Trigeminal Sensory Supply Is Essential for Motor Recovery after Facial Nerve Injury
Abstract
:1. Clinical Importance of the Afferent Fibers, including Trigeminal Nerve Fibers, for Appropriate Motor Nerve Regeneration and Recovery of Motor Performance
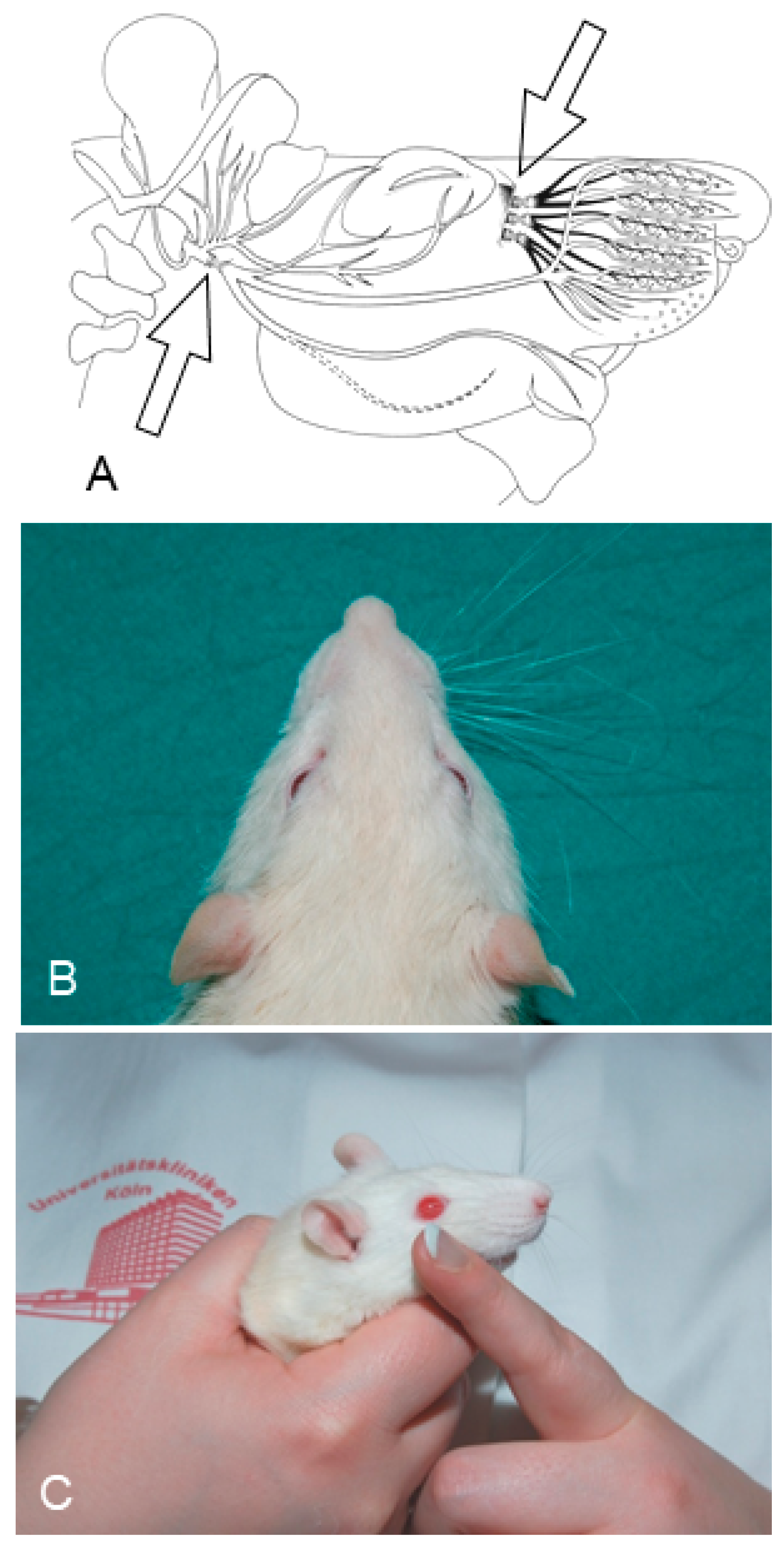
2. Ablation of Trigeminal Sensory Input by Excising the Ipsilateral Infraorbital Nerve (IOn-ipsi-ex) Impedes Recovery of Vibrissae Motor Performance after Transection and Suture of the Facial Nerve (Fn-n). Manual Stimulation (Mstim) of the Paralyzed and Deafferented Whisker Pad Worsens Recovery of Whisking. See Flow Chart Table 2 for a Synopsis
2.1. Functional Recovery
| Research Goals | Day 0 (Beginning) | Day 1: Surgeries | Day 2–63 (Two Months) | Day 64 (8 Weeks Later) | Day 65 (1 Day Later) | Day 75 (10 Days Later) | Results |
|---|---|---|---|---|---|---|---|
| Section 2: To determine recovery of vibrissae motor performance after transection and suture of the facial nerve (Fn-n), and after Ablation of trigeminal sensory input, by excising the ipsilateral infraorbital nerve (IOn-ipsi-ex). | Clipping of all vibrissal hairs except 2 on each side of the face in preparation for the videotaping. Pre-operative videotaping of 24 intact rats for video-based motion analysis of the vibrissae movements. | Right (Fn-n) in all 24 rats. 6 rats (group 1) received no other treatment. 6 rats received daily Mstim of the whisker pad (group 2). Another 6 rats received an excision of the ipsilateral infraorbital nerve (IOn-ipsi-ex) (group 3). The last 6 rats received Mstim in addition to Fn-n + IOn-ipsi-ex (group 4). | Daily manual stimulation of the whisker pad in groups 2 and 4. | Clipping of all vibrissal hairs except 2 on each side of the face in preparation for the videotaping. Postoperative videotaping of all 24 rats for video-based motion analysis of the vibrissae movements. | Perfusion fixation with 4% paraformaldehyde in phosphate-buffered saline, pH 7.4 | Cutting of the brainstems on a vibratome and determining the intensity of fluorescence after immunostaining for synaptophysin. Cutting of the ipsilateral to Fn-n levator labii superioris muscle (LLS) on a cryostat and determining the degree of polyinnervation of the motor endplates. | Amplitude: Intact: 62 ± 6° Fn-n: 19 ± 6° Fn-n + Mstim: 51 ± 19° Fn-n + IOn-ipsi-ex: 22 ± 3° Fn-n + Ion-ipsi-ex + Mstim: 14 ± 6° Polyinnervation %: Intact: 0% Fn-n: 53 ± 10%; Fn-n + Mstim: 22 ± 3%; Fn-n + Ion-ipsi-ex: 43 ± 9%; Fn-n + Ion-ipsi-ex + Mstim: 51 ± 10% |
2.2. Muscle Innervation
2.3. Conclusions
3. Mild Ipsilateral to Fn-n Trigeminal Indirect Stimulation (by Removing the Contralateral Vibrissal Hairs) and Direct Trigeminal Stimulation (by Massaging the Ipsilateral Whisker Pad) after Double Anastomotic Surgery on the Sensory Infraorbital and Motor Facial Nerves Improves the Quality of Muscle Reinnervation and Vibrissal Motor Performance. See Flow Chart Table 3 for a Synopsis
3.1. Experimental Rat Groups
| Experiment’s Research Goals | Day 0 (Beginning) | Day 1: Surgeries (1 Day Later) | Day 2–111 | Day 64–111 | Day 112 | Day 113–118 | Results |
|---|---|---|---|---|---|---|---|
| Section 3: To determine the effect of mild trigeminal indirect stimulation of sensory nerves (by removing the contralateral vibrissal hairs and massaging the ipsilateral whisker pad), after double anastomotic surgery on the sensory infraorbital and motor facial nerve, on the quality of muscle reinnervation and vibrissal motor performance. | Clipping of all vibrissal hairs except 2 on each side of the face in preparation for the videotaping. Pre-operative videotaping of 48 intact rats for video-based motion analysis of the vibrissae movements. | Right Fn-n + IOn-n) in 48 rats. 12 rats (group 1) received no other treatment. In another 12 rats (group 2) the contralateral vibrissal hairs were removed (vibrissal stimulation; Fn-n + IOn-n + Vstim). In rats from group 3, the ipsilateral whisker pads were manually stimulated (Fn-n + IOn-n + Mstim). In rats from group 4, the Vstim of the reinnervated vibrissae was followed by Mstim (Fn-n + IOn-nN + Vstim + Mstim). | Daily manual stimulation of whisker pads in group 3. | Daily manual stimulation of whisker pads in group 4. | Clipping of all vibrissal hairs except 2 on each side of the face in preparation for videotaping. Postoperative videotaping of all rats for video-based motion analysis of the vibrissae movements. Injection of 1% Fast Blue to back-label motoneuronal perikarya. | Perfusion fixation with 4% PFA in 0.1 M PBS, pH 7.4 Cutting of the brainstems on a vibratome and determining the intensity of synaptophysin fluorescence. Cutting of the ipsilateral to Fn-n LLS on a cryostat and determining the degree of polyinnervation of the motor endplates. | Amplitude Intact: 62 ± 6°; Fn-n + IOn-n: 11 ± 4°; Fn-n + IOn-n + Vstim: 28 ± 9°; Fn-n + IOn-n + Mstim: 30 ± 11°; Fn-n + Ion-n + Vstim +Mstim: 32 ± 10°. Polyinnervation %: Intact: 0%; Fn-n + IOn-n: 58 ± 8%; Fn-n + IOn-n + Vstim: 40 ± 3%; Fn-n + IOn-n + Mstim: 40 ± 2%; Fn-n + IOn-n + Vstim + Mstim: 33 ± 10%. Synaptophysin covered area:: Intact: 17 ± 2%; Fn-n + IOn-n: 12 ± 1%; Fn-n + IOn-n + Vstim: 13 ± 2% Fn-n + IOn-n + Mstim: 13 ± 2%; Fn-n + IOn-n + Vstim + Mstim: 12 ± 2%. |
3.2. Functional Recovery
3.3. Morphological Estimates in the Facial Muscles
3.4. Morphological Estimates in the Facial Nucleus
3.5. Conclusions
3.6. Clinical Application
4. Intensive Ipsilateral to Fn-n Trigeminal Indirect Stimulation, Produced by Its Forced Overuse Due to Excision of the Contralateral Infraorbital Nerve (Ion) after Surgery on the Buccal Branch of the Facial Nerve, Attenuates the Degree of Collateral Axonal Branching. See Flow Chart Table 4 for a Synopsis
4.1. Introduction
| Experimental Set Research Goals | Day 1: Surgeries | Day 28 (4 Weeks Later) | Day 38 (10 Days Later) | Day 38 | Results |
|---|---|---|---|---|---|
| Section 4: Effects of the intensive trigeminal indirect stimulation (excision of the contralateral infraorbital nerve) after surgery on the buccal branch of the facial nerve, on the degree of collateral axonal branching at the lesion site. | All groups of rats consisted of 6 animals. Rats in group 1 served as unoperated controls. Rats in groups 2–4 were subjected to identical transection and suture of the right buccal branch of the facial nerve (buccal–buccal anastomosis, Bn-n). The rats of group 3 underwent Bn-n plus excision of the ipsilateral (right) IOn (Bn-n + IOn-ipsi-ex), and those of group 4 underwent Bn-n plus excision of the contralateral (left) IOn (Bn-n + IOn-contra-ex). | Retrograde labeling of the facial motoneurons with fluorescent tracers, Fluoro-Gold (FG; Fluorochrome Inc., Englewood, CO, USA) and DiI (Molecular Probes, The Netherlands), applied to the superior and inferior buccolabial nerves (BLn) respectively. | Perfusion fixation with 4% paraformaldehyde in phosphate-buffered saline, pH 7.4 | Cutting of the brainstems on a vibratome and determining the number of double-labelled (FG + DiI) perikarya which indicates the degree of collateral axonal branching at the site of nerve injury. | Portion of double-labelled perikarya: Intact: 0%; Bn-n: 23%; Bn-n + IOn-ipsi-ex: 13%; Bn-n + IOn-contra-ex: 8% |
4.2. Identification and Localization of Facial Motoneurons Regenerating into the Branches of the Buccal Nerve after Bn-n Surgery
4.3. Results
4.3.1. Rat Group 1: Distribution of Facial Motoneurons in Both Branches of the Buccal Nerve in Unoperated Rats
4.3.2. Rat Group 2: Buccal–Buccal Anastomosis (Bn-n)
4.3.3. Rat Group 3: Bn-n Plus Excision of the Ipsilateral Infraorbital Nerve (IOn)
4.3.4. Rat Group 4: Bn-n Plus Excision of the Contralateral Infraorbital Nerve (IOn)

4.4. Conclusions
5. Intensive Ipsilateral to Fn-n Trigeminal Indirect Stimulation, Produced by Its Forced Overuse Due to Excision of the Contralateral Infraorbital Nerve (Ion) after Surgery on the Buccal Branch of the Facial Nerve, Improves the Accuracy of Muscle Reinnervation. See Flow Chart Table 5 for a Synopsis
5.1. Research Question
| Experimental Set Research Goals | 10 Days before Surgery | Day 0: Surgeries | Day 28 (4 Weeks Later) | Day 38 (10 Days Later) | Day 38 | Results |
|---|---|---|---|---|---|---|
| Section 5: Effect of intensive trigeminal indirect stimulation (excision of the contralateral IOn) after surgery on Bn on the accuracy of muscle reinnervation. | Buccal motoneurons were back labelled prior to surgeries in all 4 rat groups (each of 6 rats) to identify and localize their motoneuronal perikarya in the brainstem. A solution of 1% Fluoro-Gold (FG) was injected into the muscles of the whisker pad. | Rats in group 1 served as unoperated controls. Rats in groups 2–4 were subjected to identical transection and suture of the right buccal branch of the facial nerve (buccal–buccal anastomosis, Bn-n). The rats of group 3 underwent Bn-n plus excision of the ipsilateral (right) IOn (Bn-n + IOn-ipsi-ex), and those of group 4 underwent Bn-n plus excision of the contralateral (left) IOn (Bn-n + IOn-contra-ex). | 28 days after surgery, a 1% solution of Fast Blue (FB) was injected into the same muscle site as the earlier FG injection. | Perfusion fixation with 4% paraformaldehyde in phosphate-buffered saline, pH 7.4 | Cutting of the brainstems on a vibratome and determining the number of double-labelled (FG + DiI) perikarya which indicates accuracy of muscle reinnervation. | Portion of double-labelled perikarya: Intact: ~90–100%; Bn-n: 27%; Bn-n + IOn-ipsi-ex: 32%; Bn-n + IOn-contra-ex: 41% |
5.2. Surgeries and Rat Groups
5.3. Identification and Localization of Buccal Motoneurons Regenerating into Muscles of the Whisker Pad
5.4. Results
5.4.1. Rat Group 1
5.4.2. Rat Group 2: Bn-n
5.4.3. Rat Group 3: Bn-n Plus Excision of the Ipsilateral IOn (Bn-n + IOn-ipsi-ex)
5.4.4. Rat Group 4: Bn-n Plus Excision of the Contralateral IOn (Group BBA + ION-Contra-ex)
5.5. Conclusions
5.6. Discussion
5.7. Clinical Implications
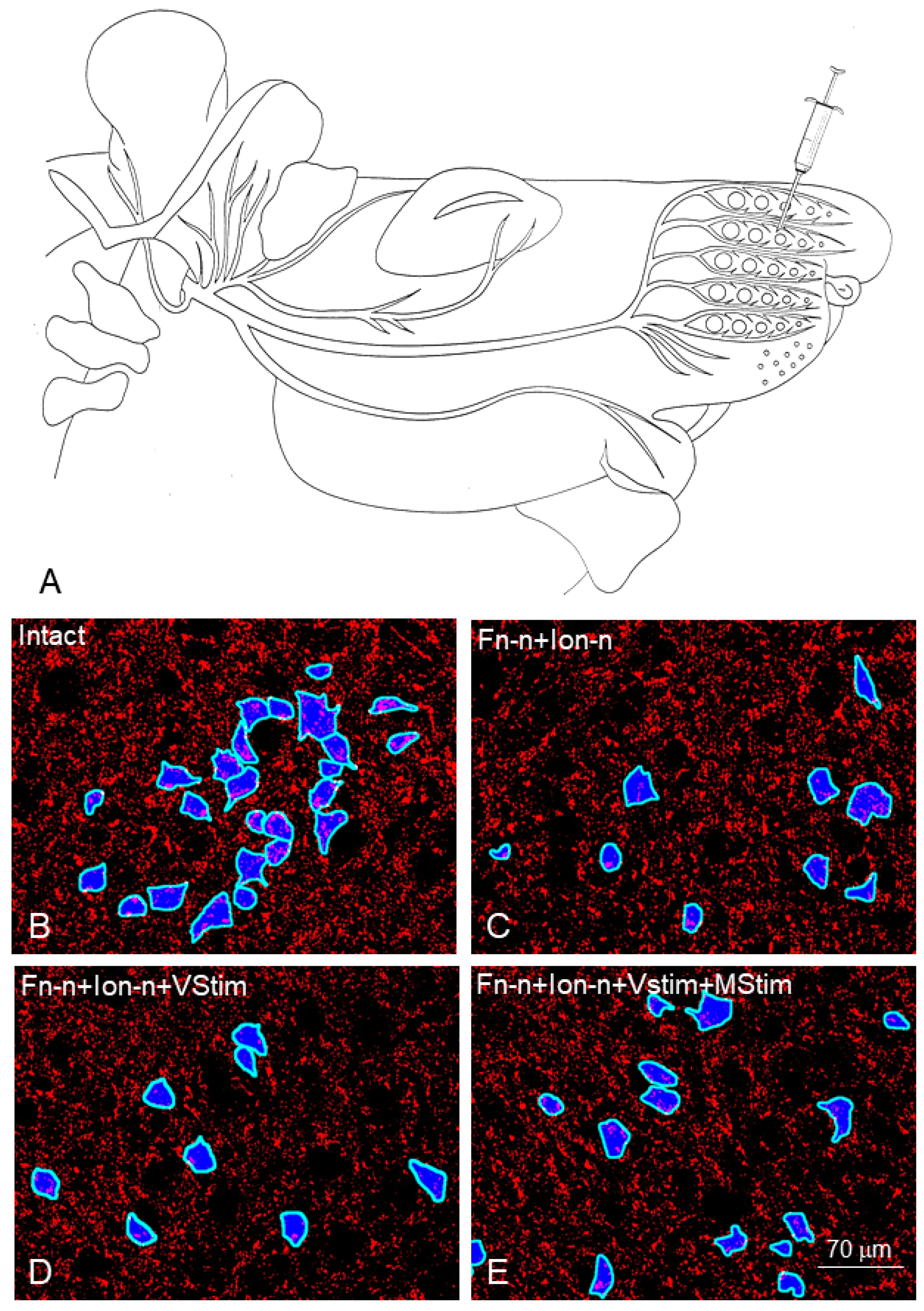
6. Direct Stimulation of the Trigeminal Nerve Afferents (by Manual Stimulation of the Whisker Pad, Massage) after Facial Nerve Surgery Restores the Synaptic Density in the Facial Nucleus, Improves the Quality of Target Reinnervation and Promotes Recovery of Vibrissal Motor Performance. See Flow Chart Table 6 for a Synopsis
6.1. Experimental Rationale
6.2. Methods
| Experiment’s Research Goal | Day 0 (Beginning) | Day 1: Surgeries (1 Day Later) | Day 2–56 (2 Months) | Day 57 | Day 58–67 | Results |
|---|---|---|---|---|---|---|
| Section 6: Effect of direct stimulation of the trigeminal nerve afferents (by manual stimulation of the whisker pad, massage) after facial nerve surgery on the synaptic density in the facial nucleus. | Clipping of all vibrissal hairs except 2 on each side of the face in preparation for the videotaping. Pre-operative videotaping of all intact rats for video-based motion analysis of the vibrissae movements. | 6 animals in group 1 were intact controls. The facial nerve was cut and re-sutured (Fn-n) in the experimental groups 2 and 3. In group 2 (6 rats; Fn-n + handling), the rats were held in the experimenter’s hand, not receiving any stimulation of the vibrissal muscles. In group 3 (r rats), Fn-n was followed by manual stimulation of the whisker pad (Fn-n + Mstim). | Daily manual stimulation of whisker pads in group 3. | Clipping of all vibrissal hairs except 2 on each side of the face in preparation for videotaping. Postoperative videotaping of all rats for video-based motion analysis of the vibrissae movements. | Perfusion fixation with 4% PFA in 0.1 M PBS, pH 7.4 Cutting of the brainstems on a vibratome and determining the intensity of synaptophysin fluorescence Cutting of the ipsilateral to Fn-n levator labii superioris muscle (LLS) on a cryostat and determining the degree of polyinnervation of the motor endplates. | Amplitude Intact: 57 ± 13° Fn-n + handl: 19 ± 6°; Fn-n + Mstim: 51 ± 19°; Polyinnervation %: Intact: 0% Fn-n + handl: 53 ± 10%; Fn-n + Mstim: 22 ± 5%; Amount of synaptophysin-positive terminals in the facial nucleus: Intact: 34.3 × 106 ± 2.3 × 106; Fn-n + handl: 29.2 × 106 ± 1.8 × 106; Fn-n + Mstim: 33 × 106 ± 2.6 × 10 |
6.3. Results
6.3.1. Recovery of Whisking
6.3.2. Loss of Synapses in the Lesioned Facial Nucleus
6.4. Discussion
6.5. Mechanisms
7. Muscle Neurotrophic Factors Are Unlikely to Play a Role in the Reduced Polyneuronal Muscle Reinnervation by Manual Stimulation of the Denervated Whisker Pad (Mstim). See Flow Chart Table 7 for a Synopsis
7.1. Experimental Rationale and Questions
| Experiment’s Research Goal | Day 0 (Beginning) | Day 1: Surgeries (1 Day Later) | Day 2–56 (2 Months) | Day 57 | Day 58–67 | Results |
|---|---|---|---|---|---|---|
| Section 7: To determine the role of muscle neurotrophic factors in the reduced polyneuronal muscle reinnervation by manual stimulation of the denervated whisker pad (Mstim) | Clipping of all vibrissal hairs except 2 on each side of the face in preparation for the videotaping. Pre-operative videotaping of all intact rats for video-based motion analysis of the vibrissae movements. | 6 animals of group 1 were intact controls. The facial nerve was cut and re-sutured (Fn-n) in the experimental groups 2 and 3. In group 2 (6 rats, Fn-n + handling), the rats were held in the experimenter’s hand, not receiving any stimulation of the vibrissal muscles. In group 3, (6 rats) Fn-n was followed by manual stimulation of the whisker pad (Fn-n + Mstim). | Daily manual stimulation of whisker pads in group 3. | Clipping of all vibrissal hairs except 2 on each side of the face in preparation for videotaping. Postoperative videotaping of all rats for video-based motion analysis of the vibrissae movements. | Blood rinse by 0.1 M PBS, pH 7.4. Samples from LLS were taken and frozen. The translation of the proteins FGF2, IGF1 and NGF was determined using sandwich ELISA-Kits following the manufacturer’s instructions. | FGF2 Intact: 75 ± 16 pg/mg, FGF2 Fn-n+ handling: 69 ± 24 pg/mg FGF2 Fn-n + Mstim: 56 ± 11 pg/mg IGF1 Intact: 1492 ± 87 pg/mg IGF1 Fn-n + handling 2079 ± 300 pg/mg, IGF1 Fn-n + Mstim: 1821 ± 784 pg/mg NGF Intact: 61 ± 22 pg/mg NGF Fn-n + handling: 51 ± 21 pg/mg NGF Fn-n + Mstim: 33 ± 13 pg/mg. |
7.2. Methods
7.3. Results
7.4. Conclusions
8. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
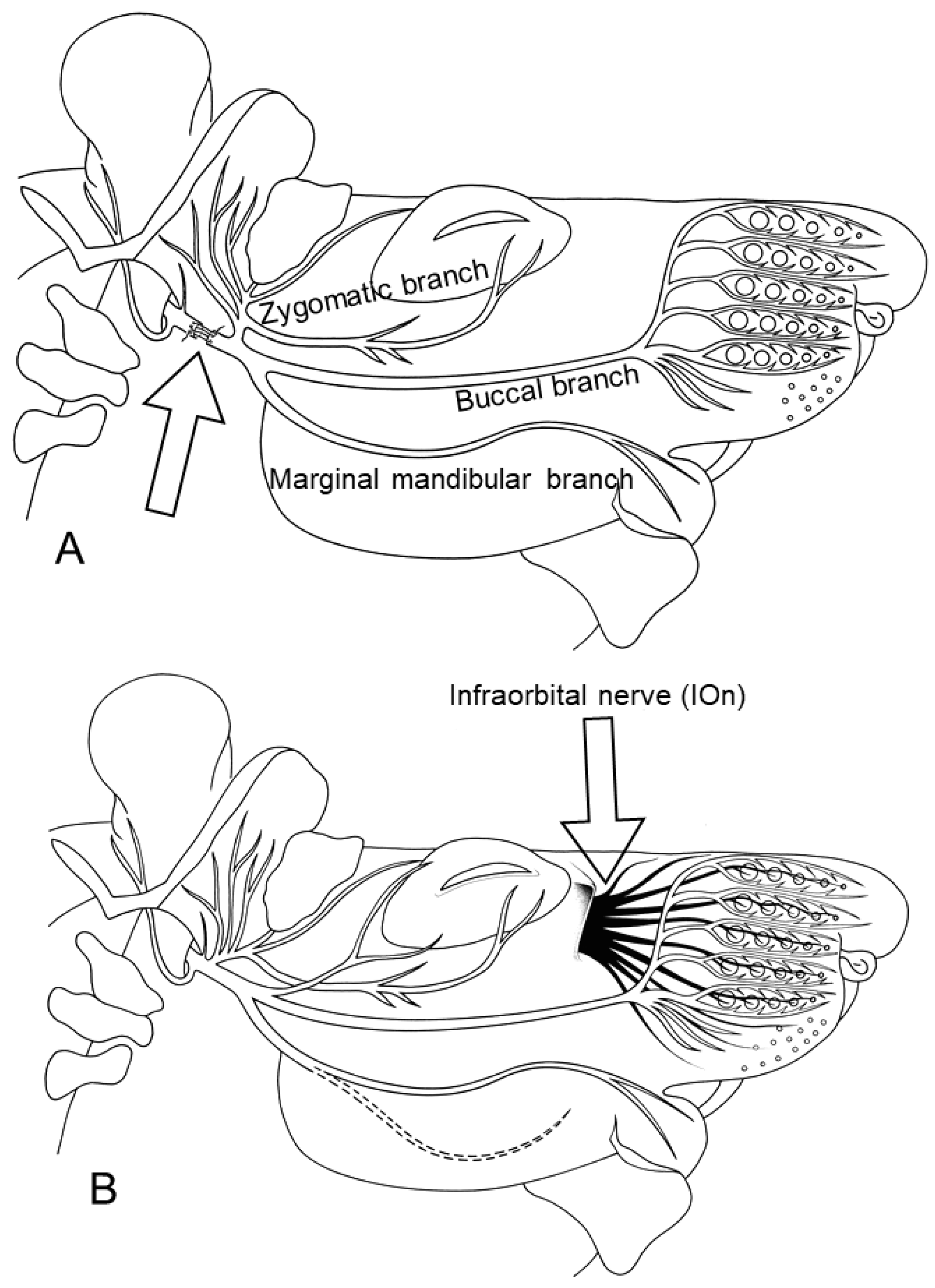
| Nerves and muscles | |
| BLn | buccolabial nerve |
| Bn | buccal nerve (more correct: buccal branch of the facial nerve, ramus buccalis, motor) |
| Fn | facial nerve (motor) |
| IOn | infraorbital nerve (sensory) |
| LLS | levator labii superioris muscle |
| NMJ | neuromuscular junction |
| Surgeries and procedures | |
| Bn-n | buccal nerve transection and end-to-end suture (anastomosis) |
| Fn-n | facial nerve transection and suture (anastomosis) |
| Fn-n + Hand | facial nerve transection and suture plus handling |
| IOn-contra-ex | excision of the contralateral infraorbital nerve |
| IOn-ipsi-ex | excision of the ipsilateral infraorbital nerve |
| IOn-n | infraorbital nerve transection and suture (anastomosis) |
| Mstim | manual stimulation, massage |
| Vstim | vibrissal stimulation |
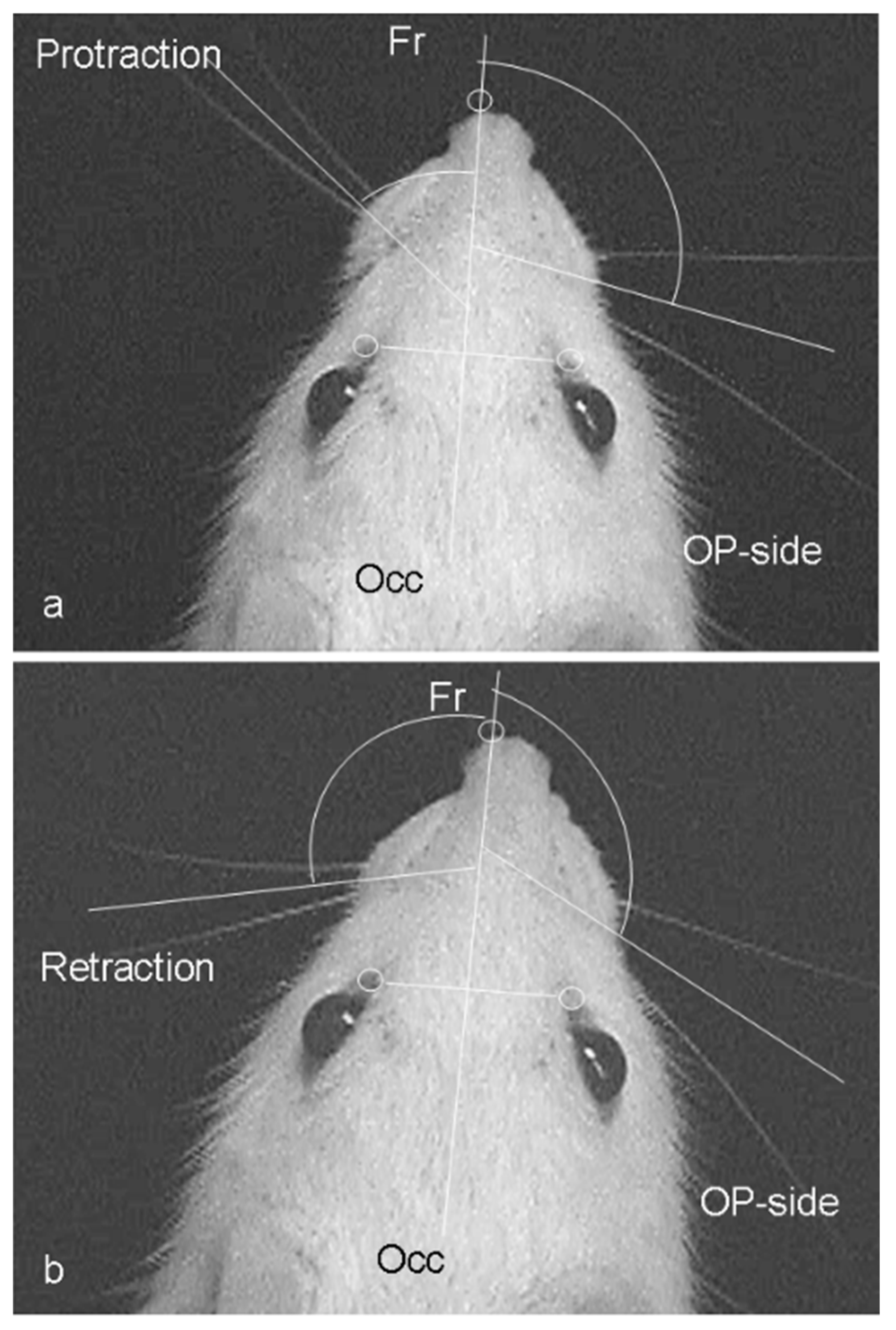
| Occ | occipital direction |
| OP-side | operation side |
| Dyes | |
| DiI | 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate |
| FB | Fast Blue |
| FG | Fluoro-Gold |
| Statistics | |
| SD | standard deviation |
| n | number |
References
- Markiewicz, M.R.; Callahan, N.; Miloro, M. Management of Traumatic Trigeminal and Facial Nerve Injuries. Oral Maxillofac. Surg. Clin. N. Am. 2021, 33, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.J.; Bernstein, J.M. Bell’s palsy. BMJ Clin. Evid. 2014, 2014, 1204. [Google Scholar] [PubMed]
- Zhang, W.; Xu, L.; Luo, T.; Wu, F.; Zhao, B.; Li, X. The etiology of Bell’s palsy: A review. J. Neurol. 2020, 267, 1896–1905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anonsen, C.K.; Trachy, R.E.; Hibbert, J.; Cummings, C.W. Assessment of facial reinnervation by use of chronic electromyographic monitoring. Otolaryngol. Neck Surg. Off. J. Am. Acad. Otolaryngol. Neck Surg. 1986, 94, 32–36. [Google Scholar] [CrossRef]
- Ferreira, M.C.; Besteiro, J.M.; Tuma Júnior, P. Results of reconstruction of the facial nerve. Microsurgery 1994, 15, 5–8. [Google Scholar] [CrossRef]
- Vaughan, E.D.; Richardson, D. Facial nerve reconstruction following ablative parotid surgery. Br. J. Oral Maxillofac. Surg. 1993, 31, 274–280. [Google Scholar] [CrossRef]
- Xie, Y.; Schneider, K.J.; Ali, S.A.; Hogikyan, N.D.; Feldman, E.L.; Brenner, M.J. Current landscape in motoneuron regeneration and reconstruction for motor cranial nerve injuries. Neural Regen. Res. 2020, 15, 1639–1649. [Google Scholar] [CrossRef]
- Yoo, M.C.; Chon, J.; Jung, J.; Kim, S.S.; Bae, S.; Kim, S.H.; Yeo, S.G. Potential Therapeutic Strategies and Substances for Facial Nerve Regeneration Based on Preclinical Studies. Int. J. Mol. Sci. 2021, 22, 4926. [Google Scholar] [CrossRef]
- Kimura, J.; Rodnitzky, R.L.; Okawara, S.H. Electrophysiologic analysis of aberrant regeneration after facial nerve paralysis. Neurology 1975, 25, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Bento, R.F.; Miniti, A. Anastomosis of the intratemporal facial nerve using fibrin tissue adhesive. Ear. Nose. Throat J. 1993, 72, 663–672. [Google Scholar] [CrossRef]
- Montserrat, L.; Benito, M. Facial synkinesis and aberrant regeneration of facial nerve. Adv. Neurol. 1988, 49, 211–224. [Google Scholar]
- Sumner, A.J. Aberrant reinnervation. Muscle Nerve 1990, 13, 801–803. [Google Scholar] [CrossRef]
- Sadjadpour, K. Postfacial palsy phenomena: Faulty nerve regeneration or ephaptic transmission? Brain Res. 1975, 95, 403–406. [Google Scholar] [CrossRef]
- Bratzlavsky, M.; Eecken, H. Vander Altered synaptic organization in facial nucleus following facial nerve regeneration: An electrophysiological study in man. Ann. Neurol. 1977, 2, 71–73. [Google Scholar] [CrossRef]
- Graeber, M.B.; Bise, K.; Mehraein, P. Synaptic stripping in the human facial nucleus. Acta Neuropathol. 1993, 86, 179–181. [Google Scholar] [CrossRef]
- Moran, C.J.; Neely, J.G. Patterns of facial nerve synkinesis. Laryngoscope 1996, 106, 1491–1496. [Google Scholar] [CrossRef]
- Eccles, J.C.; Libet, B.; Young, R.R. The behaviour of chromatolysed motoneurones studied by intracellular recording. J. Physiol. 1958, 143, 11–40. [Google Scholar] [CrossRef]
- Ferguson, J.H. Hemifacial spasm and the facial nucleus. Ann. Neurol. 1978, 4, 97–103. [Google Scholar] [CrossRef]
- Lux, H.D.; Schubert, P. Some aspects of the electroanatomy of dendrites. Adv. Neurol. 1975, 12, 29–44. [Google Scholar]
- Sumner, B.E.H.; Watson, W.E. Retraction and Expansion of the Dendritic Tree of Motor Neurones of Adult Rats induced in vivo. Nature 1971, 233, 273–275. [Google Scholar] [CrossRef]
- Lieberman, A.R. The axon reaction: A review of the principal features of perikaryal responses to axon injury. Int. Rev. Neurobiol. 1971, 14, 49–124. [Google Scholar] [CrossRef] [PubMed]
- Blinzinger, K.; Kreutzberg, G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z. Zellforsch. Mikrosk. Anat. 1968, 85, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Sinis, N.; Schaller, H.-E.; Schulte-Eversum, C.; Schlosshauer, B.; Doser, M.; Dietz, K.; Rösner, H.; Müller, H.-W.; Haerle, M. Nerve regeneration across a 2-cm gap in the rat median nerve using a resorbable nerve conduit filled with Schwann cells. J. Neurosurg. 2005, 103, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.J.; Jacoby, C.; Terenghi, G.; Mennen, U.; Ljungberg, C.; Wiberg, M. End-to-side nerve coaptation: A qualitative and quantitative assessment in the primate. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.J.; Titus-Mitchell, H.E.; Bullinger, K.L.; Kraszpulski, M.; Nardelli, P.; Cope, T.C. Permanent central synaptic disconnection of proprioceptors after nerve injury and regeneration. I. Loss of VGLUT1/IA synapses on motoneurons. J. Neurophysiol. 2011, 106, 2450–2470. [Google Scholar] [CrossRef] [PubMed]
- Rotterman, T.M.; Nardelli, P.; Cope, T.C.; Alvarez, F.J. Normal distribution of VGLUT1 synapses on spinal motoneuron dendrites and their reorganization after nerve injury. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 3475–3492. [Google Scholar] [CrossRef] [Green Version]
- Gordon, T.; English, A.W. Strategies to promote peripheral nerve regeneration: Electrical stimulation and/or exercise. Eur. J. Neurosci. 2016, 43, 336–350. [Google Scholar] [CrossRef] [Green Version]
- Titmus, M.J.; Faber, D.S. Axotomy-induced alterations in the electrophysiological characteristics of neurons. Prog. Neurobiol. 1990, 35, 1–51. [Google Scholar] [CrossRef]
- Gordon, T.; Hoffer, J.A.; Jhamandas, J.; Stein, R.B. Long-term effects of axotomy on neural activity during cat locomotion. J. Physiol. 1980, 303, 243–263. [Google Scholar] [CrossRef] [Green Version]
- Mackinnon, S.E.; Dellon, A.L.; Hudson, A.R.; Hunter, D.A. A primate model for chronic nerve compression. J. Reconstr. Microsurg. 1985, 1, 185–195. [Google Scholar] [CrossRef]
- Terzis, J.K.; Papakonstantinou, K.C. The surgical treatment of brachial plexus injuries in adults. Plast. Reconstr. Surg. 2000, 106, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Bontioti, E.; Kanje, M.; Lundborg, G.; Dahlin, L.B. End-to-side nerve repair in the upper extremity of rat. J. Peripher. Nerv. Syst. 2005, 10, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, G. Nerve Injury and Repair: Regeneration, Reconstruction, and Cortical Remodeling. J. Hand Surg. 2005, 30, 870–871. [Google Scholar]
- Gordon, T.; Hoffer, J.A.; Stein, R.B. Continued generation of action potentials in nerves without peripheral connexions [proceedings]. J. Physiol. 1977, 272, 39P–40P. [Google Scholar] [PubMed]
- Gordon, T.; Stein, R.B. Reorganization of motor-unit properties in reinnervated muscles of the cat. J. Neurophysiol. 1982, 48, 1175–1190. [Google Scholar] [CrossRef] [PubMed]
- Brännström, T.; Kellerth, J.O. Recovery of synapses in axotomized adult cat spinal motoneurons after reinnervation into muscle. Exp. Brain Res. 1999, 125, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Devor, M.; Keller, C.H.; Deerinck, T.J.; Levinson, S.R.; Ellisman, M.H. Na+ channel accumulation on axolemma of afferent endings in nerve end neuromas in Apteronotus. Neurosci. Lett. 1989, 102, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.; Bray, D. Movement and extension of isolated growth cones. Exp. Cell Res. 1977, 104, 55–62. [Google Scholar] [CrossRef]
- Diamond, J.; Coughlin, M.; Macintyre, L.; Holmes, M.; Visheau, B. Evidence that endogenous beta nerve growth factor is responsible for the collateral sprouting, but not the regeneration, of nociceptive axons in adult rats. Proc. Natl. Acad. Sci. USA 1987, 84, 6596–6600. [Google Scholar] [CrossRef] [Green Version]
- Castro-Lopes, J.; Tavares, I.; Coimbra, A. GABA decreases in the spinal cord dorsal horn after peripheral neurectomy. Brain Res. 1993, 620, 287–291. [Google Scholar] [CrossRef]
- Pavlov, S.P.; Grosheva, M.; Streppel, M.; Guntinas-Lichius, O.; Irintchev, A.; Skouras, E.; Angelova, S.K.; Kuerten, S.; Sinis, N.; Dunlop, S.A.; et al. Manually-stimulated recovery of motor function after facial nerve injury requires intact sensory input. Exp. Neurol. 2008, 211, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.R.; Jannetta, P.J. Blink reflex in patients with hemifacial spasm. Observations during microvascular decompression operations. J. Neurol. Sci. 1986, 72, 171–182. [Google Scholar] [CrossRef]
- Valls-Sole, J.; Tolosa, E.S. Blink reflex excitability cycle in hemifacial spasm. Neurology 1989, 39, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Deschênes, M.; Kurnikova, A.; Elbaz, M.; Kleinfeld, D. Circuits in the Ventral Medulla That Phase-Lock Motoneurons for Coordinated Sniffing and Whisking. Neural Plast. 2016, 2016, 7493048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, J.; Lyon, L.W. Orbicularis oculi reflex in the Wallenberg syndrome: Alteration of the late reflex by lesions of the spinal tract and nucleus of the trigeminal nerve. J. Neurol. Neurosurg. Psychiatry 1972, 35, 228–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erzurumlu, R.S.; Killackey, H.P. Efferent connections of the brainstem trigeminal complex with the facial nucleus of the rat. J. Comp. Neurol. 1979, 188, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Stennert, E.; Limberg, C.H. Central connections between fifth, seventh, and twelfth cranial nerves and their clinical significance. In Disorders of the Facial Nerve; Raven Press: New York, NY, USA, 1982; pp. 57–65. [Google Scholar]
- Hinrichsen, C.F.; Watson, C.D. Brain stem projections to the facial nucleus of the rat. Brain Behav. Evol. 1983, 22, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.B.; Norgren, R. Afferent projections to the oral motor nuclei in the rat. J. Comp. Neurol. 1983, 220, 280–298. [Google Scholar] [CrossRef]
- Isokawa-Akesson, M.; Komisaruk, B.R. Difference in projections to the lateral and medial facial nucleus: Anatomically separate pathways for rhythmical vibrissa movement in rats. Exp. Brain Res. 1987, 65, 385–398. [Google Scholar] [CrossRef]
- Sharp, F.R.; Gonzalez, M.F.; Morgan, C.W.; Morton, M.T.; Sharp, J.W. Common fur and mystacial vibrissae parallel sensory pathways: 14 C 2-deoxyglucose and WGA-HRP studies in the rat. J. Comp. Neurol. 1988, 270, 446–469. [Google Scholar] [CrossRef]
- Jacquin, M.F.; Zahm, D.S.; Henderson, T.A.; Golden, J.P.; Johnson, E.M.; Renehan, W.E.; Klein, B.G. Structure-function relationships in rat brainstem subnucleus interpolaris. X. Mechanisms underlying enlarged spared whisker projections after infraorbital nerve injury at birth. J. Neurosci. Off. J. Soc. Neurosci. 1993, 13, 2946–2964. [Google Scholar] [CrossRef] [Green Version]
- Munger, B.L.; Renehan, W.E. Degeneration and regeneration of peripheral nerve in the rat trigeminal system: III. Abnormal sensory reinnervation of rat guard hairs following nerve transection and crush. J. Comp. Neurol. 1989, 283, 169–176. [Google Scholar] [CrossRef]
- Rice, F.L.; Kinnman, E.; Aldskogius, H.; Johansson, O.; Arvidsson, J. The innervation of the mystacial pad of the rat as revealed by PGP 9.5 immunofluorescence. J. Comp. Neurol. 1993, 337, 366–385. [Google Scholar] [CrossRef]
- Semba, K.; Szechtman, H.; Komisaruk, B.R. Synchrony among rhythmical facial tremor, neocortical “alpha” waves, and thalamic non-sensory neuronal bursts in intact awake rats. Brain Res. 1980, 195, 281–298. [Google Scholar] [CrossRef]
- Bermejo, R.; Harvey, M.; Gao, P.; Zeigier, H.P. Conditioned Whisking in the Rat. Somatosens. Mot. Res. 1996, 13, 225–233. [Google Scholar] [CrossRef]
- Komisaruk, B. Synchrony between limbic system theta activity and rhythmic behavior. J. Comp. Physiol. Psychol. 1970, 70, 482–492. [Google Scholar] [CrossRef]
- Carvell, G.E.; Simons, D.J. Biometric analyses of vibrissal tactile discrimination in the rat. J. Neurosci. 1990, 10, 2638–2648. [Google Scholar] [CrossRef] [Green Version]
- Guntinas-Lichius, O.; Wewetzer, K.; Tomov, T.L.; Azzolin, N.; Kazemi, S.; Streppel, M.; Neiss, W.F.; Angelov, D.N. Transplantation of olfactory mucosa minimizes axonal branching and promotes the recovery of vibrissae motor performance after facial nerve repair in rats. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 7121–7131. [Google Scholar] [CrossRef] [Green Version]
- Tomov, T.L.; Guntinas-Lichius, O.; Grosheva, M.; Streppel, M.; Schraermeyer, U.; Neiss, W.F.; Angelov, D.N. An Example of Neural Plasticity Evoked by Putative Behavioral Demand and Early Use of Vibrissal Hairs after Facial Nerve Transection. Exp. Neurol. 2002, 178, 207–218. [Google Scholar] [CrossRef]
- Berg, R.W.; Kleinfeld, D. Rhythmic whisking by rat: Retraction as well as protraction of the vibrissae is under active muscular control. J. Neurophysiol. 2003, 89, 104–117. [Google Scholar] [CrossRef] [Green Version]
- Angelov, D.N.; Ceynowa, M.; Guntinas-Lichius, O.; Streppel, M.; Grosheva, M.; Kiryakova, S.I.; Skouras, E.; Maegele, M.; Irintchev, A.; Neiss, W.F.; et al. Mechanical stimulation of paralyzed vibrissal muscles following facial nerve injury in adult rat promotes full recovery of whisking. Neurobiol. Dis. 2007, 26, 229–242. [Google Scholar] [CrossRef]
- Soha, J.M.; Yo, C.; Van Essen, D.C. Synapse elimination by fiber type and maturational state in rabbit soleus muscle. Dev. Biol. 1987, 123, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Cramer, K.S.; Van Essen, D.C. Maturation of fast and slow motor units during synapse elimination in the rabbit soleus muscle. Dev. Biol. 1995, 171, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Smith, I.; Mikesh, M. Terminal Schwann cell and vacant site mediated synapse elimination at developing neuromuscular junctions. Sci. Rep. 2019, 9, 18594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dörfl, J. The innervation of the mystacial region of the white mouse: A topographical study. J. Anat. 1985, 142, 173–184. [Google Scholar] [PubMed]
- Stal, S.; Spira, M.; Hamilton, S. Skin morphology and function. Clin. Plast. Surg. 1987, 14, 201–208. [Google Scholar] [CrossRef]
- Stal, S.; Peterson, R.; Spira, M. Aesthetic considerations and the pediatric population. Clin. Plast. Surg. 1990, 17, 133–149. [Google Scholar] [CrossRef]
- Welt, C.; Abbs, J.H. Musculotopic organization of the facial motor nucleus in Macaca fascicularis: A morphometric and retrograde tracing study with cholera toxin B-HRP. J. Comp. Neurol. 1990, 291, 621–636. [Google Scholar] [CrossRef]
- Rice, F.; Fundin, B.; Arvidsson, J.; Aldskogius, H.; Johansson, O. Comprehensive immunofluorescence and lectin binding analysis of vibrissal follicle sinus complex innervation in the mystacial pad of the rat. J. Comp. Neurol. 1997, 385, 149–184. [Google Scholar] [CrossRef]
- McComas, A.J. Oro-facial muscles: Internal structure, function and ageing. Gerodontology 1998, 15, 3–14. [Google Scholar] [CrossRef]
- Whitehead, J.; Keller-Peck, C.; Kucera, J.; Tourtellotte, W.G. Glial cell-line derived neurotrophic factor-dependent fusimotor neuron survival during development. Mech. Dev. 2005, 122, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Guntinas-Lichius, O.; Irintchev, A.; Streppel, M.; Lenzen, M.; Grosheva, M.; Wewetzer, K.; Neiss, W.F.; Angelov, D.N. Factors limiting motor recovery after facial nerve transection in the rat: Combined structural and functional analyses. Eur. J. Neurosci. 2005, 21, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Dörfl, J. The musculature of the mystacial vibrissae of the white mouse. J. Anat. 1982, 135, 147–154. [Google Scholar] [PubMed]
- Sinis, N.; Horn, F.; Genchev, B.; Skouras, E.; Merkel, D.; Angelova, S.; Kaidoglou, K.; Michael, J.; Pavlov, S.; Igelmund, P.; et al. Electrical stimulation of paralyzed vibrissal muscles reduces endplate reinnervation and does not promote motor recovery after facial nerve repair in rats. Ann. Anat. 2009, 191, 356–370. [Google Scholar] [CrossRef]
- Semba, K.; Egger, M.D. The facial “motor” nerve of the rat: Control of vibrissal movement and examination of motor and sensory components. J. Comp. Neurol. 1986, 247, 144–158. [Google Scholar] [CrossRef]
- Bendella, H.; Pavlov, S.P.; Grosheva, M.; Irintchev, A.; Angelova, S.K.; Merkel, D.; Sinis, N.; Kaidoglou, K.; Skouras, E.; Dunlop, S.A.; et al. Non-invasive stimulation of the vibrissal pad improves recovery of whisking function after simultaneous lesion of the facial and infraorbital nerves in rats. Exp. Brain Res. 2011, 212, 65–79. [Google Scholar] [CrossRef]
- Mosforth, J.; Taverner, D. Physiotherapy for Bell’s palsy. Br. Med. J. 1958, 2, 675–677. [Google Scholar] [CrossRef] [Green Version]
- Esslen, E. Electromyographic findings on two types of misdirection of regenerating axons. Electroencephalogr. Clin. Neurophysiol. 1960, 12, 738–741. [Google Scholar] [CrossRef]
- Aldskogius, H.; Thomander, L. Selective reinnervation of somatotopically appropriate muscles after facial nerve transection and regeneration in the neonatal rat. Brain Res. 1986, 375, 126–134. [Google Scholar] [CrossRef]
- Thomander, L. Reorganization of the facial motor nucleus after peripheral nerve regeneration. An HRP study in the rat. Acta Otolaryngol. 1984, 97, 619–626. [Google Scholar] [CrossRef]
- Liu, D.W.; Westerfield, M. The formation of terminal fields in the absence of competitive interactions among primary motoneurons in the zebrafish. J. Neurosci. Off. J. Soc. Neurosci. 1990, 10, 3947–3959. [Google Scholar] [CrossRef] [PubMed]
- Shawe, G.D.H. On the number of branches formed by regenerating nerve-fibres. Br. J. Surg. 1955, 42, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Brushart, T.M.; Mesulam, M.M. Alteration in connections between muscle and anterior horn motoneurons after peripheral nerve repair. Science 1980, 208, 603–605. [Google Scholar] [CrossRef]
- Ito, M.; Kudo, M. Reinnervation by axon collaterals from single facial motoneurons to multiple muscle targets following axotomy in the adult guinea pig. Acta Anat. 1994, 151, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.S.; Stava, M.W.; Nelson, K.R.; May, P.J.; Huffman, M.D.; Porter, J.D. Aberrant reinnervation of facial musculature in a subhuman primate: A correlative analysis of eyelid kinematics, muscle synkinesis, and motoneuron localization. Neurology 1994, 44, 2165–2173. [Google Scholar] [CrossRef]
- Son, Y.J.; Trachtenberg, J.T.; Thompson, W.J. Schwann cells induce and guide sprouting and reinnervation of neuromuscular junctions. Trends Neurosci. 1996, 19, 280–285. [Google Scholar] [CrossRef]
- Brushart, T.M. Motor axons preferentially reinnervate motor pathways. J. Neurosci. Off. J. Soc. Neurosci. 1993, 13, 2730–2738. [Google Scholar] [CrossRef] [Green Version]
- MacKinnon, S.E.; Dellon, A.L.; O’Brien, J.P. Changes in nerve fiber numbers distal to a nerve repair in the rat sciatic nerve model. Muscle Nerve 1991, 14, 1116–1122. [Google Scholar] [CrossRef]
- Angelov, D.N.; Skouras, E.; Guntinas-Lichius, O.; Streppel, M.; Popratiloff, A.; Walther, M.; Klein, J.; Stennert, E.; Neiss, W.F. Contralateral trigeminal nerve lesion reduces polyneuronal muscle innervation after facial nerve repair in rats. Eur. J. Neurosci. 1999, 11, 1369–1378. [Google Scholar] [CrossRef]
- Skouras, E.; Popratiloff, A.; Guntinas-Lichius, O.; Streppel, M.; Rehm, K.; Neiss, W.; Angelov, D. Altered sensory input improves the accuracy of muscle reinnervation. Restor. Neurol. Neurosci. 2002, 20, 1–14. [Google Scholar]
- Angelov, D.N.; Gunkel, A.; Stennert, E.; Neiss, W.F. Recovery of original nerve supply after hypoglossal-facial anastomosis causes permanent motor hyperinnervation of the whisker-pad muscles in the rat. J. Comp. Neurol. 1993, 338, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Angelov, D.N.; Neiss, W.F.; Streppel, M.; Andermahr, J.; Mader, K.; Stennert, E. Nimodipine accelerates axonal sprouting after surgical repair of rat facial nerve. J. Neurosci. Off. J. Soc. Neurosci. 1996, 16, 1041–1048. [Google Scholar] [CrossRef]
- Streppel, M.; Angelov, D.N.; Guntinas-Lichius, O.; Hilgers, R.-D.; Rosenblatt, J.D.; Stennert, E.; Neiss, W.F. Slow Axonal Regrowth But Extreme Hyperinnervation of Target Muscle After Suture of the Facial Nerve in Aged Rats. Neurobiol. Aging 1998, 19, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Huston, J.P.; Steiner, H.; Weiler, H.-T.; Morgan, S.; Schwarting, R.K.W. The basal ganglia-orofacial system: Studies on neurobehavioral plasticity and sensory-motor tuning. Neurosci. Biobehav. Rev. 1990, 14, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Baumel, J.J. Trigeminal-facial nerve communications. Their function in facial muscle innervation and reinnervation. Arch. Otolaryngol. 1974, 99, 34–44. [Google Scholar] [CrossRef]
- Diels, H.J. Treatment of facial paralysis using electromyographic feedback—A case study. In The Facial Nerve; Stennert, E.R., Kreutzberg, G.W., Michel, O., Jungehülsing, M., Eds.; Springer: Berlin, Heidelberg, 1994; pp. S129–S132. [Google Scholar] [CrossRef]
- Taub, E.; Miller, N.E.; Novack, T.A.; Cook, E.W., 3rd; Fleming, W.C.; Nepomuceno, C.S.; Connell, J.S.; Crago, J.E. Technique to improve chronic motor deficit after stroke. Arch. Phys. Med. Rehabil. 1993, 74, 347–354. [Google Scholar]
- Arvidsson, J.; Ygge, J.; Grant, G. Cell loss in lumbar dorsal root ganglia and transganglionic degeneration after sciatic nerve resection in the rat. Brain Res. 1986, 373, 15–21. [Google Scholar] [CrossRef]
- Wilson, P.; Kitchener, P.D. Plasticity of cutaneous primary afferent projections to the spinal dorsal horn. Prog. Neurobiol. 1996, 48, 105–129. [Google Scholar] [CrossRef]
- Woolf, C.J.; Shortland, P.; Coggeshall, R.E. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature 1992, 355, 75–78. [Google Scholar] [CrossRef]
- Owen, A.D.; Bird, M.M. Role of glutamate in the regulation of the outgrowth and motility of neurites from mouse spinal cord neurons in culture. J. Anat. 1997, 191 Pt 2, 301–307. [Google Scholar] [CrossRef]
- Barbin, G.; Pollard, H.; Gaïarsa, J.L.; Ben-Ari, Y. Involvement of GABAA receptors in the outgrowth of cultured hippocampal neurons. Neurosci. Lett. 1993, 152, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Takada, M.; Kaneko, T.; Mizuno, N. Distribution of GABAergic and glycinergic premotor neurons projecting to the facial and hypoglossal nuclei in the rat. J. Comp. Neurol. 1997, 378, 283–294. [Google Scholar] [CrossRef]
- Guntinas-Lichius, O. The facial nerve in the presence of a head and neck neoplasm: Assessment and outcome after surgical management. Curr. Opin. Otolaryngol. Head Neck Surg. 2004, 12, 133–141. [Google Scholar] [CrossRef]
- Segev, I. Sound grounds for computing dendrites. Nature 1998, 393, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Vetter, P.; Roth, A.; Häusser, M. Propagation of action potentials in dendrites depends on dendritic morphology. J. Neurophysiol. 2001, 85, 926–937. [Google Scholar] [CrossRef]
- Calhoun, M.E.; Jucker, M.; Martin, L.J.; Thinakaran, G.; Price, D.L.; Mouton, P.R. Comparative evaluation of synaptophysin-based methods for quantification of synapses. J. Neurocytol. 1996, 25, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Marqueste, T.; Decherchi, P.; Desplanches, D.; Favier, R.; Grelot, L.; Jammes, Y. Chronic electrostimulation after nerve repair by self-anastomosis: Effects on the size, the mechanical, histochemical and biochemical muscle properties. Acta Neuropathol. 2006, 111, 589–600. [Google Scholar] [CrossRef]
- Graeber, M.B.; Kreutzberg, G.W. Delayed astrocyte reaction following facial nerve axotomy. J. Neurocytol. 1988, 17, 209–220. [Google Scholar] [CrossRef]
- Neiss, W.F.; Lichius, O.G.; Angelov, D.N.; Gunkel, A.; Stennert, E. The hypoglossal-facial anastomosis as model of neuronal plasticity in the rat. Ann. Anat.-Anat. Anzeiger 1992, 174, 419–433. [Google Scholar] [CrossRef]
- Guntinas-Lichius, O.; Neiss, W.F.; Gunkel, A.; Stennert, E. Differences in glial, synaptic and motoneuron responses in the facial nucleus of the rat brainstem following facial nerve resection and nerve suture reanastomosis. Eur. Arch. Oto-Rhino-Laryngol. Off. J. Eur. Fed. Oto-Rhino-Laryngol. Soc. Affil. Ger. Soc. Oto-Rhino-Laryngol.—Head Neck Surg. 1994, 251, 410–417. [Google Scholar] [CrossRef]
- Mader, K.; Andermahr, J.; Angelov, D.; Neiss, W. Dual Mode of Signalling of the Axotomy Reaction: Retrograde Electric Stimulation or Block of Retrograde Transport Differently Mimic the Reaction of Motoneurons to Nerve Transection in the Rat Brainstem. J. Neurotrauma 2004, 21, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Al-Majed, A.A.; Tam, S.L.; Gordon, T. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons. Cell. Mol. Neurobiol. 2004, 24, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Pearse, D.D.; Pereira, F.C.; Marcillo, A.E.; Bates, M.L.; Berrocal, Y.A.; Filbin, M.T.; Bunge, M.B. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat. Med. 2004, 10, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Feng, W.; Lu, H.; Gong, S. Synaptic plasticity in the facial nucleus in rats following infraorbital nerve manipulation after facial nerve injury. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 3135–3142. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ward, P.J.; English, A.W. The effects of exercise on synaptic stripping require androgen receptor signaling. PLoS ONE 2014, 9, e98633. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.; Evans, J.T.; Mildenhall, T.; Mulligan, A.; Konieczny, A.; Rose, S.J.; English, A.W. Delaying the onset of treadmill exercise following peripheral nerve injury has different effects on axon regeneration and motoneuron synaptic plasticity. J. Neurophysiol. 2015, 113, 2390–2399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Møller, A.R.; Sen, C.N. Recordings from the facial nucleus in the rat: Signs of abnormal facial muscle response. Exp. Brain Res. 1990, 81, 18–24. [Google Scholar] [CrossRef]
- Valls-Sole, J.; Tolosa, E.S.; Pujol, M. Myokymic discharges and enhanced facial nerve reflex responses after recovery from idiopathic facial palsy. Muscle Nerve 1992, 15, 37–42. [Google Scholar] [CrossRef]
- Zerari-Mailly, F.; Pinganaud, G.; Dauvergne, C.; Buisseret, P.; Buisseret-Delmas, C. Trigemino-reticulo-facial and trigemino-reticulo-hypoglossal pathways in the rat. J. Comp. Neurol. 2001, 429, 80–93. [Google Scholar] [CrossRef]
- Hattox, A.M.; Priest, C.A.; Keller, A. Functional circuitry involved in the regulation of whisker movements. J. Comp. Neurol. 2002, 442, 266–276. [Google Scholar] [CrossRef] [Green Version]
- Kleinfeld, D.; Berg, R.W.; O’Connor, S.M. Anatomical loops and their electrical dynamics in relation to whisking by rat. Somatosens. Mot. Res. 1999, 16, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Bermejo, R.; Zeigler, H.P. Whisker Deafferentation and Rodent Whisking Patterns: Behavioral Evidence for a Central Pattern Generator. J. Neurosci. 2001, 21, 5374–5380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kis, Z.; Rákos, G.; Farkas, T.; Horváth, S.; Toldi, J. Facial nerve injury induces facilitation of responses in both trigeminal and facial nuclei of rat. Neurosci. Lett. 2004, 358, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.-T.; Kleinfeld, D. Positive Feedback in a Brainstem Tactile Sensorimotor Loop. Neuron 2005, 45, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Leiser, S.C.; Moxon, K.A. Responses of trigeminal ganglion neurons during natural whisking behaviors in the awake rat. Neuron 2007, 53, 117–133. [Google Scholar] [CrossRef] [Green Version]
- Dauvergne, C.; Pinganaud, G.; Buisseret, P.; Buisseret-Delmas, C.; Zerari-Mailly, F. Reticular premotor neurons projecting to both facial and hypoglossal nuclei receive trigeminal afferents in rats. Neurosci. Lett. 2001, 311, 109–112. [Google Scholar] [CrossRef]
- Popratiloff, A.S.; Neiss, W.F.; Skouras, E.; Streppel, M.; Guntinas-Lichius, O.; Angelov, D.N. Evaluation of muscle re-innervation employing pre- and post-axotomy injections of fluorescent retrograde tracers. Brain Res. Bull. 2001, 54, 115–123. [Google Scholar] [CrossRef]
- Sosnik, R.; Haidarliu, S.; Ahissar, E. Temporal Frequency of Whisker Movement. I. Representations in Brain Stem and Thalamus. J. Neurophysiol. 2001, 86, 339–353. [Google Scholar] [CrossRef] [Green Version]
- Minnery, B.S.; Simons, D.J. Response properties of whisker-associated trigeminothalamic neurons in rat nucleus principalis. J. Neurophysiol. 2003, 89, 40–56. [Google Scholar] [CrossRef]
- Barbara, M.; Monini, S.; Buffoni, A.; Cordier, A.; Ronchetti, F.; Harguindey, A.; Di Stadio, A.; Cerruto, R.; Filipo, R. Early rehabilitation of facial nerve deficit after acoustic neuroma surgery. Acta Otolaryngol. 2003, 123, 932–935. [Google Scholar] [CrossRef]
- Cronin, G.W.; Steenerson, R.L. The effectiveness of neuromuscular facial retraining combined with electromyography in facial paralysis rehabilitation. Otolaryngol.-Head Neck Surg. 2003, 128, 534–538. [Google Scholar] [CrossRef] [PubMed]
- VanSwearingen, J.M.; Brach, J.S. Changes in facial movement and synkinesis with facial neuromuscular reeducation. Plast. Reconstr. Surg. 2003, 111, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Hennig, R.; Dietrichs, E. Transient reinnervation of antagonistic muscles by the same motoneuron. Exp. Neurol. 1994, 130, 331–336. [Google Scholar] [CrossRef]
- Reynolds, M.L.; Woolf, C.J. Terminal Schwann cells elaborate extensive processes following denervation of the motor endplate. J. Neurocytol. 1992, 21, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Madison, R.D.; Archibald, S.J.; Lacin, R.; Krarup, C. Factors Contributing to Preferential Motor Reinnervation in the Primate Peripheral Nervous System. J. Neurosci. 1999, 19, 11007–11016. [Google Scholar] [CrossRef] [Green Version]
- Jergović, D.; Stål, P.; Lidman, D.; Lindvall, B.; Hildebrand, C. Changes in a rat facial muscle after facial nerve injury and repair. Muscle Nerve 2001, 24, 1202–1212. [Google Scholar] [CrossRef]
- Ijkema-Paassen, J.; Meek, M.F.; Gramsbergen, A. Reinnervation of muscles after transection of the sciatic nerve in adult rats. Muscle Nerve 2002, 25, 891–897. [Google Scholar] [CrossRef]
- Grant, G.A.; Rostomily, R.R.; Kim, D.K.; Mayberg, M.R.; Farrell, D.; Avellino, A.; Duckert, L.G.; Gates, G.A.; Winn, H.R. Delayed facial palsy after resection of vestibular schwannoma. J. Neurosurg. 2002, 97, 93–96. [Google Scholar] [CrossRef]
- Choi, D.; Raisman, G. Disorganization of the facial nucleus after nerve lesioning and regeneration in the rat: Effects of transplanting candidate reparative cells to the site of injury. Neurosurgery 2005, 56, 1093–1100. [Google Scholar]
- Schröder, J.M. Supernumerary schwann cells during remyelination of regenerated and segmentally demyelinated axons in peripheral nerves. Verh. Dtsch. Ges. Pathol. 1968, 52, 222–228. [Google Scholar]
- Gorio, A.; Carmignoto, G.; Finesso, M.; Polato, P.; Nunzi, M.G. Muscle reinnervation—II. Sprouting, synapse formation and repression. Neuroscience 1983, 8, 403-IN1. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.A.; Ribchester, R.R. Persistent polyneuronal innervation in partially denervated rat muscle after reinnervation and recovery from prolonged nerve conduction block. J. Neurosci. Off. J. Soc. Neurosci. 1995, 15, 6327–6339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimby, G.; Einarsson, G.; Hedberg, M.; Aniansson, A. Muscle adaptive changes in post-polio subjects. Scand. J. Rehabil. Med. 1989, 21, 19–26. [Google Scholar]
- Trojan, D.A.; Gendron, D.; Cashman, N.R. Electrophysiology and electrodiagnosis of the post-polio motor unit. Orthopedics 1991, 14, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.L.; Gordon, T. Mechanisms controlling axonal sprouting at the neuromuscular junction. J. Neurocytol. 2003, 32, 961–974. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.C.; Holland, R.L.; Hopkins, W.G.; Keynes, R.J. An assessment of the spread of the signal for terminal sprouting within and between muscles. Brain Res. 1981, 210, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.L.; Archibald, V.; Jassar, B.; Tyreman, N.; Gordon, T. Increased neuromuscular activity reduces sprouting in partially denervated muscles. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 654–667. [Google Scholar] [CrossRef]
- Deschenes, M.R.; Tenny, K.A.; Wilson, M.H. Increased and decreased activity elicits specific morphological adaptations of the neuromuscular junction. Neuroscience 2006, 137, 1277–1283. [Google Scholar] [CrossRef]
- Grosheva, M.; Nohroudi, K.; Schwarz, A.; Rink, S.; Bendella, H.; Sarikcioglu, L.; Klimaschewski, L.; Gordon, T.; Angelov, D.N. Comparison of trophic factors’ expression between paralyzed and recovering muscles after facial nerve injury. A quantitative analysis in time course. Exp. Neurol. 2016, 279, 137–148. [Google Scholar] [CrossRef]
- He, Q.-R.; Cong, M.; Yu, F.-H.; Ji, Y.-H.; Yu, S.; Shi, H.-Y.; Ding, F. Peripheral nerve fibroblasts secrete neurotrophic factors to promote axon growth of motoneurons. Neural Regen. Res. 2022, 17, 1833–1840. [Google Scholar] [CrossRef]

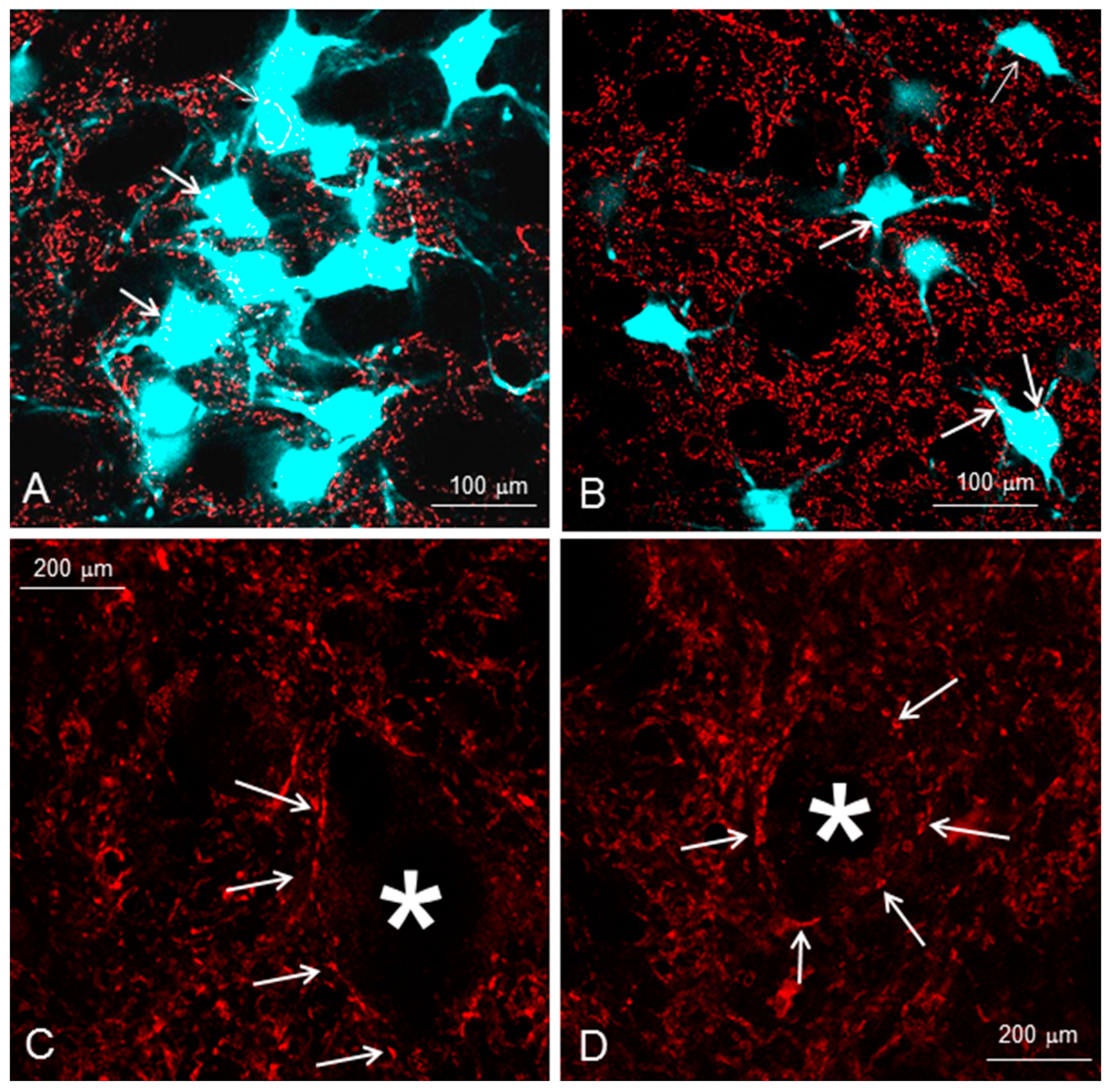

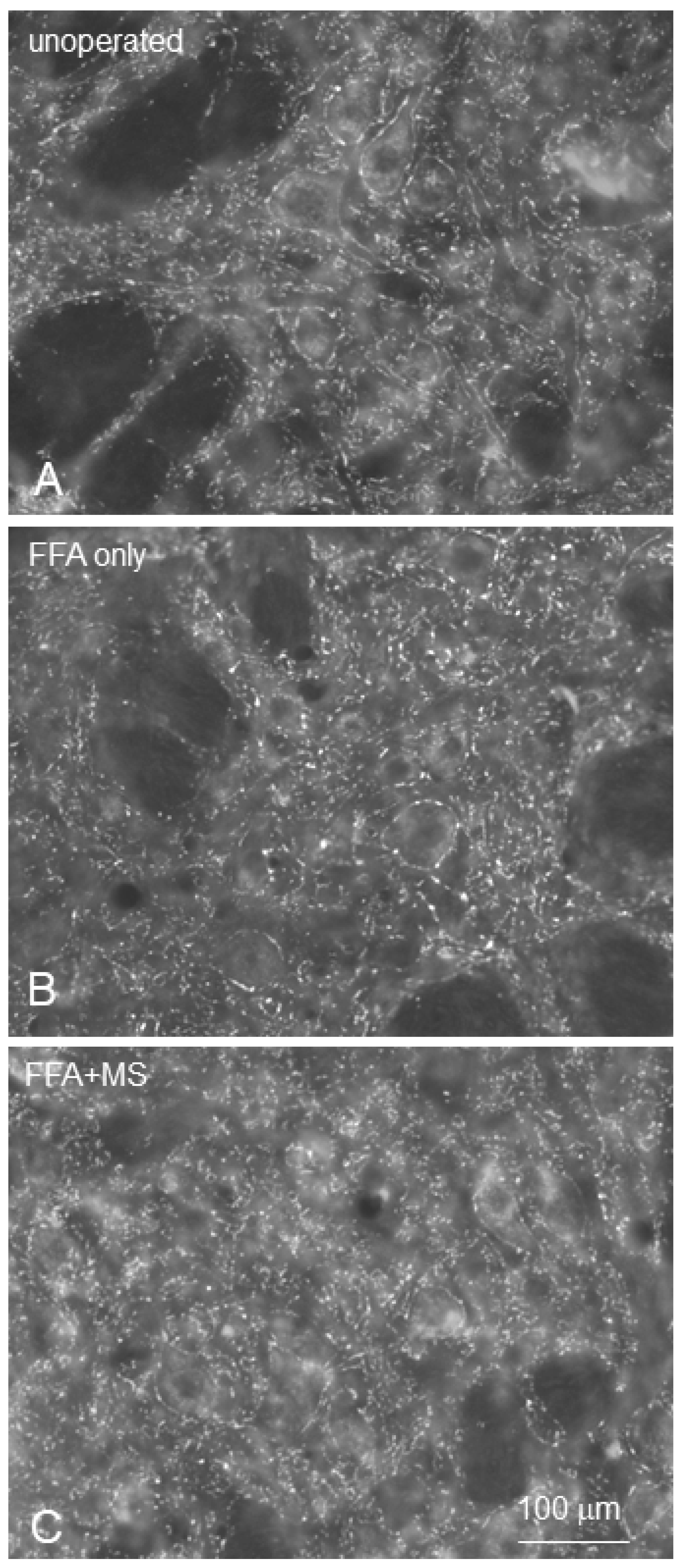
| Section 2 (2 months) | Whisking Amplitude | NMJ Polyinnervation | ||
| Rat groups | ||||
| INTACT | 62 ± 6° | 0% | ||
| Fn-n | 19 ± 6° | 53 ± 10% | ||
| Fn-n + Mstim | 51 ± 19° | 22 ± 3% | ||
| Fn-n + IOn-ipsi-ex | 22 ± 3° | 43 + 9% | ||
| Fn-n + IOn-ipsi-ex + Mstim | 14 ± 6° | 51 ± 10% | ||
| Section 3 (4 months) Rat Groups | Whisking Amplitude | NMJ Polyinnervation | Facial Motoneurons Perisomatic Terminal Fractional Area | |
| INTACT | 62 ± 6° | 0% | 17 ± 2% | |
| Fn-n + IOn-n | 11 ± 4° | 58 ± 8% | 12 ± 1% | |
| Fn-n + IOn-n + Vstim | 28 + 9° | 40 ± 3% | 13 ± 2% | |
| Fn-n + IOn-n + Mstim | 30 ± 11° | 40 ± 2% | 13 ± 2% | |
| Fn-n + IOn-n + Vstim + Mstim | 32 ± 10° | 33 ± 10% | 12 ± 2% | |
| Section 4 (4 weeks) | Buccal Branch Pathfinding | Buccal Branch Pathfinding | Buccal Branch Pathfinding | |
| Rat groups | Superior-FG | Inferior-DiI | Sup + Inferior | |
| INTACT | 91% | 9% | 0% | |
| Bn-n | 56% | 21% | 23% | |
| Bn-n + ipsi IOn-ex | 48% | 39% | 13% | |
| Bn-n + contr IOn-ex | 69% | 23% | 9% | |
| Section 5 (4 weeks) Rat Groups | Accuracy of Whisker pad Reinnervation | |||
| INTACT | 100% | |||
| Bn-n | 27% | |||
| Bn-n + ipsi IOn-ex | 32% | |||
| Bn-n + contr IOn-ex | 41% | |||
| Section 6 (2 months) Rat Groups | Whisking Amplitude | LLS Polyinnervation of NMJ | Terminal Density (×106) on Facial Motoneurons | |
| INTACT | 57 ± 13° | 0% | 34.3 ± 2.3 | |
| Fn-n + Handling | 19 + 6° | 53 ± 10% | 29.2 ± 1.8 | |
| Fn-n + Mstim | 51 ± 19° | 22 ± 5% | 33 ± 2.6 | |
| Section 7 (2 months) Rat Groups | FGF2 Protein pg/mg | IGF1 Protein pg/mg | NGF Protein pg/mg | |
| INTACT | 75 ± 16 | 1492 ± 87 | 61 ± 22 | |
| Fn-n + Handling | 69 ± 24 | 2079 ± 300 | 51 ± 21 | |
| Fn-n + Mstim | 56 ± 11 | 1821 ± 784 | 33 ± 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rink-Notzon, S.; Reuscher, J.; Nohroudi, K.; Manthou, M.; Gordon, T.; Angelov, D.N. Trigeminal Sensory Supply Is Essential for Motor Recovery after Facial Nerve Injury. Int. J. Mol. Sci. 2022, 23, 15101. https://doi.org/10.3390/ijms232315101
Rink-Notzon S, Reuscher J, Nohroudi K, Manthou M, Gordon T, Angelov DN. Trigeminal Sensory Supply Is Essential for Motor Recovery after Facial Nerve Injury. International Journal of Molecular Sciences. 2022; 23(23):15101. https://doi.org/10.3390/ijms232315101
Chicago/Turabian StyleRink-Notzon, Svenja, Jannika Reuscher, Klaus Nohroudi, Marilena Manthou, Tessa Gordon, and Doychin N. Angelov. 2022. "Trigeminal Sensory Supply Is Essential for Motor Recovery after Facial Nerve Injury" International Journal of Molecular Sciences 23, no. 23: 15101. https://doi.org/10.3390/ijms232315101






