Loss of P2Y12 Has Behavioral Effects in the Adult Mouse
Abstract
:1. Introduction
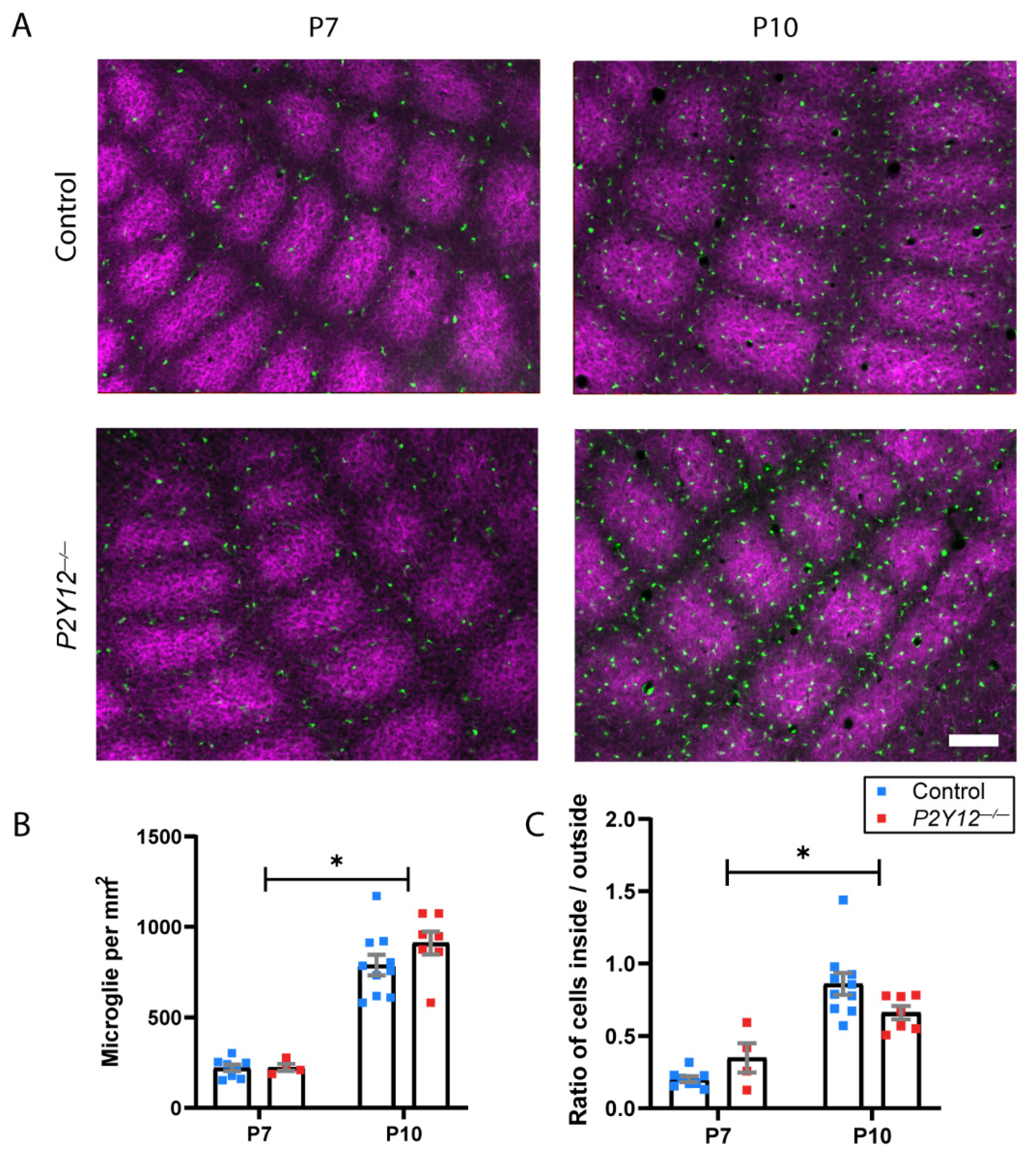
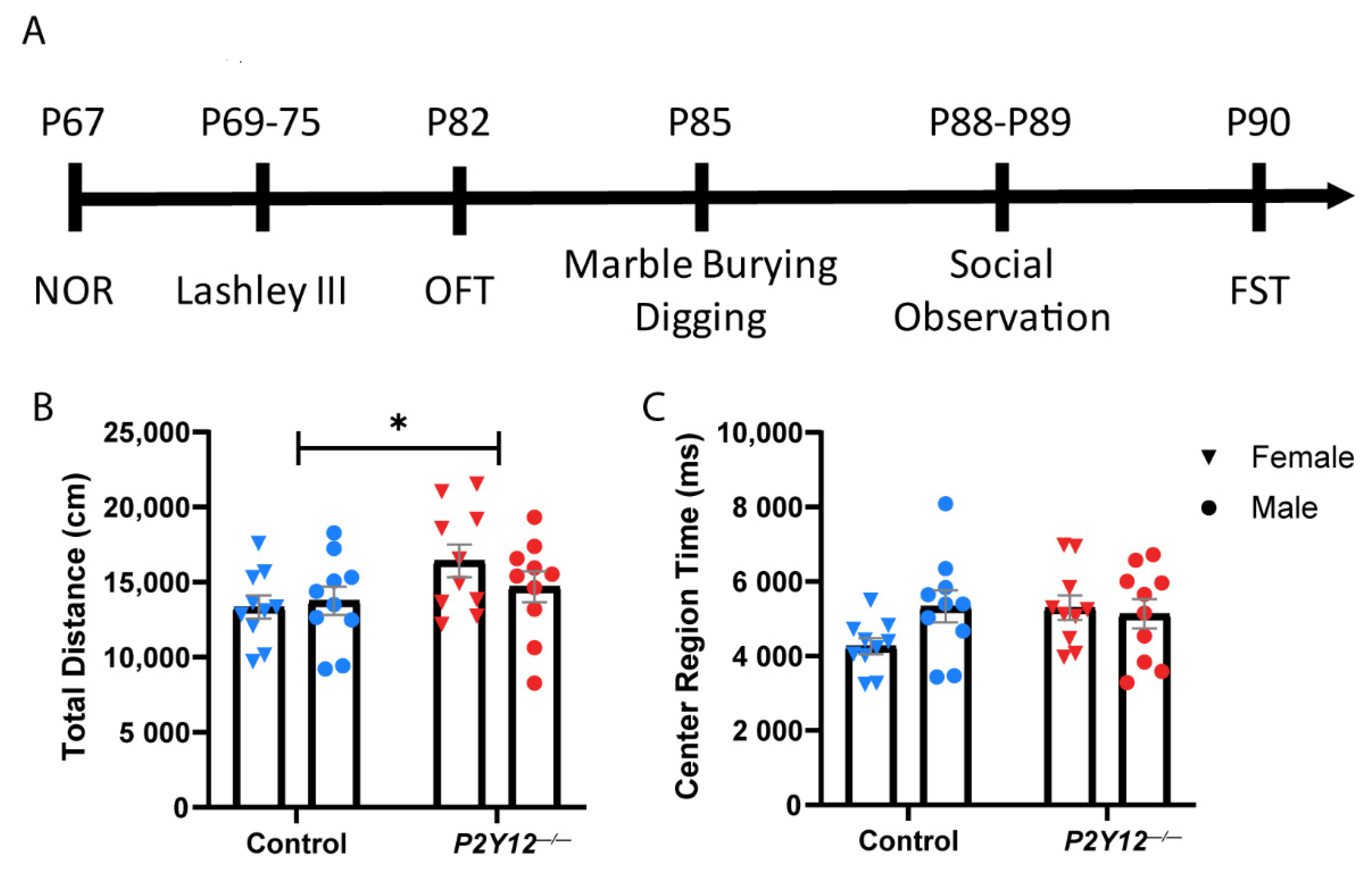
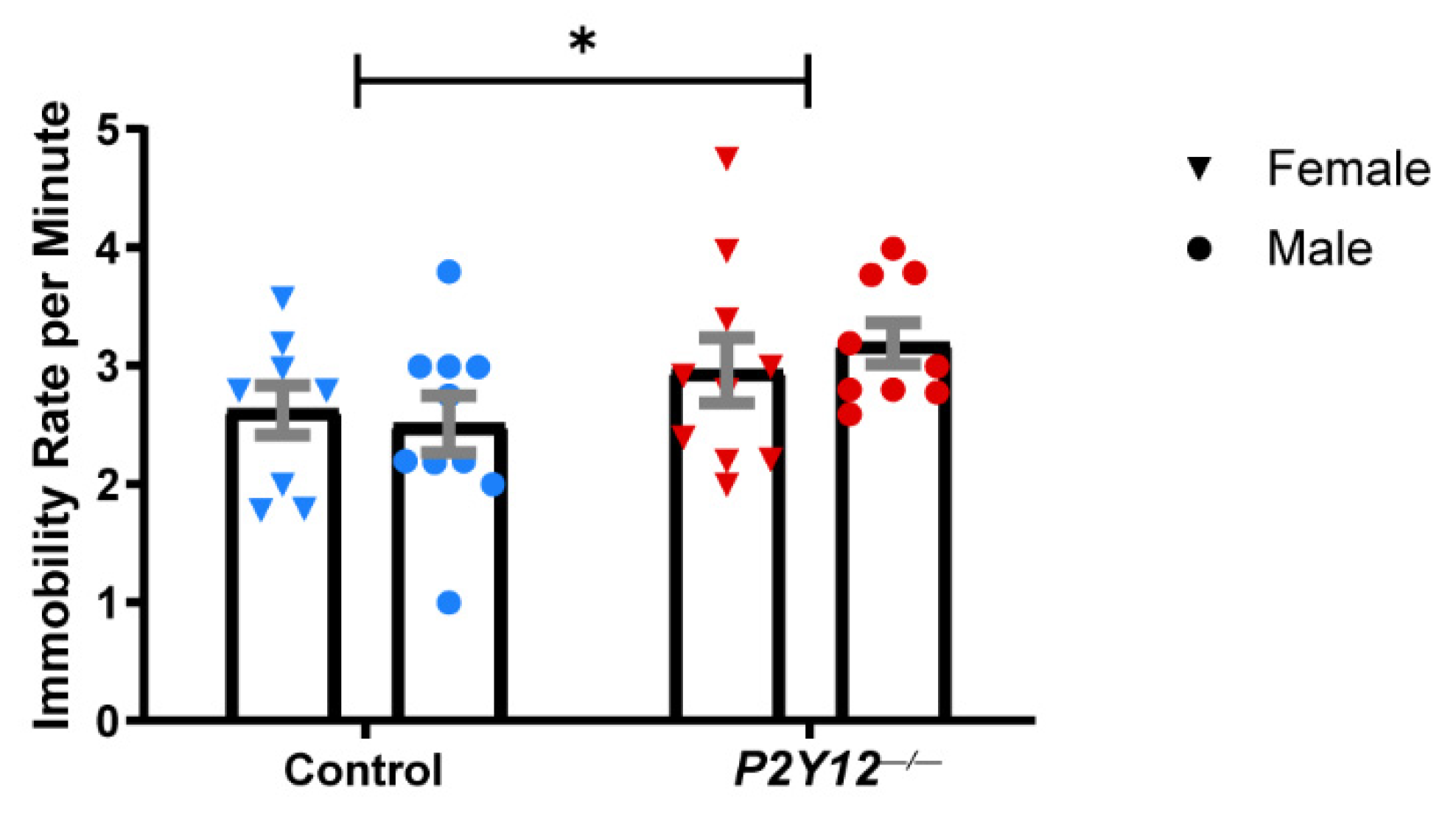
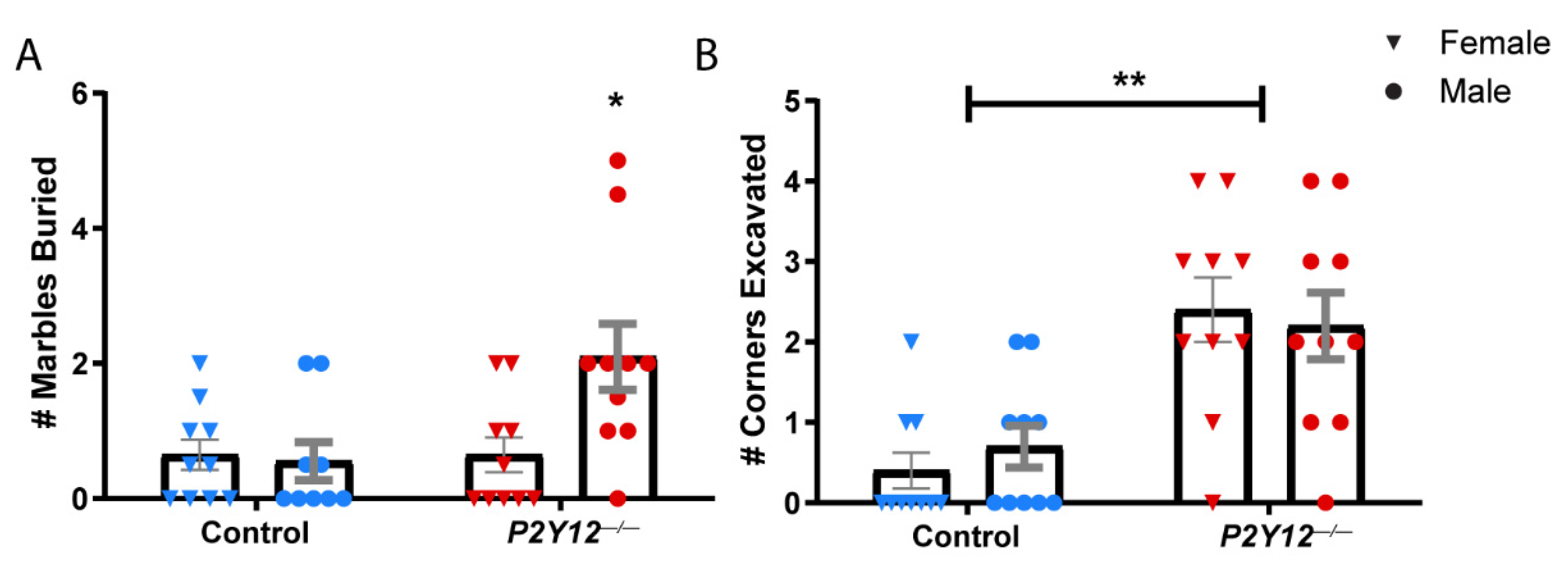
2. Results
3. Discussion
3.1. P2Y12 as a Heterogeneous Regulator of Synaptic Plasticity
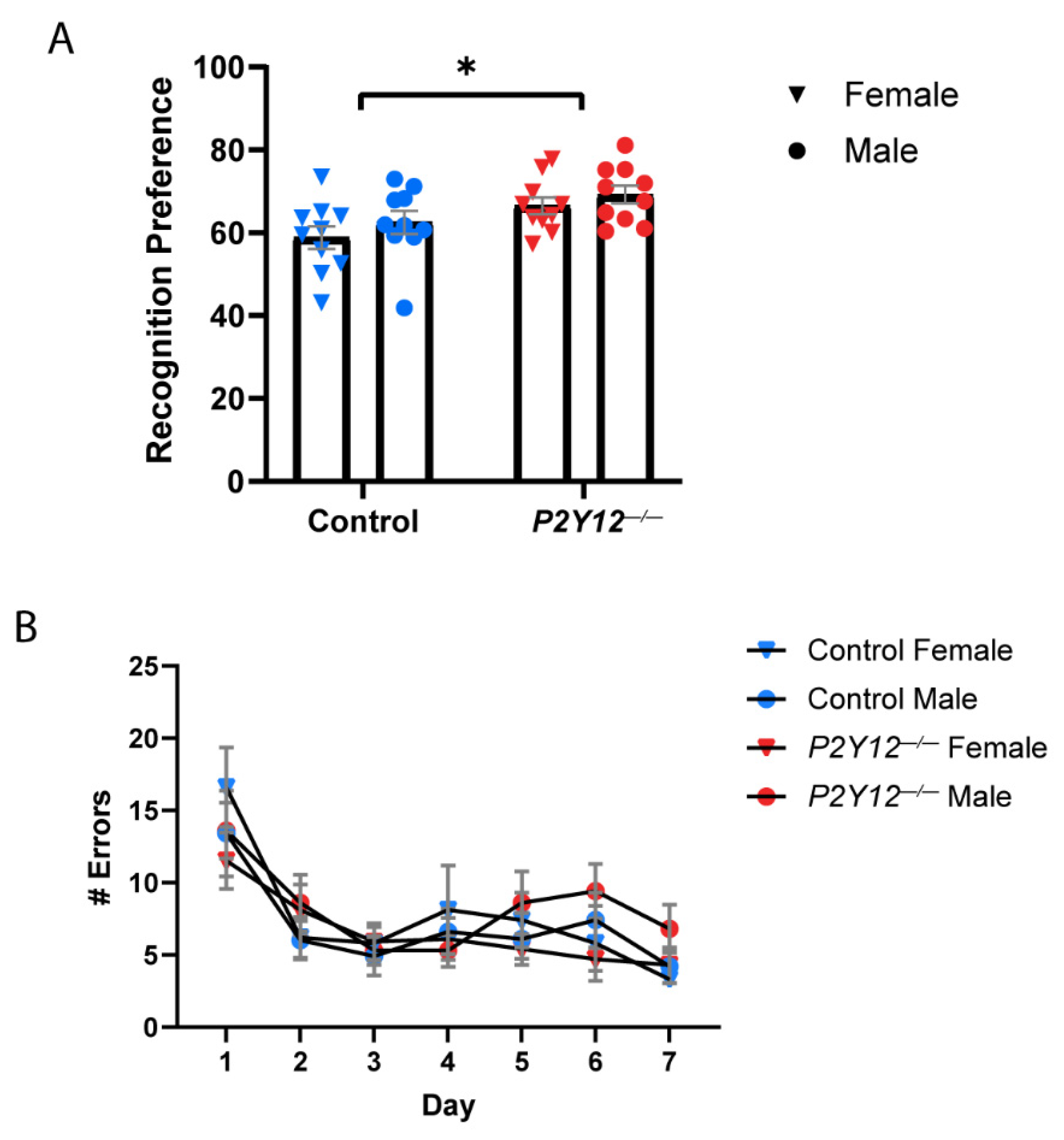
3.2. P2Y12 and Behavior
3.3. Purinergic Signaling and Synaptic Plasticity
4. Material and Methods
4.1. Experimental Subjects
4.2. Behavioral Testing
4.3. Intraocular Injections
4.4. LGN Projection Analysis
4.5. Immunohistochemistry
4.6. Barrel Field Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sierra, A.; Paolicelli, R.C.; Kettenmann, H. Cien Años de Microglía: Milestones in a Century of Microglial Research. Trends Neurosci. 2019, 42, 778–792. [Google Scholar] [CrossRef] [Green Version]
- Gasque, P. Complement: A unique innate immune sensor for danger signals. Mol. Immunol. 2004, 41, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, G.K.; Murphy, K.J. Neuron–glia crosstalk in health and disease: Fractalkine and CX 3 CR1 take centre stage. Open Biol. 2013, 3, 130181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic Pruning by Microglia Is Necessary for Normal Brain Development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schafer, D.P.; Lehrman, E.K.; Kautzman, A.G.; Koyama, R.; Mardinly, A.R.; Yamasaki, R.; Ransohoff, R.M.; Greenberg, M.E.; Barres, B.A.; Stevens, B. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef] [Green Version]
- Gunner, G.; Cheadle, L.; Johnson, K.M.; Ayata, P.; Badimon, A.; Mondo, E.; Nagy, M.A.; Liu, L.; BeMiller, S.M.; Kim, K.-W.; et al. Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling. Nat. Neurosci. 2019, 22, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Hoshiko, M.; Arnoux, I.; Avignone, E.; Yamamoto, N.; Audinat, E. Deficiency of the Microglial Receptor CX3CR1 Impairs Postnatal Functional Development of Thalamocortical Synapses in the Barrel Cortex. J. Neurosci. 2012, 32, 15106–15111. [Google Scholar] [CrossRef] [PubMed]
- Lowery, R.L.; Tremblay, M.-E.; Hopkins, B.E.; Majewska, A.K. The microglial fractalkine receptor is not required for activity-dependent plasticity in the mouse visual system. Glia 2017, 65, 1744–1761. [Google Scholar] [CrossRef] [PubMed]
- Reshef, R.; Kreisel, T.; Kay, D.B.; Yirmiya, R. Microglia and their CX3CR1 signaling are involved in hippocampal- but not olfactory bulb-related memory and neurogenesis. Brain Behav. Immun. 2014, 41, 239–250. [Google Scholar] [CrossRef]
- Schecter, R.W.; Maher, E.E.; Welsh, C.A.; Stevens, B.; Erisir, A.; Bear, M.F. Experience-Dependent Synaptic Plasticity in V1 Occurs without Microglial CX3CR1. J. Neurosci. 2017, 37, 10541–10553. [Google Scholar] [CrossRef]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef] [Green Version]
- Welsh, C.A.; Stephany, C.E.; Sapp, R.W.; Stevens, B. Ocular Dominance Plasticity in Binocular Primary Visual Cortex Does Not Require C1q. J. Neurosci. 2020, 40, 769–783. [Google Scholar] [CrossRef]
- Darabid, H.; St-Pierre-See, A.; Robitaille, R. Purinergic-Dependent Glial Regulation of Synaptic Plasticity of Competing Terminals and Synapse Elimination at the Neuromuscular Junction. Cell Rep. 2018, 25, 2070–2082.e2076. [Google Scholar] [CrossRef] [Green Version]
- Sipe, G.O.; Lowery, R.L.; Tremblay, M.-È.; Kelly, E.A.; LaMantia, C.E.; Majewska, A.K. Microglial P2Y12 is necessary for synaptic plasticity in mouse visual cortex. Nat. Commun. 2016, 7, 10905. [Google Scholar] [CrossRef]
- Tozaki-Saitoh, H.; Tsuda, M.; Miyata, H.; Ueda, K.; Kohsaka, S.; Inoue, K. P2Y12 Receptors in Spinal Microglia Are Required for Neuropathic Pain after Peripheral Nerve Injury. J. Neurosci. 2008, 28, 4949–4956. [Google Scholar] [CrossRef]
- Yu, T.; Zhang, X.; Shi, H.; Tian, J.; Sun, L.; Hu, X.; Cui, W.; Du, D. P2Y12 regulates microglia activation and excitatory synaptic transmission in spinal lamina II neurons during neuropathic pain in rodents. Cell Death Dis. 2019, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, Y.; Hoshi, M.; Akazawa, C.; Nakamura, Y.; Tsuzuki, H.; Inoue, K.; Kohsaka, S. Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia 2003, 44, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Haynes, S.E.; Hollopeter, G.; Yang, G.; Kurpius, D.; Dailey, M.E.; Gan, W.-B.; Julius, D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 2006, 9, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Sasaki, Y.; Ohsawa, K.; Imai, Y.; Nakamura, Y.; Inoue, K.; Kohsaka, S. Extracellular ATP or ADP Induce Chemotaxis of Cultured Microglia through Gi/o-Coupled P2Y Receptors. J. Neurosci. 2001, 21, 1975–1982. [Google Scholar] [CrossRef] [Green Version]
- Butovsky, O.; Jedrychowski, M.P.; Moore, C.S.; Cialic, R.; Lanser, A.J.; Gabriely, G.; Koeglsperger, T.; Dake, B.; Wu, P.M.; Doykan, C.E.; et al. Identification of a unique TGF-β–dependent molecular and functional signature in microglia. Nat. Neurosci. 2014, 17, 131–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chen, K.; Sloan, S.A.; Bennett, M.L.; Scholze, A.R.; O’Keeffe, S.; Phatnani, H.P.; Guarnieri, P.; Caneda, C.; Ruderisch, N.; et al. An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J. Neurosci. 2014, 34, 11929–11947. [Google Scholar] [CrossRef] [PubMed]
- Crawley, J.N. Translational animal models of autism and neurodevelopmental disorders. Dialogues Clin. Neurosci. 2012, 14, 293–305. [Google Scholar] [PubMed]
- Isaksson, J.; Westeinde, A.V.; Cauvet, É.; Kuja-Halkola, R.; Lundin, K.; Neufeld, J.; Willfors, C.; Bölte, S. Social Cognition in Autism and Other Neurodevelopmental Disorders: A Co-twin Control Study. J. Autism Dev. Disord. 2019, 49, 2838–2848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoghbi, H.Y.; Bear, M.F. Synaptic Dysfunction in Neurodevelopmental Disorders Associated with Autism and Intellectual Disabilities. Cold Spring Harb. Perspect. Biol. 2012, 4, a009886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, J.; Liu, Y.; Umpierre, A.D.; Xie, M.; Tian, D.-S.; Richardson, J.R.; Wu, L.-J. Microglial P2Y12 receptor regulates ventral hippocampal CA1 neuronal excitability and innate fear in mice. Mol. Brain 2019, 12, 71. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Zhou, Q.; Cao, Y.; Shi, H.; Wu, H.; Zhang, B.; Huang, F.; Wu, X. P2Y12 deficiency in mouse impairs noradrenergic system in brain, and alters anxiety-like neurobehavior and memory. Genes Brain Behav. 2019, 18, e12458. [Google Scholar] [CrossRef] [PubMed]
- Hellwig, S.; Brioschi, S.; Dieni, S.; Frings, L.; Masuch, A.; Blank, T.O.; Biber, K. Altered microglia morphology and higher resilience to stress-induced depression-like behavior in CX3CR1-deficient mice. Brain Behav. Immun. 2016, 55, 126–137. [Google Scholar] [CrossRef]
- Parkhurst, C.N.; Yang, G.; Ninan, I.; Savas, J.N.; Yates, J.R., III; Lafaille, J.J.; Hempstead, B.L.; Littman, D.R.; Gan, W.-B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 2013, 155, 1596–1609. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Y.; Paolicelli, R.C.; Sforazzini, F.; Weinhard, L.; Bolasco, G.; Pagani, F.; Vyssotski, A.L.; Bifone, A.; Gozzi, A.; Ragozzino, D.A.; et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014, 17, 400–406. [Google Scholar] [CrossRef]
- André, P.; Delaney, S.M.; LaRocca, T.; Vincent, D.; DeGuzman, F.; Jurek, M.; Koller, B.; Phillips, D.R.; Conley, P.B. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J. Clin. Investig. 2003, 112, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Kyrargyri, V.; Madry, C.; Rifat, A.; Arancibia-Carcamo, I.L.; Jones, S.P.; Chan, V.T.T.; Xu, Y.; Robaye, B.; Attwell, D. P2Y 13 receptors regulate microglial morphology, surveillance, and resting levels of interleukin 1β release. Glia 2020, 68, 328–344. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, A.M.; Hawk, J.D.; Abel, T.; Havekes, R. Post-training reversible inactivation of the hippocampus enhances novel object recognition memory. Learn. Mem. 2010, 17, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Sanderson, D.J.; Bannerman, D.M. The role of habituation in hippocampus-dependent spatial working memory tasks: Evidence from GluA1 AMPA receptor subunit knockout mice. Hippocampus 2012, 22, 981–994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenckova, M.; Blok, L.E.; Asztalos, L.; Goodman, D.P.; Cizek, P.; Singgih, E.L.; Glennon, J.C.; IntHout, J.; Zweier, C.; Eichler, E.E.; et al. Habituation Learning Is a Widely Affected Mechanism in Drosophila Models of Intellectual Disability and Autism Spectrum Disorders. Biol. Psychiatry 2019, 86, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Hudac, C.M.; Deschamps, T.D.; Arnett, A.B.; Cairney, B.E.; Ma, R.; Webb, S.J.; Bernier, R.A. Early enhanced processing and delayed habituation to deviance sounds in autism spectrum disorder. Brain Cogn. 2018, 123, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Kleinhans, N.M.; Johnson, L.C.; Richards, T.; Mahurin, R.; Greenson, J.; Dawson, G.; Aylward, E. Reduced Neural Habituation in the Amygdala and Social Impairments in Autism Spectrum Disorders. Am. J. Psychiatry 2009, 166, 467–475. [Google Scholar] [CrossRef]
- Guang, S.; Pang, N.; Deng, X.; Yang, L.; He, F.; Wu, L.; Chen, C.; Yin, F.; Peng, J. Synaptopathology Involved in Autism Spectrum Disorder. Front. Cell. Neurosci. 2018, 12, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madry, C.; Kyrargyri, V.; Arancibia-Cárcamo, I.L.; Jolivet, R.; Kohsaka, S.; Bryan, R.M.; Attwell, D. Microglial Ramification, Surveillance, and Interleukin-1β Release Are Regulated by the Two-Pore Domain K+ Channel THIK-1. Neuron 2018, 97, 299–312.e6. [Google Scholar] [CrossRef] [PubMed]
- Dissing-Olesen, L.; LeDue, J.M.; Rungta, R.L.; Hefendehl, J.K.; Choi, H.B.; MacVicar, B.A. Activation of Neuronal NMDA Receptors Triggers Transient ATP-Mediated Microglial Process Outgrowth. J. Neurosci. 2014, 34, 10511–10527. [Google Scholar] [CrossRef] [Green Version]
- Eyo, U.B.; Peng, J.; Swiatkowski, P.; Mukherjee, A.; Bispo, A.; Wu, L.-J. Neuronal Hyperactivity Recruits Microglial Processes via Neuronal NMDA Receptors and Microglial P2Y12 Receptors after Status Epilepticus. J. Neurosci. 2014, 34, 10528–10540. [Google Scholar] [CrossRef] [Green Version]
- Bezzi, P.; Volterra, A. A neuron–glia signalling network in the active brain. Curr. Opin. Neurobiol. 2001, 11, 387–394. [Google Scholar] [CrossRef]
- Fields, R.D. Nonsynaptic and nonvesicular ATP release from neurons and relevance to neuron–glia signaling. Semin. Cell Dev. Biol. 2011, 22, 214–219. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Gonçalves, F.Q.; Cristovão, G.; Rodrigues, L.; Fernandes, J.R.M.; Gonçalves, T.; Cunha, R.A.; Gomes, C.A. Different danger signals differently impact on microglial proliferation through alterations of ATP release and extracellular metabolism. Glia 2015, 63, 1636–1645. [Google Scholar] [CrossRef] [PubMed]
- Imura, Y.; Morizawa, Y.; Komatsu, R.; Shibata, K.; Shinozaki, Y.; Kasai, H.; Moriishi, K.; Moriyama, Y.; Koizumi, S. Microglia release ATP by exocytosis. Glia 2013, 61, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Pankratov, Y.; Lalo, U.; Verkhratsky, A.; North, R.A. Vesicular release of ATP at central synapses. Pflügers Archiv. 2006, 452, 589–597. [Google Scholar] [CrossRef]
- Davalos, D.; Grutzendler, J.; Yang, G.; Kim, J.V.; Zuo, Y.; Jung, S.; Littman, D.R.; Dustin, M.L.; Gan, W.-B. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005, 8, 752–758. [Google Scholar] [CrossRef]
- Burnstock, G. Cotransmission. Curr. Opin. Pharmacol. 2004, 4, 47–52. [Google Scholar] [CrossRef]
- Dani, J.W.; Chernjavsky, A.; Smith, S.J. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron 1992, 8, 429–440. [Google Scholar] [CrossRef]
- Pasti, L.; Volterra, A.; Pozzan, T.; Carmignoto, G. Intracellular Calcium Oscillations in Astrocytes: A Highly Plastic, Bidirectional Form of Communication between Neurons and AstrocytesIn Situ. J. Neurosci. 1997, 17, 7817–7830. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lou, N.; Xu, Q.; Tian, G.-F.; Peng, W.G.; Han, X.; Kang, J.; Takano, T.; Nedergaard, M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat. Neurosci. 2006, 9, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; Wang, H.-K.; Ye, C.-Q.; Ge, W.; Chen, Y.; Jiang, Z.-L.; Wu, C.-P.; Poo, M.-M.; Duan, S. ATP Released by Astrocytes Mediates Glutamatergic Activity-Dependent Heterosynaptic Suppression. Neuron 2003, 40, 971–982. [Google Scholar] [CrossRef] [Green Version]
- Perea, G. Properties of Synaptically Evoked Astrocyte Calcium Signal Reveal Synaptic Information Processing by Astrocytes. J. Neurosci. 2005, 25, 2192–2203. [Google Scholar] [CrossRef]
- Dou, Y.; Wu, H.-J.; Li, H.-Q.; Qin, S.; Wang, Y.-E.; Li, J.; Lou, H.-F.; Chen, Z.; Li, X.-M.; Luo, Q.-M.; et al. Microglial migration mediated by ATP-induced ATP release from lysosomes. Cell Res. 2012, 22, 1022–1033. [Google Scholar] [CrossRef] [Green Version]
- Lehrman, E.K.; Wilton, D.K.; Litvina, E.Y.; Welsh, C.A.; Chang, S.T.; Frouin, A.; Walker, A.J.; Heller, M.D.; Umemori, H.; Chen, C.; et al. CD47 Protects Synapses from Excess Microglia-Mediated Pruning during Development. Neuron 2018, 100, 120–134.e126. [Google Scholar] [CrossRef] [Green Version]
- Badimon, A.; Strasburger, H.J.; Ayata, P.; Chen, X.; Nair, A.; Ikegami, A.; Hwang, P.; Chan, A.T.; Graves, S.M.; Uweru, J.O.; et al. Negative feedback control of neuronal activity by microglia. Nat. Cell Biol. 2020, 586, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Merlini, M.; Rafalski, V.A.; Ma, K.; Kim, K.-Y.; Bushong, E.A.; Coronado, P.E.R.; Yan, Z.; Mendiola, A.S.; Sozmen, E.G.; Ryu, J.K.; et al. Microglial Gi-dependent dynamics regulate brain network hyperexcitability. Nat. Neurosci. 2021, 24, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Stowell, R.D.; Sipe, G.O.; Dawes, R.P.; Batchelor, H.N.; Lordy, K.A.; Whitelaw, B.S.; Stoessel, M.B.; Bidlack, J.M.; Brown, E.; Sur, M.; et al. Noradrenergic signaling in the wakeful state inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. Nat. Neurosci. 2019, 22, 1782–1792. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.U.; Ying, Y.; Li, Y.; Eyo, U.B.; Chen, T.; Zheng, J.; Umpierre, A.D.; Zhu, J.; Bosco, D.B.; Dong, H.; et al. Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat. Neurosci. 2019, 22, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Gyoneva, S.; Traynelis, S.F. Norepinephrine Modulates the Motility of Resting and Activated Microglia via Different Adrenergic Receptors. J. Biol. Chem. 2013, 288, 15291–15302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, S.; Aliberti, J.; Graemmel, P.; Sunshine, M.J.; Kreutzberg, G.W.; Sher, A.; Littman, D.R. Analysis of Fractalkine Receptor CX3CR1 Function by Targeted Deletion and Green Fluorescent Protein Reporter Gene Insertion. Mol. Cell. Biol. 2000, 20, 4106–4114. [Google Scholar] [CrossRef] [Green Version]
- Koch, S.M.; Ullian, E.M. Neuronal pentraxins mediate silent synapse conversion in the developing visual system. J. Neurosci. 2010, 30, 5404–5414. [Google Scholar] [CrossRef] [PubMed]
- Muir-Robinson, G.; Hwang, B.J.; Feller, M.B. Retinogeniculate axons undergo eye-specific segregation in the absence of eye-specific layers. J. Neurosci. 2002, 22, 5259–5264. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.M.; Cruz, C.G.D.; Hnasko, T.S.; Edwards, R.H.; Huberman, A.D.; Ullian, E.M. Pathway-Specific Genetic Attenuation of Glutamate Release Alters Select Features of Competition-Based Visual Circuit Refinement. Neuron 2011, 71, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lowery, R.L.; Mendes, M.S.; Sanders, B.T.; Murphy, A.J.; Whitelaw, B.S.; Lamantia, C.E.; Majewska, A.K. Loss of P2Y12 Has Behavioral Effects in the Adult Mouse. Int. J. Mol. Sci. 2021, 22, 1868. https://doi.org/10.3390/ijms22041868
Lowery RL, Mendes MS, Sanders BT, Murphy AJ, Whitelaw BS, Lamantia CE, Majewska AK. Loss of P2Y12 Has Behavioral Effects in the Adult Mouse. International Journal of Molecular Sciences. 2021; 22(4):1868. https://doi.org/10.3390/ijms22041868
Chicago/Turabian StyleLowery, Rebecca L., Monique S. Mendes, Brandon T. Sanders, Allison J. Murphy, Brendan S. Whitelaw, Cassandra E. Lamantia, and Ania K. Majewska. 2021. "Loss of P2Y12 Has Behavioral Effects in the Adult Mouse" International Journal of Molecular Sciences 22, no. 4: 1868. https://doi.org/10.3390/ijms22041868





