Structures and Anti-Inflammatory Evaluation of Phenylpropanoid Derivatives from the Aerial Parts of Dioscorea polystachya
Abstract
:1. Introduction
2. Results
2.1. Isolation of New Compounds 1–7 from the Aerial Parts of D. polystachya
2.2. Structure Identification of New Compounds 1–7
2.3. Inhibitory Effects of New Compounds 1–7 on NO Production of LPS-Activated RAW 264.7 Cells
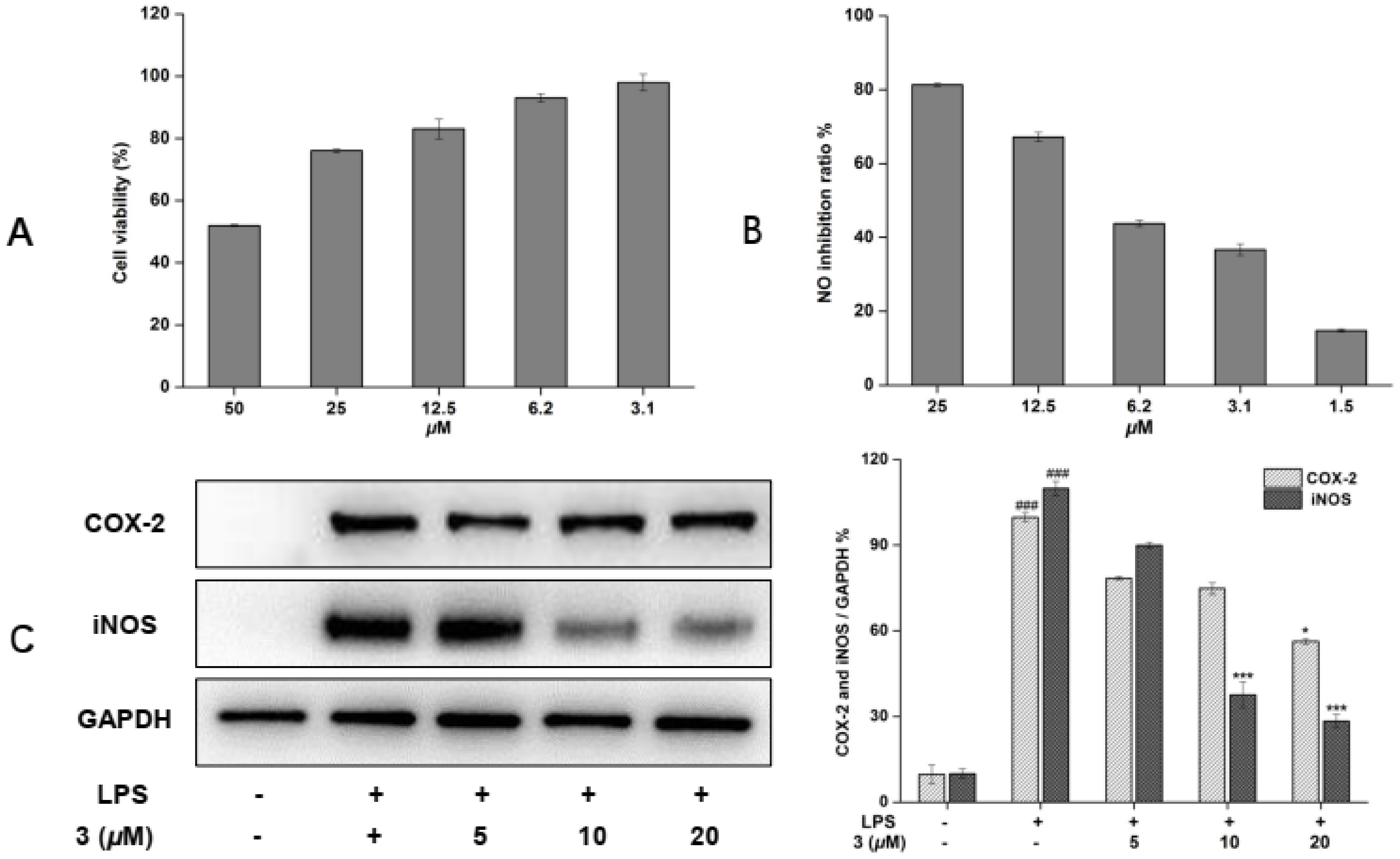
2.4. Inhibitory Effects of New Compound 3 on LPS-Enhanced Inflammatory Mediators
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Materials
4.3. Extraction and Isolation
4.4. Cell Viability Assay
4.5. Cell Culture and NO Production Measurements
4.6. Western Blot Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, J.S.; Oh, J.; Yun, H.S.; Lee, J.S.; Hahn, D.; Kim, J.S. Anti-neuroinflammatory activity of 6,7-dihydroxy-2,4-dimethoxy phenanthrene isolated from Dioscorea batatas decne partly through suppressing the p38 MAPK/NF-kappa B pathway in BV2 microglial cells. J. Ethnopharmacol. 2022, 282, 114633. [Google Scholar] [CrossRef] [PubMed]
- Adomeniene, A.; Venskutonis, P.R. Dioscorea spp.: Comprehensive review of antioxidant properties and their relation to phytochemicals and health benefits. Molecules 2022, 27, 2530. [Google Scholar] [CrossRef] [PubMed]
- Sautour, M.; Mitaine-Offer, A.C.; Lacaille-Dubois, M.A. The Dioscorea genus: A review of bioactive steroid saponins. J. Nat. Med. 2007, 61, 91–101. [Google Scholar] [CrossRef]
- Fan, Y.J.; He, Q.Y.; Luo, A.S.; Wang, M.Y.; Luo, A.X. Characterization and antihyperglycemic activity of a polysaccharide from Dioscorea opposita Thunb roots. Int. J. Mol. Sci. 2015, 16, 6391–6401. [Google Scholar] [CrossRef]
- Koo, H.J.; Park, H.J.; Byeon, H.E.; Kwak, J.H.; Um, S.H.; Kwon, S.T.; Rhee, D.K.; Pyo, S. Chinese yam extracts containing beta-sitosterol and ethyl linoleate protect against atherosclerosis in apolipoprotein E-deficient mice and inhibit muscular expression of VCAM-1 in vitro. J. Food Sci. 2014, 79, 719–729. [Google Scholar] [CrossRef]
- Liu, Y.H.; Lin, Y.S.; Liu, D.Z.; Han, C.H.; Chen, C.T.; Fan, M.; Hou, W.C. Effects of different types of Yam (Dioscorea alata) products on the blood pressure of spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2009, 73, 1371–1376. [Google Scholar] [CrossRef]
- Byeon, S.; Oh, J.; Lim, J.S.; Lee, J.S.; Kim, J.S. Protective effects of Dioscorea batatas flesh and peel extracts against ethanol-induced gastric ulcer in mice. Nutrients 2018, 10, 1860. [Google Scholar] [CrossRef]
- Koo, H.J.; Lee, S.; Chang, K.J.; Sohn, E.; Sohn, E.H.; Kang, S.C.; Pyo, S. Hepatic anti-inflammatory effect of hexane extracts of Dioscorea batatas decne: Possible suppression of toll-like receptor 4-mediated signaling. Biomed. Pharmacother. 2017, 92, 157–167. [Google Scholar] [CrossRef]
- Go, H.K.; Rahman, M.M.; Kim, G.B.; Na, C.S.; Song, C.H.; Kim, J.S.; Kim, S.J.; Kang, H.S. Antidiabetic effects of Yam (Dioscorea batatas) and its active constituent, allantoin, in a rat model of streptozotocin-induced diabetes. Nutrients 2015, 7, 8532–8544. [Google Scholar] [CrossRef]
- Gugu, F.S.; Paul, S.; Simon, G. Constituents of two Dioscorea species that potentiate antibiotic activity against MRSA. J. Nat. Prod. 2020, 83, 1696–1700. [Google Scholar] [CrossRef]
- Ma, C.; Wang, W.; Chen, Y.Y.; Liu, R.N.; Wang, R.F.; Du, L.J. Neuroprotective and antioxidant activity of compounds from the aerial parts of Dioscorea opposite. J. Nat. Prod. 2005, 68, 1259–1261. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.H.; Nikolic, D.; Simmler, C.; Qu, F.; van Breemen, R.B.; Soejarto, D.D.; Pauli, G.F.; Chen, S.N. Diarylheptanoids from Dioscorea villosa (Wild Yam). J. Nat. Prod. 2013, 76, 2005–2007. [Google Scholar] [CrossRef]
- Wang, J.T.; Ge, D.; Qu, H.F.; Wang, G.K.; Wang, G. Chemical constituents of Curcuma kwangsiensis and their antimigratory activities in RKO cells. Nat. Prod. Res. 2019, 33, 3493–3499. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.; Zhang, P.L.; Liu, J.S.; Wang, G.K.; Xu, F.Q.; Chen, L.; Yu, Y.; Wang, G. Aspergilates A to E, second metabolites from Aspergillus sp. isolated from Paeonia ostia. Fitoterapia 2018, 131, 204–208. [Google Scholar] [CrossRef]
- Yue, J.Y.; Wang, R.; Xu, T.; Wang, J.T.; Yu, Y.; Cai, B.X. Novel phenolic metabolites isolated from plant endophytic fungus Fusarium guttiforme. Nat. Prod. Res. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.M.; Zheng, C.J.; Gan, L.S.; Chen, G.Y.; Zhang, X.P.; Song, X.P.; Li, G.N.; Sun, C.G. Bioactive phenanthrene and bibenzyl derivatives from the stems of Dendrobium nobile. J. Nat. Prod. 2016, 79, 1791–1797. [Google Scholar] [CrossRef]
- Wang, W.J.; Wang, L.; Liu, Z.; Jiang, R.W.; Liu, Z.W.; Li, M.M.; Zhang, Q.W.; Dai, Y.; Li, Y.L.; Zhang, X.Q.; et al. Antiviral benzofurans from Eupatorium chinense. Phytochemistry 2016, 122, 238–245. [Google Scholar] [CrossRef]
- Hong, C.J.; Chen, S.Y.; Hsu, Y.H.; Yen, G.C. Protective effect of fermented okara on the regulation of inflammation, the gut microbiota, and SCFAs production in rats with TNBS-induced colitis. Food Res. Int. 2022, 157, 111390. [Google Scholar] [CrossRef]
- Smith, T.L.; Oubaha, M.; Cagnone, G.; Boscher, C.; Kim, J.S.; El Bakkouri, Y.; Zhang, Y.; Chidiac, R.; Corriveau, J.; Delisle, C.; et al. eNOS controls angiogenic sprouting and retinal neovascularization through the regulation of endothelial cell polarity. Cell. Mol. Life Sci. 2022, 79, 37. [Google Scholar] [CrossRef]
- Erkoc, P.; Schmitt, M.; Ingelfinger, R.; Bischoff-Kont, I.; Kopp, L.; Bode, H.B.; Schiffmann, S.; Furst, R. Xenocoumacin 2 reduces protein biosynthesis and inhibits inflammatory and angiogenesis-related processes in endothelial cells. Biomed. Pharmacother. 2021, 140, 111765. [Google Scholar] [CrossRef]
- Cai, B.X.; Song, L.X.; Hu, H.J.; Han, Z.Z.; Zhou, Y.; Wang, Z.T.; Yang, L. Structures and biological evaluation of phenylpropanoid derivatives from Dendrobium Sonia. Nat. Prod. Res. 2021, 35, 5120–5124. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.N.; de Mejia, E.G.; Wu, J.S.B. Inhibitory effect of a glycoprotein isolated from golden oyster mushroom (Pleurotus citrinopileatus) on the lipopolysaccharide-induced inflammatory reaction in RAW 264.7 Macrophage. J. Agric. Food Chem. 2011, 59, 7092–7097. [Google Scholar] [CrossRef] [PubMed]
- Hankittichai, P.; Buacheen, P.; Pitchakarn, P.; Takuathung, M.N.; Wikan, N.; Smith, D.R.; Potikanond, S.; Nimlamool, W. Artocarpus lakoocha extract inhibits LPS-induced inflammatory response in RAW 264.7 macrophage cells. Int. J. Mol. Sci. 2020, 21, 1355. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Position | 1 a | 2 b | 3 a | |||
|---|---|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | - | 134.1, C | - | 139.5, C | - | 134.2, C |
| 2 | 6.37 (s) | 106.7, CH | 6.33 (d, 1.6) | 110.1, CH | 6.30 (d, 1.4) | 109.9, CH |
| 3 | - | 149.0, C | - | 151.2, C | - | 146.3, C |
| 4 | - | 134.5, C | - | 135.8, C | - | 133.1, C |
| 5 | - | 149.0, C | - | 154.2, C | - | 149.4, C |
| 6 | 6.37 (s) | 106.7, CH | 6.27 (d, 1.6) | 105.2, CH | 6.23 (d, 1.4) | 104.9, C |
| 1″ | 2.78 (m) | 38.1, CH2 | 2.73 (m) | 38.2, CH2 | 2.69 (m) | 38.1, CH2 |
| 2″ | 2.84 (m) | 33.2, CH2 | 2.83 (m) | 33.1, CH2 | 2.82 (m) | 33.3, CH2 |
| 1′ | - | 136.6, C | - | 136.6, C | - | 136.7, C |
| 2′ | - | 147.2, C | - | 147.2, C | - | 148.3, C |
| 3′ | - | 151.0, C | - | 151.2, C | - | 154.0, C |
| 4′ | 6.67 (dd, 7.8, 1.4) | 115.4, CH | 6.67 (dd, 7.7, 1.2) | 115.5, CH | 6.80 (d, 7.8) | 111.6, CH |
| 5′ | 6.80 (t, 7.8) | 125.0, CH | 6.81 (t, 7.7) | 125.1, CH | 6.92 (t, 7.8) | 124.9, CH |
| 6′ | 6.59 (dd, 7.8, 1.4) | 122.1, CH | 6.62 (dd, 7.7, 1.2) | 121.9, CH | 6.70 (d, 7.8) | 123.2, CH |
| 3-OCH3 | 3.75 (s) | 56.6, CH3 | - | - | - | - |
| 4-OCH3 | - | - | 3.72 (s) | 60.9, CH3 | - | - |
| 5-OCH3 | 3.75 (s) | 56.6, CH3 | 3.75 (s) | 56.2, CH3 | 3.71 (s) | 61.0, CH3 |
| 2′-OCH3 | 3.70 (s) | 60.8, CH3 | 3.72 (s) | 60.9, CH3 | 3.71 (s) | 61.0, CH3 |
| 3′-OCH3 | - | - | - | - | 3.79 (s) | 56.1, CH3 |
| Position | δH (J in Hz) | |||
|---|---|---|---|---|
| 4 a | 5 b | 6 a | 7 a | |
| 2 | 7.38 (d, 8.5) | 7.04 (d, 2.5) | 7.06 (s) | 6.58 (d, 7.8) |
| 3 | 6.82 (dd, 8.5, 2.0) | - | - | 7.06 (t, 7.8) |
| 4 | - | 6.81 (dd, 8.8, 2.5) | - | 6.98 (d, 7.8) |
| 5 | 7.07 (d, 2.0) | 7.33 (d, 8.8) | 7.14 (s) | - |
| 1″ | 6.85 (s) | 6.89 (s) | 6.83 (s) | 7.07 (s) |
| 2′ | 6.96 (s) | 6.98 (d, 1.8) | 6.95 (s) | 6.99 (s) |
| 6′ | 6.96 (s) | 6.99 (d, 1.8) | 6.95 (s) | 6.99 (s) |
| 3-OCH3 | - | 3.81 (s) | 3.85 (s) | - |
| 4-OCH3 | 3.83 (s) | - | 3.87 (s) | - |
| 3′-OCH3 | 3.91 (s) | 3.92 (s) | 3.91 (s) | 3.91 (s) |
| 4′-OCH3 | - | - | - | 3.81 (s) |
| Position | δC | |||
|---|---|---|---|---|
| 4 a | 5 b | 6 a | 7 a | |
| 1 | 124.2, C | 131.5, C | 123.2, C | 119.8, C |
| 2 | 121.6, CH | 104.1, CH | 104.0, CH | 108.7, CH |
| 3 | 112.6, CH | 157.6, C | 147.9, C | 126.0, CH |
| 4 | 159.2, C | 113.2, CH | 149.1, C | 103.6, CH |
| 5 | 96.6, CH | 112.0, CH | 100.7, CH | 152.0, C |
| 6 | 156.9, C | 150.9, C | 150.7, C | 152.1, C |
| 1″ | 100.3, CH | 100.7, CH | 96.6, CH | 99.2, CH |
| 2″ | 156.9, C | 158.6, C | 157.0, C | 157.7, C |
| 1′ | 123.1, C | 122.8, C | 123.1, C | 127.7, C |
| 2′ | 106.5, CH | 106.8, CH | 106.4, CH | 106.6, CH |
| 3′ | 149.9, C | 149.9, C | 149.8, C | 154.9, C |
| 4′ | 135.9, C | 136.4, C | 135.8, C | 138.1, C |
| 5′ | 146.8, C | 146.9, C | 146.8, C | 155.3, C |
| 6′ | 101.2, CH | 101.5, CH | 101.1, CH | 101.4, CH |
| 3-OCH3 | - | 56.2, CH3 | 57.0, CH3 | - |
| 4-OCH3 | 56.2, CH3 | - | 56.8, CH3 | - |
| 3′-OCH3 | 56.7, CH3 | 56.7, CH3 | 56.6, CH3 | 56.5, CH3 |
| 4′-OCH3 | - | - | - | 61.0, CH3 |
| Compound | IC50 (μM) a |
|---|---|
| 1 | 32.3 ± 0.82 |
| 2 | 28.6 ± 1.41 |
| 3 | 9.3 ± 1.03 |
| 4 | >50 |
| 5 | >50 |
| 6 | 24.1 ± 1.21 |
| 7 | >50 |
| AH b | 19.2 ± 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, B.; Cai, X.; Xu, T.; Wang, J.; Yu, Y. Structures and Anti-Inflammatory Evaluation of Phenylpropanoid Derivatives from the Aerial Parts of Dioscorea polystachya. Int. J. Mol. Sci. 2022, 23, 10954. https://doi.org/10.3390/ijms231810954
Cai B, Cai X, Xu T, Wang J, Yu Y. Structures and Anti-Inflammatory Evaluation of Phenylpropanoid Derivatives from the Aerial Parts of Dioscorea polystachya. International Journal of Molecular Sciences. 2022; 23(18):10954. https://doi.org/10.3390/ijms231810954
Chicago/Turabian StyleCai, Baixiang, Xinyin Cai, Tao Xu, Jutao Wang, and Yang Yu. 2022. "Structures and Anti-Inflammatory Evaluation of Phenylpropanoid Derivatives from the Aerial Parts of Dioscorea polystachya" International Journal of Molecular Sciences 23, no. 18: 10954. https://doi.org/10.3390/ijms231810954







