011, 59, 10964–10970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruggisser, R.; von Daekiken, K.; Jundt, G.; Schaffner, W.; Tullberg-Reiner, H. Interference of plant extracts, phytoestrogens and antioxidants with the MTT tetrazolium assay. Planta Med. 2002, 68, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Azorín-Ortuño, M.; Yáñez-Gascón, M.J.; Vallejo, F.; Pallarés, F.J.; Larrosa, M.; Lucas, R.; Morales, J.C.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Metabolites and tissue distribution of resveratrol in the pig. Mol. Nutr. Food Res. 2011, 55, 1154–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajha, H.N.; Paule, A.; Aragonès, G.; Barbosa, M.; Caddeo, C.; Debs, E.; Dinkova, R.; Eckert, G.P.; Fontana, A.; Gebrayel, P.; et al. Recent Advances in Research on Polyphenols: Effects on Microbiota, Metabolism, and Health. Mol. Nutr. Food Res. 2022, 66, 2100670. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; Rosano, C.; Madeo, A.; Albanito, L.; Plastina, P.; Gabriele, B.; Forti, L.; Stivala, L.A.; Iacopetta, D.; Dolce, V.; et al. Structure-activity relationships of resveratrol and derivatives in breast cancer cells. Mol. Nutr. Food Res. 2009, 53, 845–858. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Gálvez, M.Á.; García-Villalba, R.; Martínez-Díaz, F.; Ocaña-Castillo, B.; Monedero-Saiz, T.; Torrecillas-Sánchez, A.; Abellán, B.; González-Sarrías, A.; Espín, J.C. Metabolic Profiling of Dietary Polyphenols and Methylxanthines in Normal and Malignant Mammary Tissues from Breast Cancer Patients. Mol. Nutr. Food Res. 2019, 63, e1801239. [Google Scholar] [CrossRef]
- Ávila-Gálvez, M.Á.; González-Sarrías, A.; Martínez-Díaz, F.; Abellán, B.; Martínez-Torrano, A.J.; Fernández-López, A.J.; Giménez-Bastida, J.A.; Espín, J.C. Disposition of Dietary Polyphenols in Breast Cancer Patients’ Tumors, and Their Associated Anticancer Activity: The Particular Case of Curcumin. Mol. Nutr. Food Res. 2021, 65, e2100163. [Google Scholar] [CrossRef]
- Córdova-Gómez, M.; Galano, A.; Alvarez-Idaboy, J.R. Piceatannol, a better peroxyl radical scavenger than resveratrol. RSC Adv. 2013, 3, 20209–20218. [Google Scholar] [CrossRef]
- Zimányi, L.; Thekkan, S.; Eckert, B.; Condren, A.R.; Dmitrenko, O.; Kuhn, L.R.; Alabugin, I.V.; Saltiel, J. Determination of the pKa Values of trans-Resveratrol, a Triphenolic Stilbene, by Singular Value Decomposition. Comparison with Theory. J. Phys. Chem. A 2020, 124, 6294–6302. [Google Scholar] [CrossRef]
- Sprous, D.G.; Palmer, R.K.; Swanson, J.T.; Lawless, M. QSAR in the pharmaceutical research setting: QSAR models for broad, large problems. Curr. Top. Med. Chem. 2010, 10, 619–637. [Google Scholar] [CrossRef]
- Pecyna, P.; Wargula, J.; Murias, M.; Kucinska, M. More Than Resveratrol: New Insights into Stilbene-Based Compounds. Biomolecules 2020, 10, 1111. [Google Scholar] [CrossRef] [PubMed]
- Chittenden, J.T.; Riviere, J.E. Assessment of penetrant and vehicle mixture properties on transdermal permeability using a mixed effect pharmacokinetic model of ex vivo porcine skin. Biopharm. Drug Dispos. 2016, 37, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, Y.; Nakata, R.; Fukuhara, K.; Yamashita, H.; Kubodera, H.; Inoue, H. The 4’-hydroxyl group of resveratrol is functionally important for direct activation of PPARα. PLoS ONE 2015, 10, e0120865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larrosa, M.; Tomás-Barberán, F.A.; Espín, J.C. The grape and wine polyphenol piceatannol is a potent inducer of apoptosis in human SK-Mel-28 melanoma cells. Eur. J. Nutr. 2004, 43, 275–284. [Google Scholar] [CrossRef]
- Dai, Y.; Lim, J.X.; Yeo, S.C.M.; Xiang, X.; Tan, K.S.; Fu, J.H.; Huang, L.; Lin, H.S. Biotransformation of Piceatannol, a Dietary Resveratrol Derivative: Promises to Human Health. Mol. Nutr. Food Res. 2020, 64, e1900905. [Google Scholar] [CrossRef]
- Grimes, K.L.; Stuart, C.M.; McCarthy, J.J.; Kaur, B.; Cantu, E.J.; Forester, S.C. Enhancing the Cancer Cell Growth Inhibitory Effects of Table Grape Anthocyanins. J. Food Sci. 2018, 83, 2369–2374. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Ávila-Gálvez, M.Á.; Espín, J.C.; González-Sarrías, A. The gut microbiota metabolite urolithin A, but not other relevant urolithins, induces p53-dependent cellular senescence in human colon cancer cells. Food Chem. Toxicol. 2020, 139, 111260. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Iglesias-Aguirre, C.E.; Cortés-Martín, A.; Vallejo, F.; Cattivelli, A.; Del Pozo-Acebo, L.; Del Saz, A.; López de Las Hazas, M.C.; Dávalos, A.; Espín, J.C. Milk-Derived Exosomes as Nanocarriers to Deliver Curcumin and Resveratrol in Breast Tissue and Enhance Their Anticancer Activity. Int. J. Mol. Sci. 2022, 23, 2860. [Google Scholar] [CrossRef]












| Compounds | Caco-2 (48 h) | HT-29 (48 h) | Caco-2 (72 h) | HT-29 (72 h) |
|---|---|---|---|---|
| 4HST | 26.9 ± 8.0 | 34.0 ± 8.1 | 11.4 ± 10.1 | 24.4 ± 11.3 |
| RSV | 50.4 ± 3.4 | 68.9 ± 22.6 | 18.8 ± 1.6 | 59.1 ± 9.9 |
| DHST | 57.1 ± 10.2 | 64.8 ± 15.6 | 22.7 ± 6.4 | 48.3 ± 17.8 |
| PINO | 55.3 ± 12.7 | 37.5 ± 14.3 | 26.4 ± 7.1 | 38.1 ± 8.0 |
| DHRSV | 55.4 ± 10.8 | >100 | 33.8 ± 17.8 | 69.0 ± 9.3 |
| PICE | 55.4 ± 10.8 | 78.5 ± 5.7 | 42.0 ± 9.8 | 69.5 ± 4.6 |
| 3HDB | >100 | >100 | 42.9 ± 4.1 | 81.7 ± 15.5 |
| PTERO | 67.6 ± 12.3 | 45.1 ± 13.7 | 43.0 ± 11.6 | 37.9 ± 5.4 |
| LUNU | 84.5 ± 11.7 | >100 | 50.5 ± 23.1 | 53.2 ± 6.8 |
| Oxy-RSV | 74.7 ± 11.5 | 96.7 ± 2.4 | 55.4 ± 2.5 | 83.4 ± 5.8 |
| 4HDB | >100 | >100 | 56.9 ± 8.0 | 96.7 ± 6.7 |
| 4STMe | 88.3 ± 14.6 | 85.4 ± 16.5 | 59.9 ± 23.1 | 57.1 ± 18.9 |
| DHP | 54.0 ± 3.1 | >100 | 73.9 ± 13.8 | 82.4 ± 12.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Sarrías, A.; Espín-Aguilar, J.C.; Romero-Reyes, S.; Puigcerver, J.; Alajarín, M.; Berná, J.; Selma, M.V.; Espín, J.C. Main Determinants Affecting the Antiproliferative Activity of Stilbenes and Their Gut Microbiota Metabolites in Colon Cancer Cells: A Structure–Activity Relationship Study. Int. J. Mol. Sci. 2022, 23, 15102. https://doi.org/10.3390/ijms232315102
González-Sarrías A, Espín-Aguilar JC, Romero-Reyes S, Puigcerver J, Alajarín M, Berná J, Selma MV, Espín JC. Main Determinants Affecting the Antiproliferative Activity of Stilbenes and Their Gut Microbiota Metabolites in Colon Cancer Cells: A Structure–Activity Relationship Study. International Journal of Molecular Sciences. 2022; 23(23):15102. https://doi.org/10.3390/ijms232315102
Chicago/Turabian StyleGonzález-Sarrías, Antonio, Juan Carlos Espín-Aguilar, Salvador Romero-Reyes, Julio Puigcerver, Mateo Alajarín, José Berná, María Victoria Selma, and Juan Carlos Espín. 2022. "Main Determinants Affecting the Antiproliferative Activity of Stilbenes and Their Gut Microbiota Metabolites in Colon Cancer Cells: A Structure–Activity Relationship Study" International Journal of Molecular Sciences 23, no. 23: 15102. https://doi.org/10.3390/ijms232315102