Estrogen Regulates the Expression and Localization of YAP in the Uterus of Mice
Abstract
:1. Introduction
2. Results
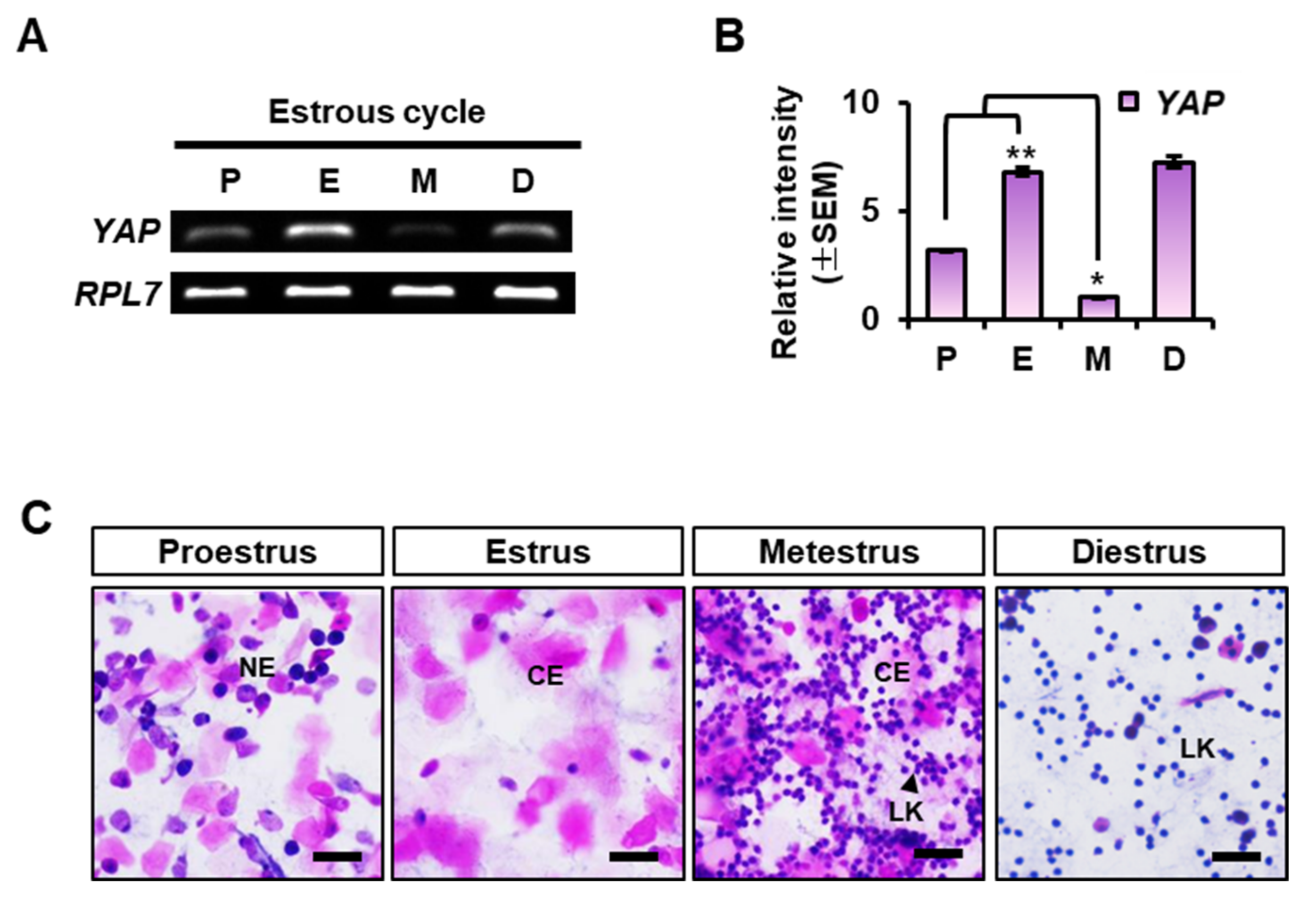
2.1. YAP Expression in the Mouse Uterus at Each Stage of the Estrous Cycle
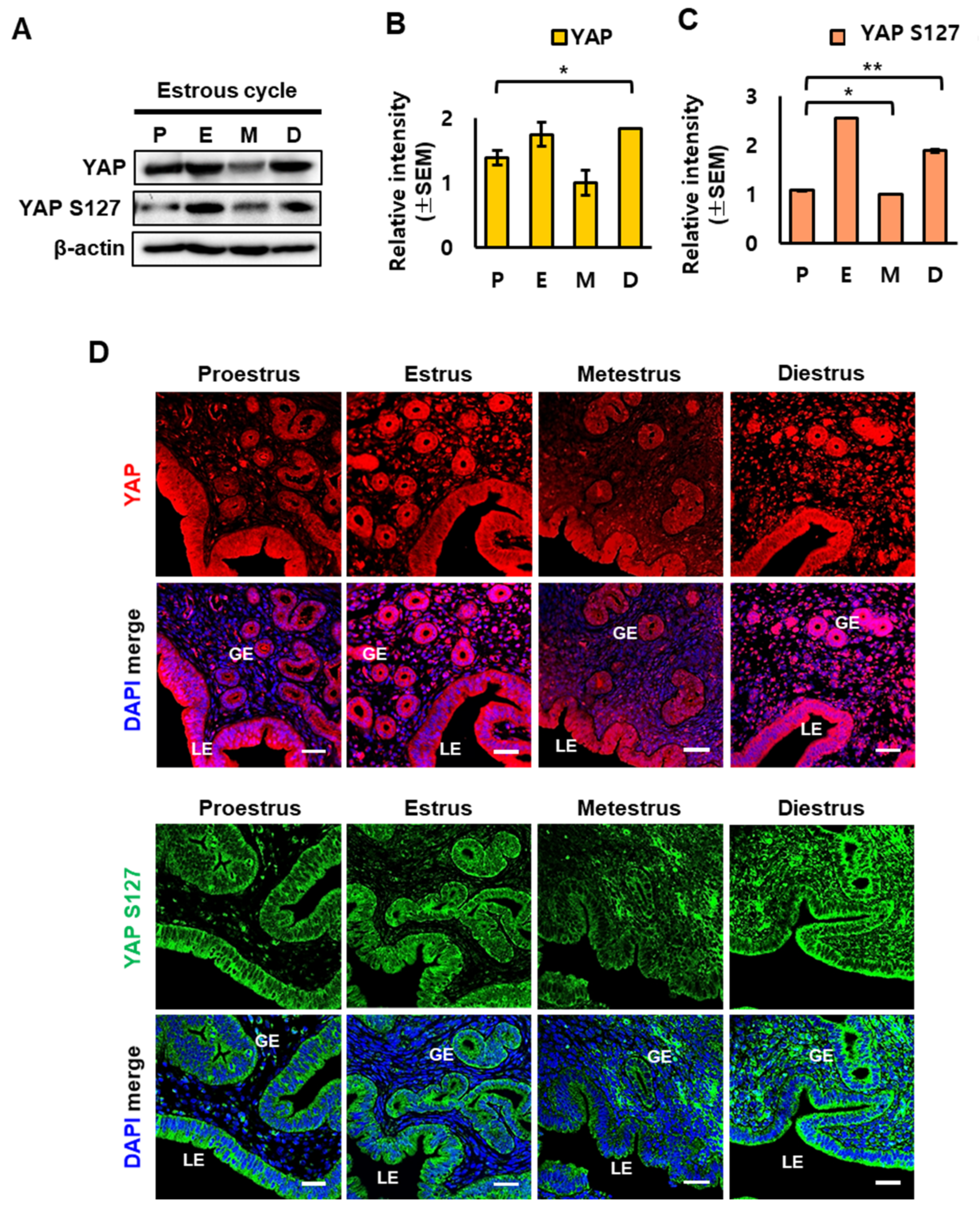
2.2. YAP Localization in Mouse Uterus during the Estrous Cycle
2.3. Regulation of YAP Expression by Estrogen in the Mouse Uterus
2.4. Effect of the Estrogen Receptor Antagonist ICI on the Expression of YAP in OVX Mouse Uterus
2.5. Knockdown of YAP in Human Endometrial Epithelial Cell Lines
3. Discussion
4. Materials and Methods
4.1. Animal Management
4.2. Vaginal Smear Assay
4.3. Ovariectomy and Hormone Treatments
4.4. Immunofluorescence
4.5. RNA Preparation
4.6. Reverse Transcription Polymerase Chain Reaction (RT-PCR) and Real-Time PCR (qPCR)
4.7. Western Blotting
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, K.; Choi, Y. Role of estrogen and RAS signaling in repeated implantation failure. BMB Rep. 2018, 51, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Hwang, S.; Kim, B.; Lee, S.; Kim, H.; Lee, G.; Hong, K.; Song, H.; Choi, Y. Hippo Signaling in the Endometrium. Int. J. Mol. Sci. 2022, 23, 3852. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.C.; Guo, J.H.; Liu, X.; Zhang, R.; Tsang, L.L.; Dong, J.D.; Chen, H.; Yu, M.K.; Jiang, X.; Zhang, X.H.; et al. Activation of the epithelial Na+ channel triggers prostaglandin E(2) release and production required for embryo implantation. Nat. Med. 2012, 18, 1112–1117. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.K.; Lim, H.; Das, S.K.; Reese, J.; Paria, B.C.; Daikoku, T.; Wang, H. Molecular cues to implantation. Endocr. Rev. 2004, 25, 341–373. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Tumaneng, K.; Guan, K.L. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat. Cell Biol. 2011, 13, 877–883. [Google Scholar] [CrossRef]
- Haskins, J.W.; Nguyen, D.X.; Stern, D.F. Neuregulin 1-activated ERBB4 interacts with YAP to induce Hippo pathway target genes and promote cell migration. Sci. Signal. 2014, 7, ra116. [Google Scholar] [CrossRef]
- Shome, D.; von Woedtke, T.; Riedel, K.; Masur, K. The HIPPO Transducer YAP and Its Targets CTGF and Cyr61 Drive a Paracrine Signalling in Cold Atmospheric Plasma-Mediated Wound Healing. Oxidative Med. Cell. Longev. 2020, 2020, 4910280. [Google Scholar] [CrossRef]
- Yu, F.X.; Zhao, B.; Guan, K.L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef]
- Zhao, B.; Li, L.; Lei, Q.; Guan, K.L. The Hippo-YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev. 2010, 24, 862–874. [Google Scholar] [CrossRef]
- Keightley, M.C.; Sales, K.J.; Jabbour, H.N. PGF2alpha-F-prostanoid receptor signalling via ADAMTS1 modulates epithelial cell invasion and endothelial cell function in endometrial cancer. BMC Cancer 2010, 10, 488. [Google Scholar] [CrossRef] [Green Version]
- Mishra, B.; Koshi, K.; Kizaki, K.; Ushizawa, K.; Takahashi, T.; Hosoe, M.; Sato, T.; Ito, A.; Hashizume, K. Expression of ADAMTS1 mRNA in bovine endometrium and placenta during gestation. Domest. Anim. Endocrinol. 2013, 45, 43–48. [Google Scholar] [CrossRef]
- Chan, S.W.; Lim, C.J.; Chong, Y.F.; Pobbati, A.V.; Huang, C.; Hong, W. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. J. Biol. Chem. 2011, 286, 7018–7026. [Google Scholar] [CrossRef]
- Wang, W.; Huang, J.; Chen, J. Angiomotin-like proteins associate with and negatively regulate YAP1. J. Biol. Chem. 2011, 286, 4364–4370. [Google Scholar] [CrossRef]
- Almodovar-Garcia, K.; Kwon, M.; Samaras, S.E.; Davidson, J.M. ANKRD1 acts as a transcriptional repressor of MMP13 via the AP-1 site. Mol. Cell. Biol. 2014, 34, 1500–1511. [Google Scholar] [CrossRef]
- Wu, Y.; Ruggiero, C.L.; Bauman, W.A.; Cardozo, C. Ankrd1 is a transcriptional repressor for the androgen receptor that is downregulated by testosterone. Biochem. Biophys. Res. Commun. 2013, 437, 355–360. [Google Scholar] [CrossRef]
- Findakly, D.; Wang, J. Molecular Profiling of Benign Metastasizing Leiomyoma of the Uterus Revealing Unique Novel Therapeutic Targets. Cureus 2020, 12, e7701. [Google Scholar] [CrossRef]
- Honda, H.; Barrueto, F.F.; Gogusev, J.; Im, D.D.; Morin, P.J. Serial analysis of gene expression reveals differential expression between endometriosis and normal endometrium. Possible roles for AXL and SHC1 in the pathogenesis of endometriosis. Reprod. Biol. Endocrinol. 2008, 6, 59. [Google Scholar] [CrossRef]
- Moon, S.; Lee, O.H.; Lee, S.; Lee, J.; Park, H.; Park, M.; Chang, E.M.; Park, K.H.; Choi, Y. STK3/4 Expression Is Regulated in Uterine Endometrial Cells during the Estrous Cycle. Cells 2019, 8, 1643. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.; Barrera, J.; Matthews, K.; Pan, D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005, 122, 421–434. [Google Scholar] [CrossRef]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007, 21, 2747–2761. [Google Scholar] [CrossRef] [Green Version]
- Moroishi, T.; Hansen, C.G.; Guan, K.L. The emerging roles of YAP and TAZ in cancer. Nat. Rev. Cancer 2015, 15, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Totaro, A.; Panciera, T.; Piccolo, S. YAP/TAZ upstream signals and downstream responses. Nat. Cell Biol. 2018, 20, 888–899. [Google Scholar] [CrossRef]
- Yuan, M.; Tomlinson, V.; Lara, R.; Holliday, D.; Chelala, C.; Harada, T.; Gangeswaran, R.; Manson-Bishop, C.; Smith, P.; Danovi, S.A.; et al. Yes-associated protein (YAP) functions as a tumor suppressor in breast. Cell Death Differ. 2008, 15, 1752–1759. [Google Scholar] [CrossRef]
- Li, H.; Wolfe, A.; Septer, S.; Edwards, G.; Zhong, X.; Abdulkarim, A.B.; Ranganathan, S.; Apte, U. Deregulation of Hippo kinase signalling in human hepatic malignancies. Liver Int. Off. J. Int. Assoc. Study Liver 2012, 32, 38–47. [Google Scholar] [CrossRef]
- Cho, M.; Lee, O.H.; Chang, E.M.; Lee, S.; Moon, S.; Lee, J.; Park, H.; Park, K.H.; Park, C.; Hong, K.; et al. BIRC5 Expression is Regulated in Uterine Epithelium During the Estrous Cycle. Genes 2020, 11, 282. [Google Scholar] [CrossRef]
- Matsumoto, H.; Sato, E. Uterine angiogenesis during implantation and decidualization in mice. Reprod. Med. Biol. 2006, 5, 81–86. [Google Scholar] [CrossRef]
- Luan, L.; Ding, T.; Stinnett, A.; Reese, J.; Paria, B.C. Adherens junction proteins in the hamster uterus: Their contributions to the success of implantation. Biol. Reprod. 2011, 85, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Zaitseva, M.; Holdsworth-Carson, S.J.; Waldrip, L.; Nevzorova, J.; Martelotto, L.; Vollenhoven, B.J.; Rogers, P.A. Aberrant expression and regulation of NR2F2 and CTNNB1 in uterine fibroids. Reproduction 2013, 146, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Cheng, Y.; Suzuki, N.; Deguchi, M.; Sato, Y.; Takae, S.; Ho, C.H.; Kawamura, N.; Tamura, M.; Hashimoto, S.; et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc. Natl. Acad. Sci. USA 2013, 110, 17474–17479. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, S.G.; Kim, J.I.; Park, J.H.; Kim, S.K.; Cho, D.J.; Kim, H. Implication of ADAM-8, -9, -10, -12, -15, -17, and ADAMTS-1 in implantational remodeling of a mouse uterus. Yonsei Med. J. 2006, 47, 558–567. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, H.; Lee, S.J.; Choi, Y.M.; Lee, S.J.; Lee, J.Y. Abundance of ADAM-8, -9, -10, -12, -15 and -17 and ADAMTS-1 in mouse uterus during the oestrous cycle. Reprod. Fertil. Dev. 2005, 17, 543–555. [Google Scholar] [CrossRef]
- Ng, Y.H.; Zhu, H.; Pallen, C.J.; Leung, P.C.; MacCalman, C.D. Differential effects of interleukin-1beta and transforming growth factor-beta1 on the expression of the inflammation-associated protein, ADAMTS-1, in human decidual stromal cells in vitro. Hum. Reprod. 2006, 21, 1990–1999. [Google Scholar] [CrossRef]
- Das, S.K.; Chakraborty, I.; Paria, B.C.; Wang, X.N.; Plowman, G.; Dey, S.K. Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol. Endocrinol. 1995, 9, 691–705. [Google Scholar] [CrossRef]
- Zhu, C.; Wei, Y.; Wei, X. AXL receptor tyrosine kinase as a promising anti-cancer approach: Functions, molecular mechanisms and clinical applications. Mol. Cancer 2019, 18, 153. [Google Scholar] [CrossRef]
- Divine, L.M.; Nguyen, M.R.; Meller, E.; Desai, R.A.; Arif, B.; Rankin, E.B.; Bligard, K.H.; Meyerson, C.; Hagemann, I.S.; Massad, M.; et al. AXL modulates extracellular matrix protein expression and is essential for invasion and metastasis in endometrial cancer. Oncotarget 2016, 7, 77291–77305. [Google Scholar] [CrossRef]
- Champlin, A.K.; Dorr, D.L.; Gates, A.H. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol. Reprod. 1973, 8, 491–494. [Google Scholar] [CrossRef]
- Bae, S.; Kwon, H.; Yoon, H.; Park, M.; Kim, H.R.; Song, H.; Hong, K.; Choi, Y. Estrogen-dependent expression of sine oculis homeobox 1 in the mouse uterus during the estrous cycle. Biochem. Biophys. Res. Commun. 2016, 472, 489–495. [Google Scholar] [CrossRef]
- Kim, H.R.; Cho, K.S.; Kim, E.; Lee, O.H.; Yoon, H.; Lee, S.; Moon, S.; Park, M.; Hong, K.; Na, Y.; et al. Rapid expression of RASD1 is regulated by estrogen receptor-dependent intracellular signaling pathway in the mouse uterus. Mol. Cell. Endocrinol. 2017, 446, 32–39. [Google Scholar] [CrossRef]
- Lee, J.; Park, H.; Moon, S.; Do, J.T.; Hong, K.; Choi, Y. Expression and Regulation of CD73 during the Estrous Cycle in Mouse Uterus. Int. J. Mol. Sci. 2021, 22, 9403. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.A.; Shim, C.; Khang, I.; Lee, K.A.; Park, Y.M.; Kang, B.M.; Kim, K. Identification of estrogen-regulated genes in the mouse uterus using a delayed-implantation model. Mol. Reprod. Dev. 2003, 64, 405–413. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Gene Symbol | NCBI ID | Sequence (5′–3′) | Annealing Temp. |
|---|---|---|---|
| Human YAP | NM_001195044.1 | Fwd: CACAGCTCAGCATCTTCGAC | 60 °C |
| Rev: TATTCTGCTGCACTGGTGGA | |||
| Mouse YAP | NM_001171147.1 | Fwd: TCCAACCAGCAGCAGCAAAT | 60 °C |
| Rev: TTCCGTATTGCCTGCCGAAA | |||
| AMOT | NM_001113490.2 | Fwd: CATCACCACCAACAACAGCA | 60 °C |
| Rev: GTCTCCACCTTCTGCAGTCT | |||
| AMOTL1 | NM_001301007.2 | Fwd: AGACAGACTCCTCCAGCCTA | 60 °C |
| Rev: CGTAGTGGAGGCTATGGAGG | |||
| AMOTL2 | NM_001363943.2 | Fwd: GCTGCAGAGAGACAATGAGC | 60 °C |
| Rev: AGTCTTGCAGCCTCCTCATT | |||
| MCL1 | NM_021960.5 | Fwd: GCTGCATCGAACCATTAGCA | 60 °C |
| Rev: ATGCCAAACCAGCTCCTACT | |||
| ANKRD1 | NM_014391.3 | Fwd: TGAATCCACAGCCATCCACT | 63 °C |
| Rev: TCCTTCTCTGTCTTTGGCGT | |||
| AXL | NM_001699.6 | Fwd: GAGGGAGAGTTTGGAGCTGT | 63 °C |
| Rev: GAAACAGACACCGATGAGCC | |||
| ADAMTS1 | NM_006988.5 | Fwd: GCAGAGCACTATGACACAGC | 60 °C |
| Rev: CACGTGGCCTAATTCATGGG | |||
| CTNNA1 | NM_001290307.3 | Fwd: ATTAGTGGGGCTGCCTTGAT | 60 °C |
| Rev: GTCCCTGGTCTTCTTGGTCA | |||
| GAPDH | AY340484.1 | Fwd: ATGGGGAAGGTGAAGGTCG | 60 °C |
| Rev: ATTGTTGCCATCAATGACCC | |||
| RPL7 | NM_011291.5 | Fwd: TTTGTCATCAGAATTCGAGG | 60 °C |
| Rev: CTGACTTCAGGTTGGGGTAC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, S.; Lee, O.-H.; Kim, B.; Park, J.; Hwang, S.; Lee, S.; Lee, G.; Kim, H.; Song, H.; Hong, K.; et al. Estrogen Regulates the Expression and Localization of YAP in the Uterus of Mice. Int. J. Mol. Sci. 2022, 23, 9772. https://doi.org/10.3390/ijms23179772
Moon S, Lee O-H, Kim B, Park J, Hwang S, Lee S, Lee G, Kim H, Song H, Hong K, et al. Estrogen Regulates the Expression and Localization of YAP in the Uterus of Mice. International Journal of Molecular Sciences. 2022; 23(17):9772. https://doi.org/10.3390/ijms23179772
Chicago/Turabian StyleMoon, Sohyeon, Ok-Hee Lee, Byeongseok Kim, Jinju Park, Semi Hwang, Siyoung Lee, Giwan Lee, Hyukjung Kim, Hyuk Song, Kwonho Hong, and et al. 2022. "Estrogen Regulates the Expression and Localization of YAP in the Uterus of Mice" International Journal of Molecular Sciences 23, no. 17: 9772. https://doi.org/10.3390/ijms23179772





