Neonatal Rat Glia Cultured in Physiological Normoxia for Modeling Neuropathological Conditions In Vitro
Abstract
:1. Introduction
2. Results
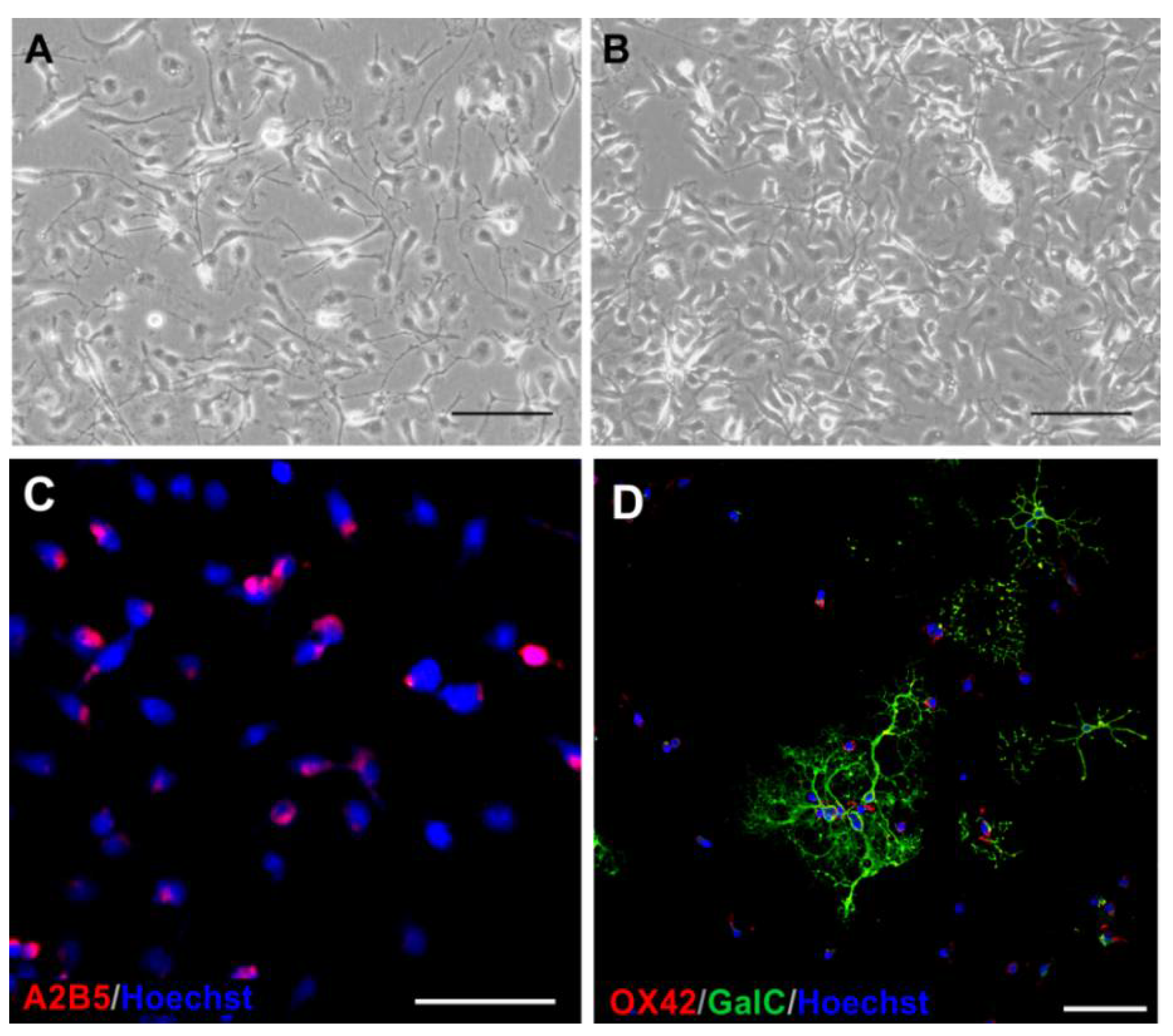
2.1. The Glial Cells of Rat Neonatal Brains
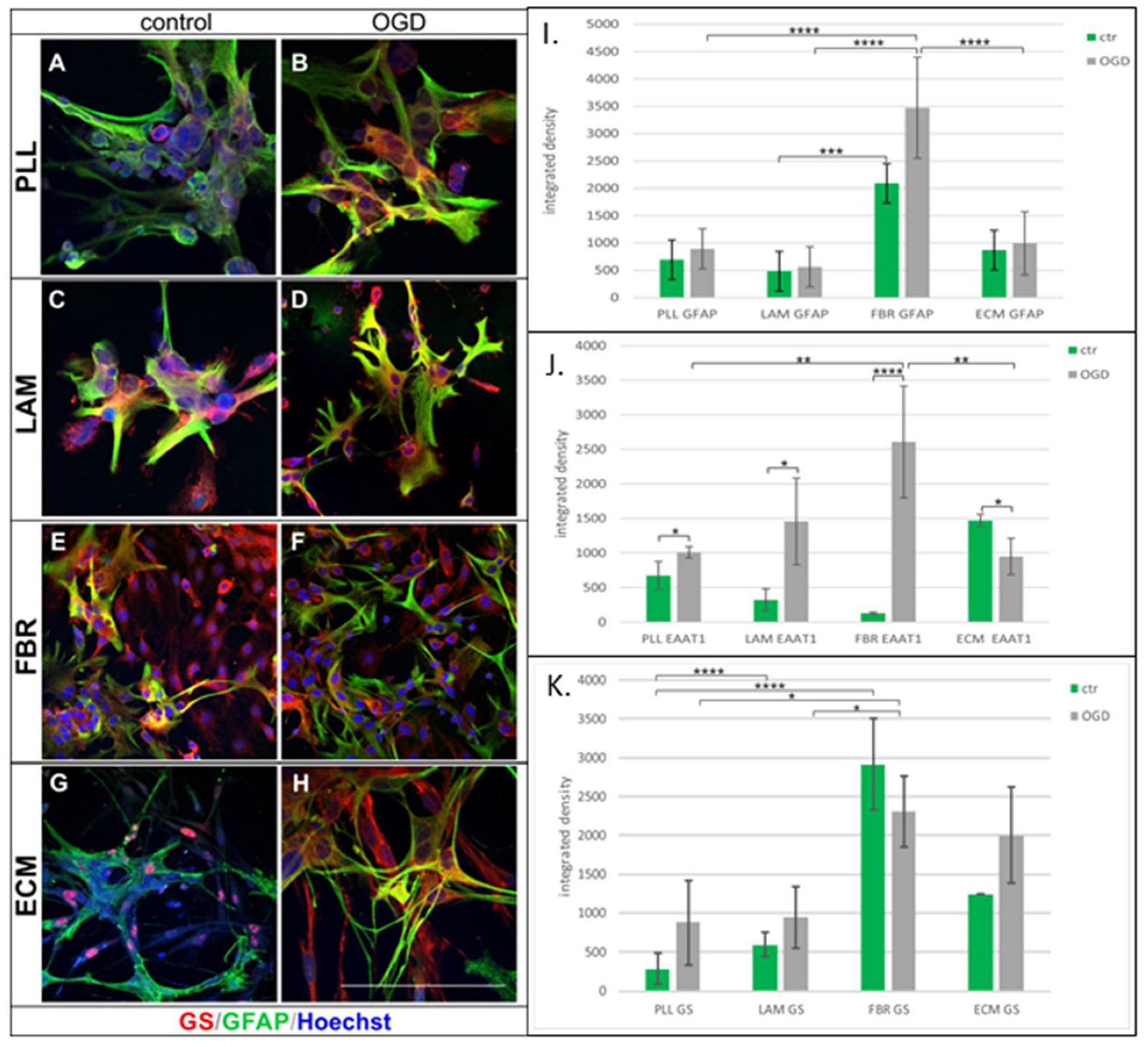
2.2. Effect of Cell Culturing on Biomaterials
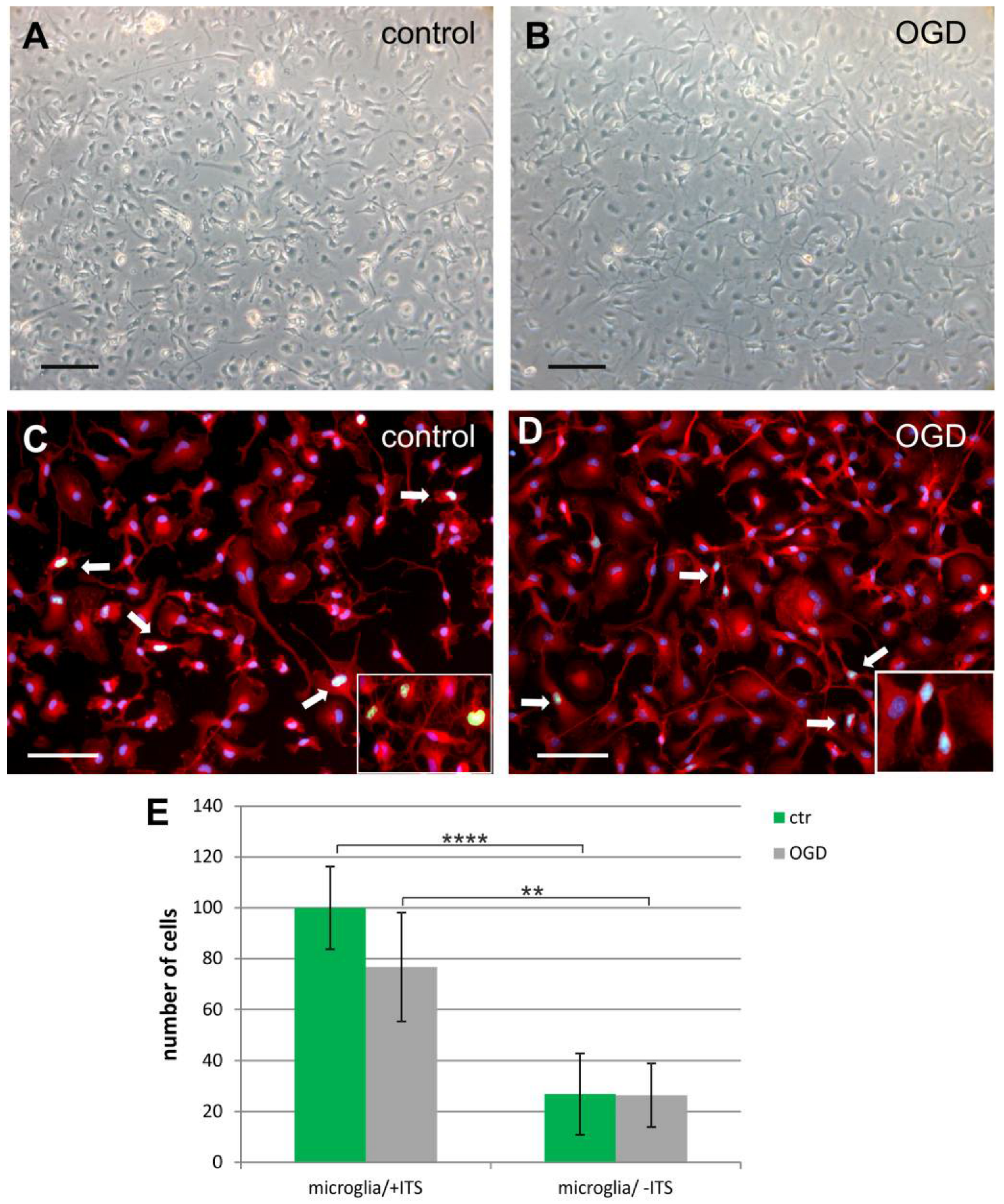
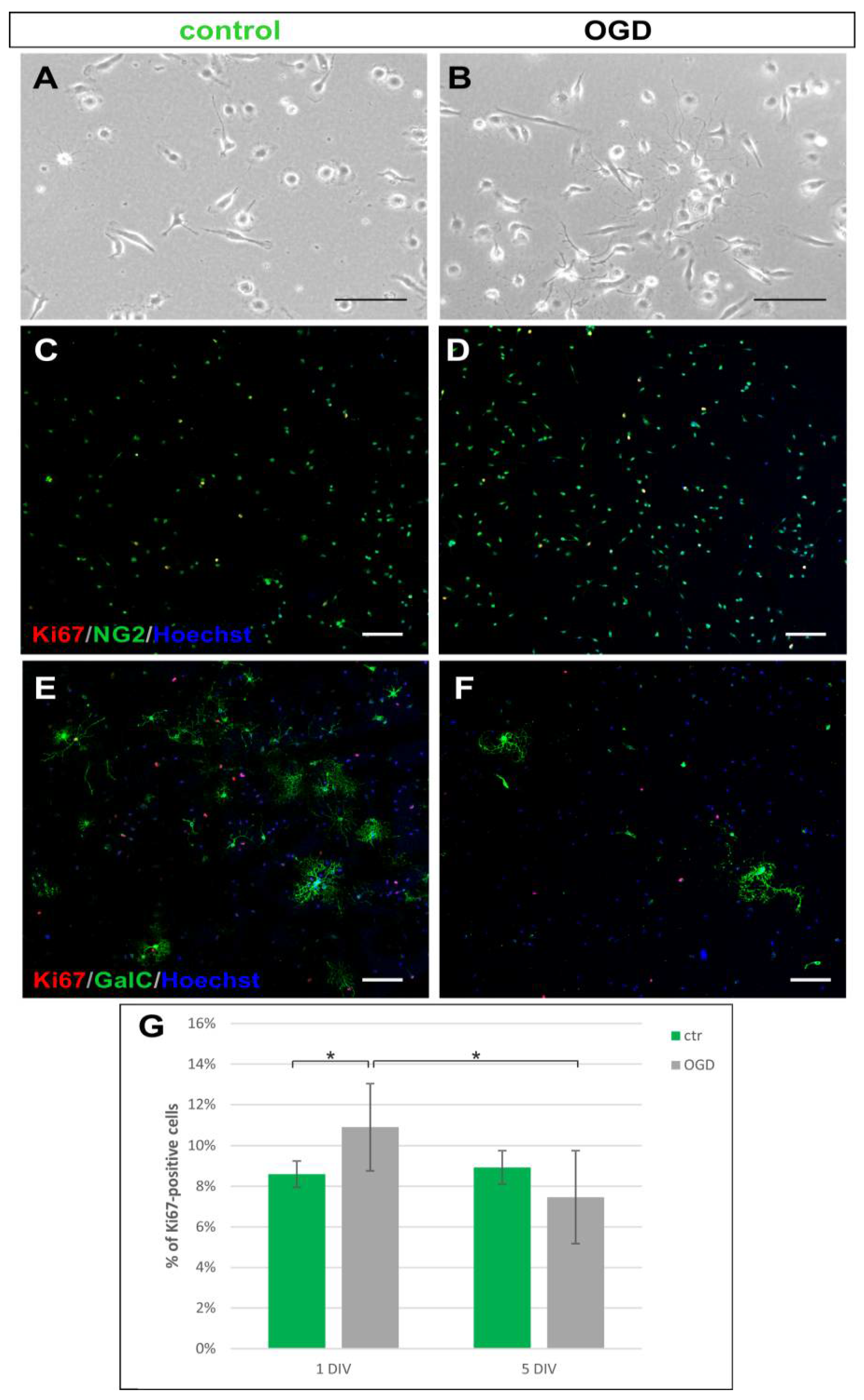
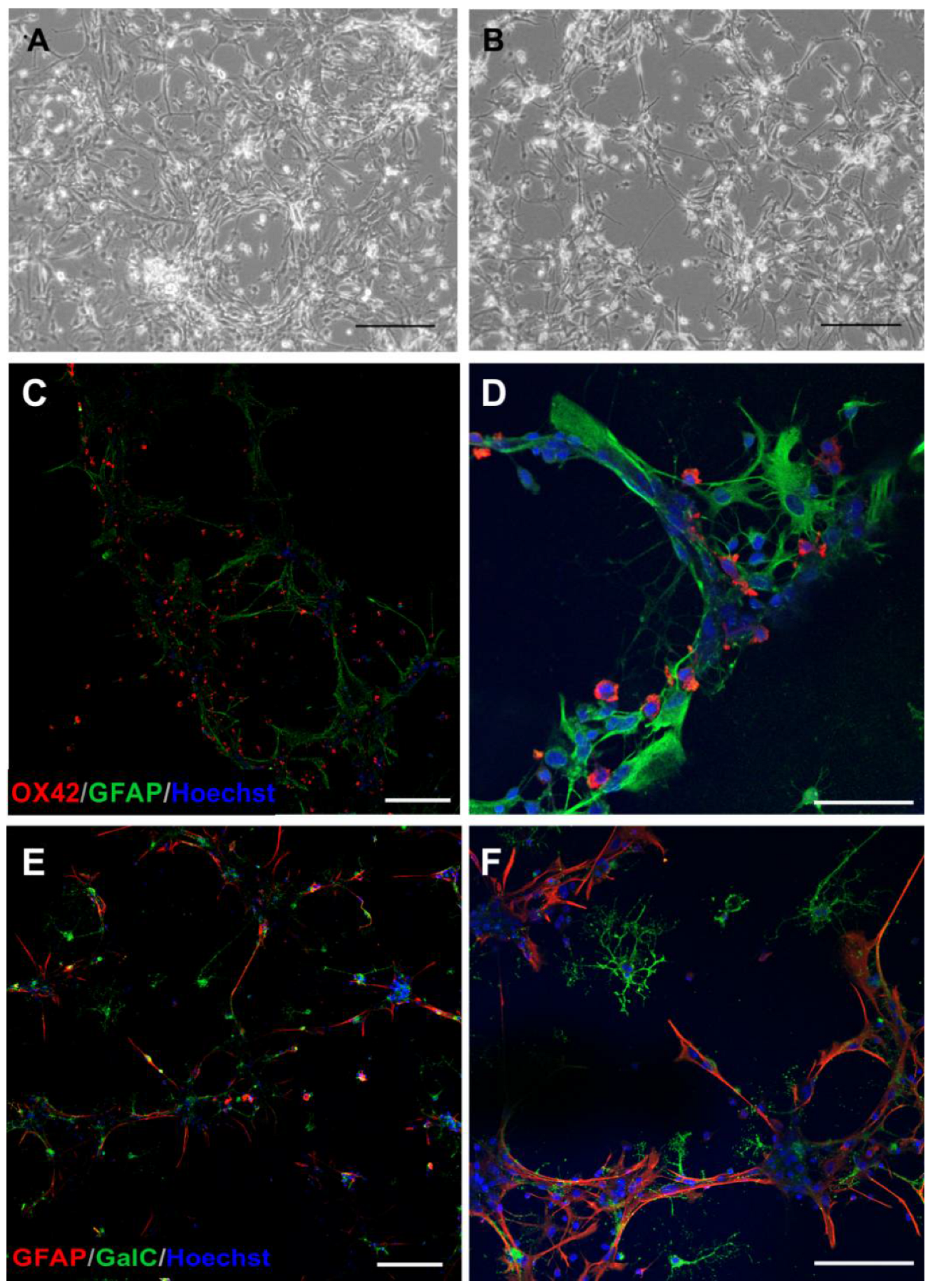
2.3. Cocultures of Glial Cells
3. Discussion
4. Materials and Methods
4.1. The Primary Mixed Glial Culture
4.2. Isolation of Microglia
4.3. Separation of Oligodendrocyte Progenitors
4.4. Detachment of Astrocytes
4.5. Cell Counting
4.6. Application of the Selected Biomaterials for Cell Culturing
4.7. Cocultures of Glial Cells
4.8. Neonatal Hypoxia-Ischemia Model In Vitro
4.9. Identification of Glial Cell Phenotype by Immunostaining
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAS | antibiotic–antimycotic solution |
| AD | Alzheimer’s Disease |
| ALS | amyotrophic lateral sclerosis |
| CNS | central nervous system |
| DIV | days in vitro |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| EAAT1 | glutamate aspartate transporter 1, GLAST-1 |
| ECM Gel | Extracellular Matrix Gel, Matrigel |
| FBR | fibronectin |
| FBS | fetal bovine serum |
| GS | glutamine synthetase |
| ITS | Insulin-Transferrin-Selenium-A Solution |
| LAM | laminin |
| LPS | lipopolysaccharide |
| MS | multiple sclerosis |
| NG2 cells | oligodendrocyte progenitors |
| OGD | oxygen-glucose deprivation |
| OPCs | oligodendrocyte progenitor cells |
| PFA | paraformaldehyde |
| PLL | poly-L-lysine hydrobromide |
| RT | room temperature |
| TNFα | tumor necrosis factor α |
References
- Verkhratsky, A.; Zorec, R. Large-Scale Proteomics Highlights Glial Role in Neurodegeneration. Cell Metab. 2020, 32, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Hanson, J.E.; Sheng, M. Microglia in Alzheimer’s Disease. J. Cell Biol. 2018, 217, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M.; De La Rocha, I.C.; Rivera, A. Oligodendroglial Cells in Alzheimer’s Disease. Adv. Exp. Med. Biol. 2019, 1175, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Sheeler, C.; Rosa, J.-G.; Ferro, A.; McAdams, B.; Borgenheimer, E.; Cvetanovic, M. Glia in Neurodegeneration: The Housekeeper, the Defender and the Perpetrator. Int. J. Mol. Sci. 2020, 21, 9188. [Google Scholar] [CrossRef] [PubMed]
- Zia, S.; Rawji, K.S.; Michaels, N.J.; Burr, M.; Kerr, B.J.; Healy, L.M.; Plemel, J.R. Microglia Diversity in Health and Multiple Sclerosis. Front. Immunol. 2020, 11, 588021. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Parpura, V.; Rodriguez-Arellano, J.J.; Zorec, R. Astroglia in Alzheimer’s Disease. Adv. Exp. Med. Biol. 2019, 1175, 273–324. [Google Scholar] [CrossRef] [PubMed]
- Arranz, A.M.; De Strooper, B. The Role of Astroglia in Alzheimer’s Disease: Pathophysiology and Clinical Implications. Lancet Neurol. 2019, 18, 406–414. [Google Scholar] [CrossRef]
- Kulkarni, B.; Cruz-Martins, N.; Kumar, D. Microglia in Alzheimer’s Disease: An Unprecedented Opportunity as Prospective Drug Target. Mol. Neurobiol. 2022, 59, 2678–2693. [Google Scholar] [CrossRef]
- Reemst, K.; Noctor, S.C.; Lucassen, P.J.; Hol, E.M. The Indispensable Roles of Microglia and Astrocytes during Brain Development. Front. Hum. Neurosci. 2016, 10, 566. [Google Scholar] [CrossRef] [Green Version]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Puebla, M.; Tapia, P.J.; Espinoza, H. Key Role of Astrocytes in Postnatal Brain and Retinal Angiogenesis. Int. J. Mol. Sci 2022, 23, 2646. [Google Scholar] [CrossRef]
- Ginhoux, F.; Lim, S.; Hoeffel, G.; Low, D.; Huber, T. Origin and Differentiation of Microglia. Front. Cell Neurosci. 2013, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Tay, T.L.; Savage, J.C.; Hui, C.W.; Bisht, K.; Tremblay, M.-È. Microglia across the Lifespan: From Origin to Function in Brain Development, Plasticity and Cognition. J. Physiol. 2017, 595, 1929–1945. [Google Scholar] [CrossRef] [Green Version]
- Utz, S.G.; See, P.; Mildenberger, W.; Thion, M.S.; Silvin, A.; Lutz, M.; Ingelfinger, F.; Rayan, N.A.; Lelios, I.; Buttgereit, A.; et al. Early Fate Defines Microglia and Non-Parenchymal Brain Macrophage Development. Cell 2020, 181, 557–573.e18. [Google Scholar] [CrossRef]
- Augusto-Oliveira, M.; Arrifano, G.P.; Takeda, P.Y.; Lopes-Araújo, A.; Santos-Sacramento, L.; Anthony, D.C.; Verkhratsky, A.; Crespo-Lopez, M.E. Astroglia-Specific Contributions to the Regulation of Synapses, Cognition and Behaviour. Neurosci. Biobehav. Rev. 2020, 118, 331–357. [Google Scholar] [CrossRef]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef] [Green Version]
- Mosser, C.-A.; Baptista, S.; Arnoux, I.; Audinat, E. Microglia in CNS Development: Shaping the Brain for the Future. Prog. Neurobiol. 2017, 149–150, 1–20. [Google Scholar] [CrossRef]
- Nelson, L.H.; Peketi, P.; Lenz, K.M. Microglia Regulate Cell Genesis in a Sex-Dependent Manner in the Neonatal Hippocampus. Neuroscience 2021, 453, 237–255. [Google Scholar] [CrossRef]
- Cunningham, C. Microglia and Neurodegeneration: The Role of Systemic Inflammation. Glia 2013, 61, 71–90. [Google Scholar] [CrossRef]
- Mazaheri, F.; Breus, O.; Durdu, S.; Haas, P.; Wittbrodt, J.; Gilmour, D.; Peri, F. Distinct Roles for BAI1 and TIM-4 in the Engulfment of Dying Neurons by Microglia. Nat. Commun. 2014, 5, 4046. [Google Scholar] [CrossRef] [Green Version]
- Wake, H.; Moorhouse, A.J.; Jinno, S.; Kohsaka, S.; Nabekura, J. Resting Microglia Directly Monitor the Functional State of Synapses in Vivo and Determine the Fate of Ischemic Terminals. J. Neurosci. 2009, 29, 3974–3980. [Google Scholar] [CrossRef] [Green Version]
- Marinelli, C.; Bertalot, T.; Zusso, M.; Skaper, S.D.; Giusti, P. Systematic Review of Pharmacological Properties of the Oligodendrocyte Lineage. Front. Cell Neurosci. 2016, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Zeis, T.; Enz, L.; Schaeren-Wiemers, N. The Immunomodulatory Oligodendrocyte. Brain Res. 2016, 1641, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Linnerbauer, M.; Rothhammer, V. Protective Functions of Reactive Astrocytes Following Central Nervous System Insult. Front. Immunol. 2020, 11, 573256. [Google Scholar] [CrossRef]
- Giovannoni, F.; Quintana, F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020, 41, 805–819. [Google Scholar] [CrossRef]
- Akay, L.A.; Effenberger, A.H.; Tsai, L.-H. Cell of All Trades: Oligodendrocyte Precursor Cells in Synaptic, Vascular, and Immune Function. Genes Dev. 2021, 35, 180–198. [Google Scholar] [CrossRef]
- Peferoen, L.; Kipp, M.; van der Valk, P.; van Noort, J.M.; Amor, S. Oligodendrocyte-Microglia Cross-Talk in the Central Nervous System. Immunology 2014, 141, 302–313. [Google Scholar] [CrossRef]
- Jha, M.K.; Jo, M.; Kim, J.-H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Linnerbauer, M.; Wheeler, M.A.; Quintana, F.J. Astrocyte Crosstalk in CNS Inflammation. Neuron 2020, 108, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, L.; Cuddapah, S.; Costa, M. Oxidative Stress under Ambient and Physiological Oxygen Tension in Tissue Culture. Curr. Pharmacol. Rep. 2016, 2, 64–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janowska, J.; Ziemka-Nalecz, M.; Sypecka, J. The Differentiation of Rat Oligodendroglial Cells Is Highly Influenced by the Oxygen Tension: In Vitro Model Mimicking Physiologically Normoxic Conditions. Int. J. Mol. Sci. 2018, 19, 331. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Lowry, W.E. Manipulation of Neural Progenitor Fate through the Oxygen Sensing Pathway. Methods 2018, 133, 44–53. [Google Scholar] [CrossRef]
- Bal-Price, A.K.; Hogberg, H.T.; Buzanska, L.; Lenas, P.; van Vliet, E.; Hartung, T. In Vitro Developmental Neurotoxicity (DNT) Testing: Relevant Models and Endpoints. Neurotoxicology 2010, 31, 545–554. [Google Scholar] [CrossRef]
- Thompson-Branch, A.; Havranek, T. Neonatal Hypoglycemia. Pediatr. Rev. 2017, 38, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Chappe, M.K. Hypoglycemia in High-Risk Infants Within the Immediate Postnatal Period. Neonatal Netw. 2020, 39, 263–267. [Google Scholar] [CrossRef]
- Fleiss, B.; Van Steenwinckel, J.; Bokobza, C.; Shearer, I.K.; Ross-Munro, E.; Gressens, P. Microglia-Mediated Neurodegeneration in Perinatal Brain Injuries. Biomolecules 2021, 11, 99. [Google Scholar] [CrossRef]
- Shao, R.; Sun, D.; Hu, Y.; Cui, D. White Matter Injury in the Neonatal Hypoxic-Ischemic Brain and Potential Therapies Targeting Microglia. J. Neurosci. Res. 2021, 99, 991–1008. [Google Scholar] [CrossRef]
- McCarthy, K.D.; de Vellis, J. Preparation of Separate Astroglial and Oligodendroglial Cell Cultures from Rat Cerebral Tissue. J. Cell Biol. 1980, 85, 890–902. [Google Scholar] [CrossRef] [Green Version]
- Janowska, J.; Gargas, J.; Ziemka-Nalecz, M.; Zalewska, T.; Sypecka, J. Oligodendrocyte Response to Pathophysiological Conditions Triggered by Episode of Perinatal Hypoxia-Ischemia: Role of IGF-1 Secretion by Glial Cells. Mol. Neurobiol. 2020, 57, 4250–4268. [Google Scholar] [CrossRef]
- Allen, N.J.; Lyons, D.A. Glia as Architects of Central Nervous System Formation and Function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Lago-Baldaia, I.; Fernandes, V.M.; Ackerman, S.D. More Than Mortar: Glia as Architects of Nervous System Development and Disease. Front. Cell Dev. Biol. 2020, 8, 611269. [Google Scholar] [CrossRef]
- Amor, S.; McNamara, N.B.; Gerrits, E.; Marzin, M.C.; Kooistra, S.M.; Miron, V.E.; Nutma, E. White Matter Microglia Heterogeneity in the CNS. Acta Neuropathol. 2022, 143, 125–141. [Google Scholar] [CrossRef]
- Wlodarczyk, A.; Holtman, I.R.; Krueger, M.; Yogev, N.; Bruttger, J.; Khorooshi, R.; Benmamar-Badel, A.; de Boer-Bergsma, J.J.; Martin, N.A.; Karram, K.; et al. A Novel Microglial Subset Plays a Key Role in Myelinogenesis in Developing Brain. EMBO J. 2017, 36, 3292–3308. [Google Scholar] [CrossRef]
- Bar, E.; Barak, B. Microglia Roles in Synaptic Plasticity and Myelination in Homeostatic Conditions and Neurodevelopmental Disorders. Glia 2019, 67, 2125–2141. [Google Scholar] [CrossRef]
- Kim, S.; Son, Y. Astrocytes Stimulate Microglial Proliferation and M2 Polarization In Vitro through Crosstalk between Astrocytes and Microglia. Int. J. Mol. Sci. 2021, 22, 8800. [Google Scholar] [CrossRef]
- Corvace, F.; Faustmann, T.J.; Faustmann, P.M.; Ismail, F.S. Anti-Inflammatory Properties of Lacosamide in an Astrocyte-Microglia Co-Culture Model of Inflammation. Eur. J. Pharmacol. 2022, 915, 174696. [Google Scholar] [CrossRef]
- Das, A.; Kim, S.H.; Arifuzzaman, S.; Yoon, T.; Chai, J.C.; Lee, Y.S.; Park, K.S.; Jung, K.H.; Chai, Y.G. Transcriptome Sequencing Reveals That LPS-Triggered Transcriptional Responses in Established Microglia BV2 Cell Lines Are Poorly Representative of Primary Microglia. J. Neuroinflamm. 2016, 13, 182. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Yao, X.; Taylor, N.; Bai, Y.; Lovenberg, T.; Bhattacharya, A. RNA Sequencing Analysis Reveals Quiescent Microglia Isolation Methods from Postnatal Mouse Brains and Limitations of BV2 Cells. J. Neuroinflamm. 2018, 15, 153. [Google Scholar] [CrossRef]
- Bohlen, C.J.; Bennett, F.C.; Tucker, A.F.; Collins, H.Y.; Mulinyawe, S.B.; Barres, B.A. Diverse Requirements for Microglial Survival, Specification, and Function Revealed by Defined-Medium Cultures. Neuron 2017, 94, 759–773.e8. [Google Scholar] [CrossRef] [Green Version]
- Collins, H.Y.; Bohlen, C.J. Isolation and Culture of Rodent Microglia to Promote a Dynamic Ramified Morphology in Serum-Free Medium. J. Vis. Exp. 2018, 133, e57122. [Google Scholar] [CrossRef]
- Yu, T.; Yu, H.; Zhang, B.; Wang, D.; Li, B.; Zhu, J.; Zhu, W. Promising Neuroprotective Function for M2 Microglia in Kainic Acid-Induced Neurotoxicity via the Down-Regulation of NF-ΚB and Caspase 3 Signaling Pathways. Neuroscience 2019, 406, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Kell, D.B.; Pretorius, E. The Role of Lipopolysaccharide-Induced Cell Signalling in Chronic Inflammation. Chronic Stress 2022, 6, 24705470221076390. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 Polarization and Metabolic States. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.; Lively, S.; Schlichter, L.C. Responses of Rat and Mouse Primary Microglia to Pro- and Anti-Inflammatory Stimuli: Molecular Profiles, K+ Channels and Migration. J. Neuroinflamm. 2017, 14, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quarta, A.; Berneman, Z.; Ponsaerts, P. Neuroprotective Modulation of Microglia Effector Functions Following Priming with Interleukin 4 and 13: Current Limitations in Understanding Their Mode-of-Action. Brain Behav. Immun. 2020, 88, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.G.; Stockton, M.E. Premyelinating Oligodendrocytes: Mechanisms Underlying Cell Survival and Integration. Front. Cell Dev. Biol. 2021, 9, 714169. [Google Scholar] [CrossRef] [PubMed]
- Dansu, D.K.; Sauma, S.; Casaccia, P. Oligodendrocyte Progenitors as Environmental Biosensors. Semin. Cell Dev. Biol. 2021, 116, 38–44. [Google Scholar] [CrossRef]
- Li, D.; Liu, X.; Liu, T.; Liu, H.; Tong, L.; Jia, S.; Wang, Y.-F. Neurochemical Regulation of the Expression and Function of Glial Fibrillary Acidic Protein in Astrocytes. Glia 2020, 68, 878–897. [Google Scholar] [CrossRef]
- Brenner, M.; Messing, A. Regulation of GFAP Expression. ASN Neuro 2021, 13, 1759091420981206. [Google Scholar] [CrossRef]
- He, Y.; Hakvoort, T.B.M.; Vermeulen, J.L.M.; Labruyère, W.T.; De Waart, D.R.; Van Der Hel, W.S.; Ruijter, J.M.; Uylings, H.B.M.; Lamers, W.H. Glutamine Synthetase Deficiency in Murine Astrocytes Results in Neonatal Death. Glia 2010, 58, 741–754. [Google Scholar] [CrossRef]
- Anlauf, E.; Derouiche, A. Glutamine Synthetase as an Astrocytic Marker: Its Cell Type and Vesicle Localization. Front. Endocrinol. 2013, 4, 144. [Google Scholar] [CrossRef] [Green Version]
- Rose, C.F.; Verkhratsky, A.; Parpura, V. Astrocyte Glutamine Synthetase: Pivotal in Health and Disease. Biochem. Soc. Trans. 2013, 41, 1518–1524. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kadluczka, J.; Kuter, K.Z. Beyond the GFAP-Astrocyte Protein Markers in the Brain. Biomolecules 2021, 11, 1361. [Google Scholar] [CrossRef]
- Van Strien, M.E.; Brevé, J.J.P.; Fratantoni, S.; Schreurs, M.W.J.; Bol, J.G.J.M.; Jongenelen, C.A.M.; Drukarch, B.; van Dam, A.-M. Astrocyte-Derived Tissue Transglutaminase Interacts with Fibronectin: A Role in Astrocyte Adhesion and Migration? PLoS ONE 2011, 6, e25037. [Google Scholar] [CrossRef] [Green Version]
- Faissner, A.; Pyka, M.; Geissler, M.; Sobik, T.; Frischknecht, R.; Gundelfinger, E.D.; Seidenbecher, C. Contributions of Astrocytes to Synapse Formation and Maturation—Potential Functions of the Perisynaptic Extracellular Matrix. Brain Res. Rev. 2010, 63, 26–38. [Google Scholar] [CrossRef]
- Song, I.; Dityatev, A. Crosstalk between Glia, Extracellular Matrix and Neurons. Brain Res. Bull. 2018, 136, 101–108. [Google Scholar] [CrossRef]
- Long, K.R.; Huttner, W.B. How the Extracellular Matrix Shapes Neural Development. Open Biol. 2019, 9, 180216. [Google Scholar] [CrossRef] [Green Version]
- Walma, D.A.C.; Yamada, K.M. The Extracellular Matrix in Development. Development 2020, 147, dev175596. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Burnside, E.R. Moving beyond the Glial Scar for Spinal Cord Repair. Nat. Commun. 2019, 10, 3879. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, Y.; Jia, H.; Qi, Y.; Tu, S.; Shao, A. Affective Immunology: The Crosstalk Between Microglia and Astrocytes Plays Key Role? Front. Immunol. 2020, 11, 1818. [Google Scholar] [CrossRef]
- Li, Z.; Song, Y.; He, T.; Wen, R.; Li, Y.; Chen, T.; Huang, S.; Wang, Y.; Tang, Y.; Shen, F.; et al. M2 Microglial Small Extracellular Vesicles Reduce Glial Scar Formation via the MiR-124/STAT3 Pathway after Ischemic Stroke in Mice. Theranostics 2021, 11, 1232–1248. [Google Scholar] [CrossRef]
- Wasseff, S.K.; Scherer, S.S. Cx32 and Cx47 Mediate Oligodendrocyte:Astrocyte and Oligodendrocyte: Oligodendrocyte Gap Junction Coupling. Neurobiol. Dis. 2011, 42, 506–513. [Google Scholar] [CrossRef] [Green Version]
- Lana, D.; Ugolini, F.; Nosi, D.; Wenk, G.L.; Giovannini, M.G. The Emerging Role of the Interplay Among Astrocytes, Microglia, and Neurons in the Hippocampus in Health and Disease. Front. Aging Neurosci. 2021, 13, 651973. [Google Scholar] [CrossRef]
- Matejuk, A.; Ransohoff, R.M. Crosstalk Between Astrocytes and Microglia: An Overview. Front. Immunol. 2020, 11, 1416. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Hu, J.; Wu, Z.; Meng, J.; Hayashi, Y.; Nakanishi, H.; Qing, H.; Ni, J. Differential Expression and Distinct Roles of Proteinase-Activated Receptor 2 in Microglia and Neurons in Neonatal Mouse Brain after Hypoxia-Ischemic Injury. Mol. Neurobiol. 2022, 59, 717–730. [Google Scholar] [CrossRef]
- Domingues, H.S.; Portugal, C.C.; Socodato, R.; Relvas, J.B. Oligodendrocyte, Astrocyte, and Microglia Crosstalk in Myelin Development, Damage, and Repair. Front. Cell Dev. Biol. 2016, 4, 71. [Google Scholar] [CrossRef]
- Yang, J.; Wang, T.; Jin, X.; Wang, G.; Zhao, F.; Jin, Y. Roles of Crosstalk between Astrocytes and Microglia in Triggering Neuroinflammation and Brain Edema Formation in 1,2-Dichloroethane-Intoxicated Mice. Cells 2021, 10, 2647. [Google Scholar] [CrossRef]
- Dambach, H.; Hinkerohe, D.; Prochnow, N.; Stienen, M.N.; Moinfar, Z.; Haase, C.G.; Hufnagel, A.; Faustmann, P.M. Glia and Epilepsy: Experimental Investigation of Antiepileptic Drugs in an Astroglia/Microglia Co-Culture Model of Inflammation. Epilepsia 2014, 55, 184–192. [Google Scholar] [CrossRef]
- Kirkley, K.S.; Popichak, K.A.; Afzali, M.F.; Legare, M.E.; Tjalkens, R.B. Microglia Amplify Inflammatory Activation of Astrocytes in Manganese Neurotoxicity. J. Neuroinflamm. 2017, 14, 99. [Google Scholar] [CrossRef]
- Ismail, F.S.; Faustmann, P.M. Experimental Investigations of Antiepileptic Drugs in Astrocytes-Microglia Co-Cultures Suggest Possible Protective Effects on Astrocytes during Early Epileptogenesis. Epilepsia 2021, 62, 2297–2298. [Google Scholar] [CrossRef]
- Soto-Verdugo, J.; Ortega, A. Critical Involvement of Glial Cells in Manganese Neurotoxicity. Biomed. Res. Int. 2021, 2021, 1596185. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Cai, Z.; Rhodes, P.G. Effects of Lipopolysaccharide on Oligodendrocyte Progenitor Cells Are Mediated by Astrocytes and Microglia. J. Neurosci. Res. 2000, 62, 510–520. [Google Scholar] [CrossRef]
- Clemente, D.; Ortega, M.C.; Melero-Jerez, C.; de Castro, F. The Effect of Glia-Glia Interactions on Oligodendrocyte Precursor Cell Biology during Development and in Demyelinating Diseases. Front. Cell Neurosci. 2013, 7, 268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Miron, V.E. Microglia-Driven Regulation of Oligodendrocyte Lineage Cells, Myelination, and Remyelination. J. Leukoc. Biol. 2017, 101, 1103–1108. [Google Scholar] [CrossRef]
- Fernández-López, D.; Natarajan, N.; Ashwal, S.; Vexler, Z.S. Mechanisms of Perinatal Arterial Ischemic Stroke. J. Cereb. Blood Flow Metab. 2014, 34, 921–932. [Google Scholar] [CrossRef]
- Giraud, A.; Guiraut, C.; Chevin, M.; Chabrier, S.; Sébire, G. Role of Perinatal Inflammation in Neonatal Arterial Ischemic Stroke. Front. Neurol. 2017, 8, 612. [Google Scholar] [CrossRef] [Green Version]
- Ziemka-Nalecz, M.; Janowska, J.; Strojek, L.; Jaworska, J.; Zalewska, T.; Frontczak-Baniewicz, M.; Sypecka, J. Impact of Neonatal Hypoxia-Ischaemia on Oligodendrocyte Survival, Maturation and Myelinating Potential. J. Cell Mol. Med. 2018, 22, 207–222. [Google Scholar] [CrossRef] [Green Version]
- Babu, M.; Singh, N.; Datta, A. In Vitro Oxygen Glucose Deprivation Model of Ischemic Stroke: A Proteomics-Driven Systems Biological Perspective. Mol. Neurobiol. 2022, 59, 2363–2377. [Google Scholar] [CrossRef]
- Yao, S.-Y.; Natarajan, C.; Sriram, S. NNOS Mediated Mitochondrial Injury in LPS Stimulated Oligodendrocytes. Mitochondrion 2012, 12, 336–344. [Google Scholar] [CrossRef]
- Ghosh, M.; Xu, Y.; Pearse, D.D. Cyclic AMP Is a Key Regulator of M1 to M2a Phenotypic Conversion of Microglia in the Presence of Th2 Cytokines. J. Neuroinflamm. 2016, 13, 9. [Google Scholar] [CrossRef] [Green Version]
- Karababa, A.; Groos-Sahr, K.; Albrecht, U.; Keitel, V.; Shafigullina, A.; Görg, B.; Häussinger, D. Ammonia Attenuates LPS-Induced Upregulation of Pro-Inflammatory Cytokine MRNA in Co-Cultured Astrocytes and Microglia. Neurochem. Res. 2017, 42, 737–749. [Google Scholar] [CrossRef]
- Silwedel, C.; Speer, C.P.; Haarmann, A.; Fehrholz, M.; Claus, H.; Buttmann, M.; Glaser, K. Novel Insights into Neuroinflammation: Bacterial Lipopolysaccharide, Tumor Necrosis Factor α, and Ureaplasma Species Differentially Modulate Atypical Chemokine Receptor 3 Responses in Human Brain Microvascular Endothelial Cells. J. Neuroinflamm. 2018, 15, 156. [Google Scholar] [CrossRef] [Green Version]
- Adán Lanceta, V.; Martín Ruiz, N.; Benito Costey, S.; Alijarde Lorente, R.; Martínez de Zabarte Fernández, J.M. A Neonatal Hypocalcemia Due to Maternal Vitamin D Deficiency. Reviewing Supplementation. An. Pediatría (Engl. Ed.) 2022, 96, 153–154. [Google Scholar] [CrossRef]
- Moss, C.R. Neonatal Hypocalcemia in the Infant of a Diabetic Mother. Neonatal Netw. 2020, 39, 200–204. [Google Scholar] [CrossRef]
- Morales-Garcia, J.A.; Alonso-Gil, S.; Santos, Á.; Perez-Castillo, A. Phosphodiesterase 7 Regulation in Cellular and Rodent Models of Parkinson’s Disease. Mol. Neurobiol. 2020, 57, 806–822. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Fan, J.-K.; Gu, L.; Yang, H.-M.; Zhan, S.-Q.; Zhang, H. Metabotropic Glutamate Receptor 5 Inhibits α-Synuclein-Induced Microglia Inflammation to Protect from Neurotoxicity in Parkinson’s Disease. J. Neuroinflamm. 2021, 18, 23. [Google Scholar] [CrossRef]
- Song, W.; Zukor, H.; Liberman, A.; Kaduri, S.; Arvanitakis, Z.; Bennett, D.A.; Schipper, H.M. Astroglial Heme Oxygenase-1 and the Origin of Corpora Amylacea in Aging and Degenerating Neural Tissues. Exp. Neurol. 2014, 254, 78–89. [Google Scholar] [CrossRef] [Green Version]
- Facci, L.; Barbierato, M.; Zusso, M.; Skaper, S.D.; Giusti, P. Serum Amyloid A Primes Microglia for ATP-Dependent Interleukin-1β Release. J. Neuroinflamm. 2018, 15, 164. [Google Scholar] [CrossRef] [Green Version]
- Jang, K.-B.; You, M.-J.; Yang, B.; Rim, C.; Kim, H.-J.; Kwon, M.-S. Persistent Acidic Environment Induces Impaired Phagocytosis via ERK in Microglia. Neurochem. Res. 2022, 47, 1341–1353. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Hsu, S.-M.; Shie, F.-S.; Shiao, Y.-J.; Chao, L.-J.; Chen, H.-W.; Yao, H.-H.; Chien, M.A.; Lin, C.-C.; Tsay, H.-J. Reduced Mitochondria Membrane Potential and Lysosomal Acidification Are Associated with Decreased Oligomeric Aβ Degradation Induced by Hyperglycemia: A Study of Mixed Glia Cultures. PLoS ONE 2022, 17, e0260966. [Google Scholar] [CrossRef]
- Blaževski, J.; Petković, F.; Momčilović, M.; Paschke, R.; Kaluđerović, G.N.; Mostarica Stojković, M.; Miljković, D. Betulinic Acid Regulates Generation of Neuroinflammatory Mediators Responsible for Tissue Destruction in Multiple Sclerosis in Vitro. Acta Pharmacol. Sin. 2013, 34, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Tatomir, A.; Tegla, C.A.; Martin, A.; Boodhoo, D.; Nguyen, V.; Sugarman, A.J.; Mekala, A.; Anselmo, F.; Talpos-Caia, A.; Cudrici, C.; et al. RGC-32 Regulates Reactive Astrocytosis and Extracellular Matrix Deposition in Experimental Autoimmune Encephalomyelitis. Immunol. Res. 2018, 66, 445–461. [Google Scholar] [CrossRef]
- Exil, V.; Ping, L.; Yu, Y.; Chakraborty, S.; Caito, S.W.; Wells, K.S.; Karki, P.; Lee, E.; Aschner, M. Activation of MAPK and FoxO by Manganese (Mn) in Rat Neonatal Primary Astrocyte Cultures. PLoS ONE 2014, 9, e94753. [Google Scholar] [CrossRef]
- Chang, J.; Yang, B.; Zhou, Y.; Yin, C.; Liu, T.; Qian, H.; Xing, G.; Wang, S.; Li, F.; Zhang, Y.; et al. Acute Methylmercury Exposure and the Hypoxia-Inducible Factor-1α Signaling Pathway under Normoxic Conditions in the Rat Brain and Astrocytes in Vitro. Environ. Health Perspect. 2019, 127, 127006. [Google Scholar] [CrossRef] [Green Version]
- Papp, A.; Horváth, T.; Igaz, N.; Gopisetty, M.K.; Kiricsi, M.; Berkesi, D.S.; Kozma, G.; Kónya, Z.; Wilhelm, I.; Patai, R.; et al. Presence of Titanium and Toxic Effects Observed in Rat Lungs, Kidneys, and Central Nervous System in Vivo and in Cultured Astrocytes in Vitro on Exposure by Titanium Dioxide Nanorods. Int. J. Nanomed. 2020, 15, 9939–9960. [Google Scholar] [CrossRef]




| Culture Density | GFAP EAAT1 GS | |||
|---|---|---|---|---|
| PLL | - | - | ↑ | - |
| LAM | - | - | ↑ | - |
| FBR | ↑ | ↑ | ↑ | - |
| ECM | - | - | ↓ | ↑ |
| Isolation Methods: | ||
|---|---|---|
| Microglia | 1 h shaking, 160 rpm, repeated | |
| OPCs | 15–20 h shaking, 160 rpm, repeated | |
| Astrocytes | mild trypsinization, 5 min, 37 °C | |
| Cell density [(cell/cm2) and % of the cultured neonatal glial population | ||
| Primary mixed glial culture | 5 × 104 | n/a |
| Microglia | 4 × 104 | 7.19 ± 3.17% |
| OPCs | 2 × 104 | 20.87 ± 8.7% |
| Astrocytes | 4 × 104 | 72 ± 10% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gargas, J.; Janowska, J.; Ziabska, K.; Ziemka-Nalecz, M.; Sypecka, J. Neonatal Rat Glia Cultured in Physiological Normoxia for Modeling Neuropathological Conditions In Vitro. Int. J. Mol. Sci. 2022, 23, 6000. https://doi.org/10.3390/ijms23116000
Gargas J, Janowska J, Ziabska K, Ziemka-Nalecz M, Sypecka J. Neonatal Rat Glia Cultured in Physiological Normoxia for Modeling Neuropathological Conditions In Vitro. International Journal of Molecular Sciences. 2022; 23(11):6000. https://doi.org/10.3390/ijms23116000
Chicago/Turabian StyleGargas, Justyna, Justyna Janowska, Karolina Ziabska, Malgorzata Ziemka-Nalecz, and Joanna Sypecka. 2022. "Neonatal Rat Glia Cultured in Physiological Normoxia for Modeling Neuropathological Conditions In Vitro" International Journal of Molecular Sciences 23, no. 11: 6000. https://doi.org/10.3390/ijms23116000







