The Roles of the Colon Cancer Associated Transcript 2 (CCAT2) Long Non-Coding RNA in Cancer: A Comprehensive Characterization of the Tumorigenic and Molecular Functions
Abstract
:1. Introduction
2. Cancer-Associated Regulatory Activity of CCAT2
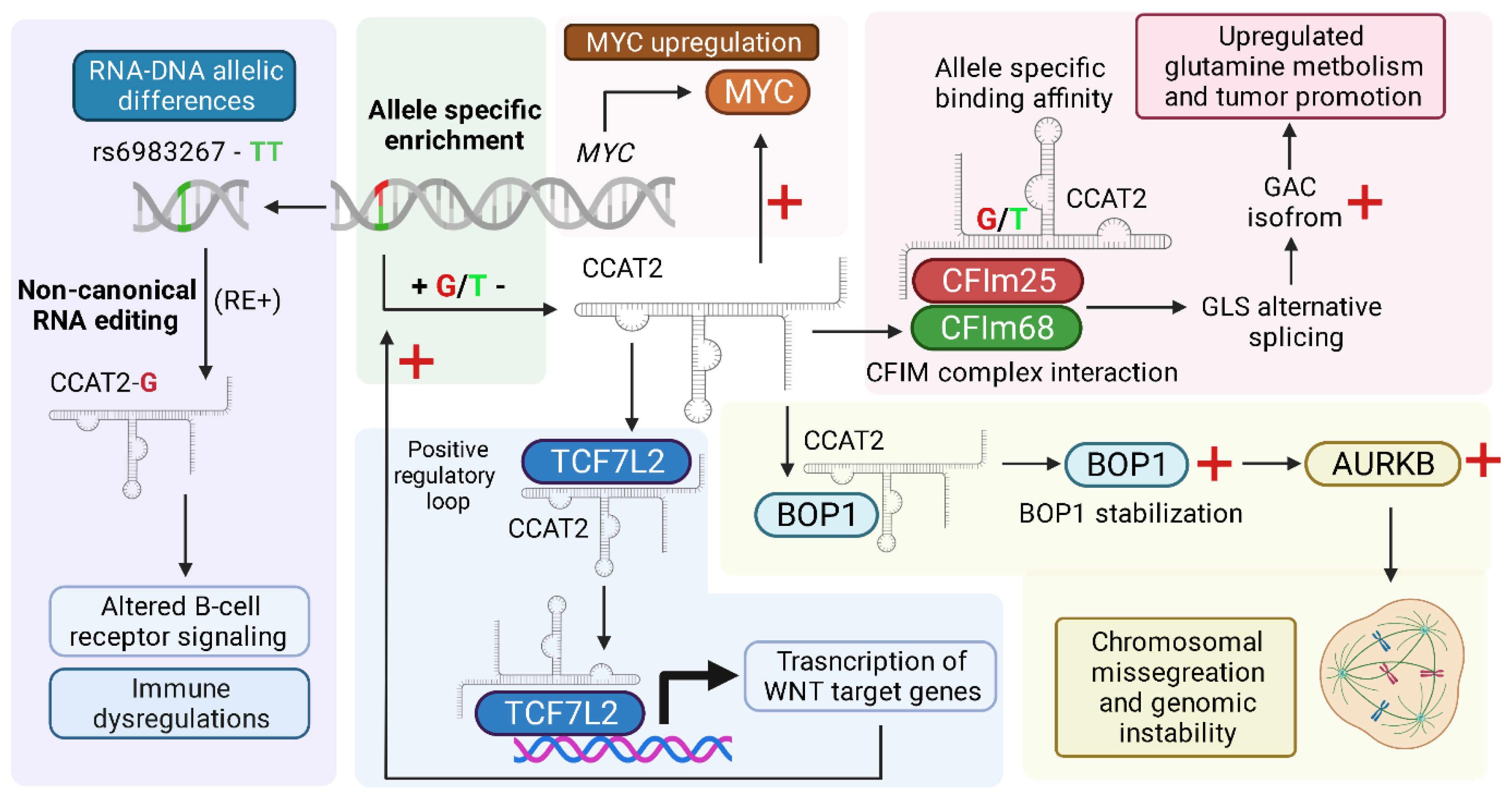
2.1. MYC and WNT Pathway Activation
2.2. Allele-Specific Metabolic Reprogramming
2.3. Chromosomal Instability
2.4. RNA Editing and CCAT2-Associated RNA–DNA Differences at the rs6983267 Locus
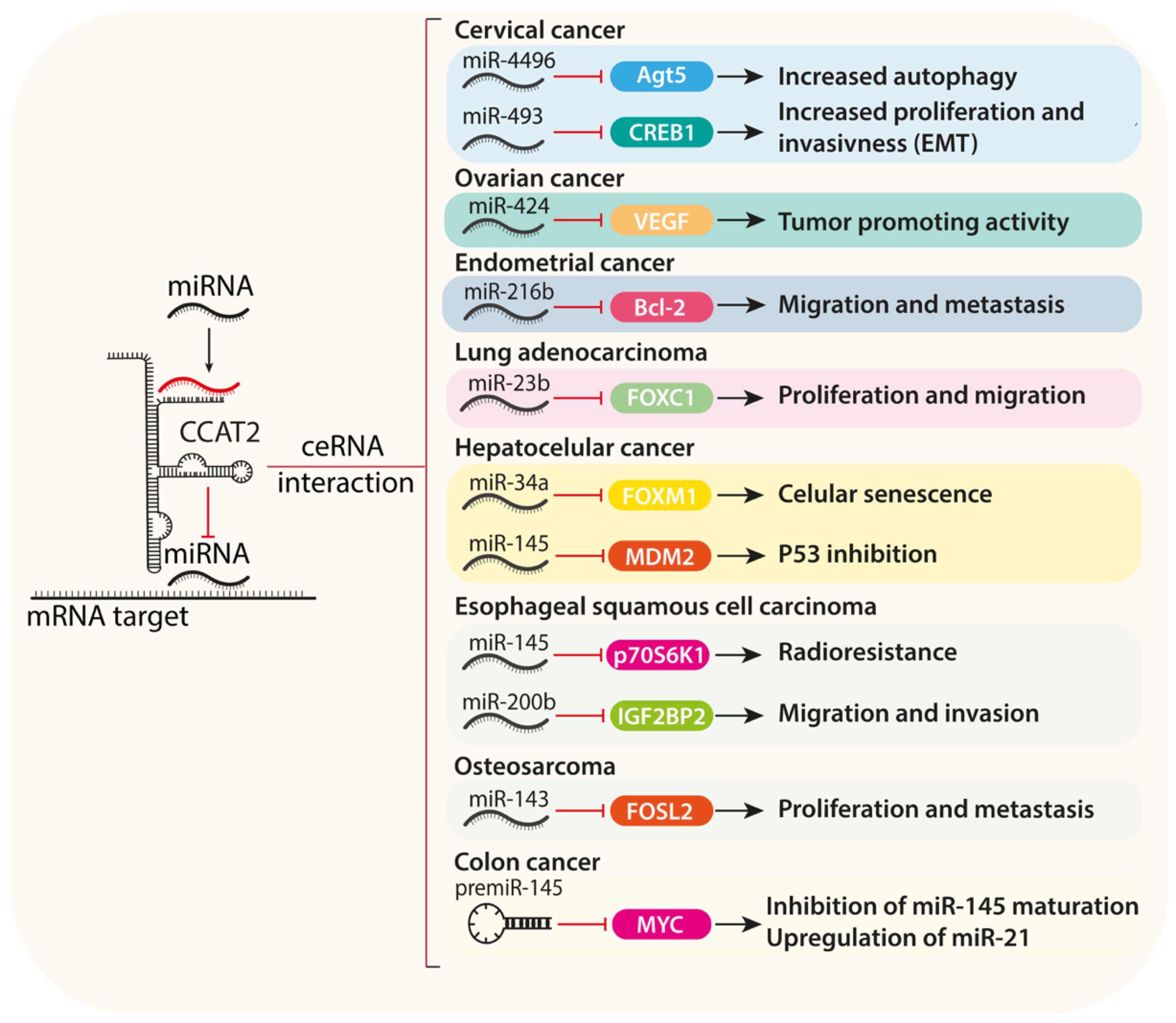
2.5. ceRNA Activity of CCAT2 and miRNA Sponging
2.6. Therapeutic Resistance
2.7. CCAT2 Enhancer Activity and Interactions with RBPs
3. CCAT2 as a Potential Biomarker
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mattick, J.S.; Rinn, J.L. Discovery and annotation of long noncoding RNAs. Nat. Struct. Mol. Biol. 2015, 22, 5–7. [Google Scholar] [CrossRef]
- van Leeuwen, S.; Mikkers, H. Long non-coding RNAs: Guardians of development. Differ. Res. Biol. Divers 2010, 80, 175–183. [Google Scholar] [CrossRef]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Wang, J.M.; Li, M.; Tang, R.; Tang, K.; Su, Y.; Hou, Y.; Zhang, J. Long non-coding RNAs: New biomarkers for prognosis and diagnosis of colon cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2017, 39, 1010428317706332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, Y.; Shinjo, K.; Katsushima, K. Long non-coding RNAs as an epigenetic regulator in human cancers. Cancer Sci. 2017, 108, 1927–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra Gupta, S.; Nandan Tripathi, Y. Potential of long non-coding RNAs in cancer patients: From biomarkers to therapeutic targets. Int. J. Cancer 2017, 140, 1955–1967. [Google Scholar] [CrossRef]
- Qiu, L.; Tang, Q.; Li, G.; Chen, K. Long non-coding RNAs as biomarkers and therapeutic targets: Recent insights into hepatocellular carcinoma. Life Sci. 2017, 191, 273–282. [Google Scholar] [CrossRef]
- Brown, C.J.; Hendrich, B.D.; Rupert, J.L.; Lafrenière, R.G.; Xing, Y.; Lawrence, J.; Willard, H.F. The human XIST gene: Analysis of a 17 kb inactive X-specific RNA that contains conserved repeats and is highly localized within the nucleus. Cell 1992, 71, 527–542. [Google Scholar] [CrossRef]
- Woo, C.J.; Kingston, R.E. HOTAIR lifts noncoding RNAs to new levels. Cell 2007, 129, 1257–1259. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, L.H.; Spieker, T.; Koschmieder, S.; Schäffers, S.; Humberg, J.; Jungen, D.; Bulk, E.; Hascher, A.; Wittmer, D.; Marra, A.; et al. The long noncoding MALAT-1 RNA indicates a poor prognosis in non-small cell lung cancer and induces migration and tumor growth. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2011, 6, 1984–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.-C.; Hung, T.; Argani, P.; Rinn, J.L.; et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef]
- Rinn, J.L.; Kertesz, M.; Wang, J.K.; Squazzo, S.L.; Xu, X.; Brugmann, S.A.; Goodnough, L.H.; Helms, J.A.; Farnham, P.J.; Segal, E.; et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell 2007, 129, 1311–1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, P.; Diederichs, S.; Wang, W.; Böing, S.; Metzger, R.; Schneider, P.M.; Tidow, N.; Brandt, B.; Buerger, H.; Bulk, E.; et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 2003, 22, 8031–8041. [Google Scholar] [CrossRef] [Green Version]
- Hutchinson, J.N.; Ensminger, A.W.; Clemson, C.M.; Lynch, C.R.; Lawrence, J.B.; Chess, A. A screen for nuclear transcripts identifies two linked noncoding RNAs associated with SC35 splicing domains. BMC Genom. 2007, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, V.; Ellis, J.D.; Shen, Z.; Song, D.Y.; Pan, Q.; Watt, A.T.; Freier, S.M.; Bennett, C.F.; Sharma, A.; Bubulya, P.A.; et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell 2010, 39, 925–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, H.; Spizzo, R.; Atlasi, Y.; Nicoloso, M.; Shimizu, M.; Redis, R.S.; Nishida, N.; Gafa, R.; Song, J.; Guo, Z.; et al. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013, 23, 1446–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghafouri-Fard, S.; Taheri, M. Colon Cancer-Associated Transcripts 1 and 2: Roles and functions in human cancers. J. Cell Physiol. 2019, 234, 14581–14600. [Google Scholar] [CrossRef]
- Nissan, A.; Stojadinovic, A.; Mitrani-Rosenbaum, S.; Halle, D.; Grinbaum, R.; Roistacher, M.; Bochem, A.; Dayanc, B.E.; Ritter, G.; Gomceli, I.; et al. Colon cancer associated transcript-1: A novel RNA expressed in malignant and pre-malignant human tissues. Int. J. Cancer 2012, 130, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, Q.; Hann, S.S. The functions and oncogenic roles of CCAT1 in human cancer. Biomed. Pharmacother. 2019, 115, 108943. [Google Scholar] [CrossRef]
- Xiang, J.-F.; Yin, Q.-F.; Chen, T.; Zhang, Y.; Zhang, X.-O.; Wu, Z.; Zhang, S.; Wang, H.-B.; Ge, J.; Lu, X.; et al. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res. 2014, 24, 513–531. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Hua, Y. CCAT1: An oncogenic long noncoding RNA in human cancers. J. Cancer Res. Clin. Oncol. 2017, 143, 555–562. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, Y.-Y.; Xie, J.-J.; Mayakonda, A.; Hazawa, M.; Chen, L.; Xiao, J.-F.; Li, C.-Q.; Huang, M.-L.; Ding, L.-W.; et al. Co-activation of super-enhancer-driven CCAT1 by TP63 and SOX2 promotes squamous cancer progression. Nat. Commun. 2018, 9, 3619. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghoussaini, M.; Song, H.; Koessler, T.; Al Olama, A.A.; Kote-Jarai, Z.; Driver, K.E.; Pooley, K.A.; Ramus, S.J.; Kjaer, S.K.; Hogdall, E.; et al. Multiple loci with different cancer specificities within the 8q24 gene desert. J. Natl. Cancer Inst. 2008, 100, 962–966. [Google Scholar] [CrossRef] [Green Version]
- Wright, J.B.; Brown, S.J.; Cole, M.D. Upregulation of c-MYC in cis through a large chromatin loop linked to a cancer risk-associated single-nucleotide polymorphism in colorectal cancer cells. Mol. Cell Biol. 2010, 30, 1411–1420. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Li, F.; Zhou, W.; Huang, J. Knockdown of long non-coding RNA CCAT2 suppresses growth and metastasis of esophageal squamous cell carcinoma by inhibiting the β-catenin/WISP1 signaling pathway. J. Int. Med. Res. 2021, 49, 3000605211019938. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Xiong, G.; Guo, W.; Jiang, G.; Li, Y.; Li, H. Long non-coding RNA CCAT2 promotes prostate cancer cell proliferation and invasion by regulating the Wnt/β-catenin signaling pathway. Oncol. Lett. 2020, 20, 97. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, M.; Zhuang, R.; Jiang, J.; Gao, J.; Wang, H.; Chen, H.; Zhang, Z.; Kuang, Y.; Li, P. Long non-coding RNA CCAT2 promotes epithelial-mesenchymal transition involving Wnt/beta-catenin pathway in epithelial ovarian carcinoma cells. Oncol. Lett. 2018, 15, 3369–3375. [Google Scholar]
- Huang, J.-L.; Liao, Y.; Qiu, M.-X.; Li, J.; An, Y. Long non-coding RNA CCAT2 promotes cell proliferation and invasion through regulating Wnt/beta-catenin signaling pathway in clear cell renal cell carcinoma. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2017, 39, 1010428317711314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Y.; He, J.; Zhang, D. Long noncoding RNA CCAT2 promotes breast tumor growth by regulating the Wnt signaling pathway. OncoTargets Ther. 2015, 8, 2657–2664. [Google Scholar]
- Redis, R.S.; Vela, L.E.; Lu, W.; Ferreira de Oliveira, J.; Ivan, C.; Rodriguez-Aguayo, C.; Adamoski, D.; Pasculli, B.; Taguchi, A.; Chen, Y.; et al. Allele-Specific Reprogramming of Cancer Metabolism by the Long Non-coding RNA CCAT2. Mol. Cell. 2016, 61, 520–534. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Dragomir, M.P.; Fabris, L.; Bayraktar, R.; Knutsen, E.; Liu, X.; Tang, C.; Li, Y.; Shimura, T.; Ivkovic, T.C.; et al. The Long Noncoding RNA CCAT2 Induces Chromosomal Instability Through BOP1-AURKB Signaling. Gastroenterology 2020, 159, 2146–2162.e33. [Google Scholar] [CrossRef]
- Baysal, B.E.; Sharma, S.; Hashemikhabir, S.; Janga, S.C. RNA Editing in Pathogenesis of Cancer. Cancer Res. 2017, 77, 3733–3739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christofi, T.; Zaravinos, A. RNA editing in the forefront of epitranscriptomics and human health. J. Transl. Med. 2019, 17, 319. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.Y.; Ferracin, M.; Pileczki, V.; Chen, B.; Redis, R.; Fabris, L.; Zhang, X.; Ivan, C.; Shimizu, M.; Rodriguez-Aguayo, C.; et al. Cancer-associated rs6983267 SNP and its accompanying long noncoding RNA CCAT2 induce myeloid malignancies via unique SNP-specific RNA mutations. Genome Res. 2018, 28, 432–447. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.-S.; Zhang, E.-X.; Sun, Q.-F.; Ye, Z.-J.; Liu, J.-W.; Zhou, D.-H.; Tang, Y. Integrated analysis of lncRNA-miRNA-mRNA ceRNA network in squamous cell carcinoma of tongue. BMC Cancer 2019, 19, 779. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, H.; Zheng, B.; Sun, L.; Yuan, Y.; Xing, C. Competitive Endogenous RNA (ceRNA) Regulation Network of lncRNA-miRNA-mRNA in Colorectal Carcinogenesis. Dig. Dis. Sci. 2019, 64, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qian, W.; Wang, S.; Ji, D.; Wang, Q.; Li, J.; Peng, W.; Gu, J.; Hu, T.; Ji, B.; et al. Analysis of lncRNA-Associated ceRNA Network Reveals Potential lncRNA Biomarkers in Human Colon Adenocarcinoma. Cell Physiol. Biochem. Int. J. Exp. Cell Physiol. Biochem. Pharmacol. 2018, 49, 1778–1791. [Google Scholar] [CrossRef]
- Hua, F.; Li, C.-H.; Chen, X.-G.; Liu, X.-P. Long Noncoding RNA CCAT2 Knockdown Suppresses Tumorous Progression by Sponging miR-424 in Epithelial Ovarian Cancer. Oncol. Res. 2018, 26, 241–247. [Google Scholar] [CrossRef]
- Thomson, D.W.; Dinger, M.E. Endogenous microRNA sponges: Evidence and controversy. Nat. Rev. Genet. 2016, 17, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Cao, H.; Li, Y.; Wang, J.; Cui, Z. Knockdown of lncRNA CCAT2 inhibits endometrial cancer cells growth and metastasis via sponging miR-216b. Cancer Biomark. Sect Dis. Markers 2017, 21, 123–133. [Google Scholar] [CrossRef]
- Hu, G.-D.; Wang, C.-X.; Wang, H.-Y.; Wang, Y.-Q.; Hu, S.; Cao, Z.-W.; Min, B.; Li, L.; Tian, X.-F.; Hu, H.-B. Long noncoding RNA CCAT2 functions as a competitive endogenous RNA to regulate FOXC1 expression by sponging miR-23b-5p in lung adenocarcinoma. J. Cell Biochem. 2018, 120, 7998–8007. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-L.; Shu, Y.-G.; Tao, M.-Y. LncRNA CCAT2 promotes angiogenesis in glioma through activation of VEGFA signalling by sponging miR-424. Mol. Cell Biochem. 2020, 468, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Zhang, L.; Chen, S.; Cao, H.; Xu, C.; Wang, X. lncRNA CCAT2 Enhanced Resistance of Glioma Cells Against Chemodrugs by Disturbing the Normal Function of miR-424. OncoTargets Ther. 2020, 13, 1431–1445. [Google Scholar] [CrossRef] [Green Version]
- Niu, C.; Wang, L.; Ye, W.; Guo, S.; Bao, X.; Wang, Y.; Xia, Z.; Chen, R.; Liu, C.; Lin, X.; et al. CCAT2 contributes to hepatocellular carcinoma progression via inhibiting miR-145 maturation to induce MDM2 expression. J. Cell Physiol. 2020, 235, 6307–6320. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Qureshi, M.Z.; Attar, R.; Alhewairini, S.S.; Fayyaz, S.; Sabitaliyevich, U.Y.; Duisenbayevich, T.M.; Alaaeddine, N. MicroRNA-143 as a new weapon against cancer: Overview of the mechanistic insights and long non-coding RNA mediated regulation of miRNA-143 in different cancers. Cell Mol. Biol. 2019, 65, 1–5. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, Y.; Zhu, J.; Wu, T.; Ma, J.; Du, C.; Chen, S.; Li, T.; Han, J.; Wang, X. Overexpression of long non-coding RNA colon cancer-associated transcript 2 is associated with advanced tumor progression and poor prognosis in patients with colorectal cancer. Oncol. Lett. 2017, 14, 6907–6914. [Google Scholar] [CrossRef] [PubMed]
- Kasagi, Y.; Oki, E.; Ando, K.; Ito, S.; Iguchi, T.; Sugiyama, M.; Nakashima, Y.; Ohgaki, K.; Saeki, H.; Mimori, K.; et al. The Expression of CCAT2, a Novel Long Noncoding RNA Transcript, and rs6983267 Single-Nucleotide Polymorphism Genotypes in Colorectal Cancers. Oncology 2017, 92, 48–54. [Google Scholar] [CrossRef]
- Yu, Y.; Nangia-Makker, P.; Farhana, L.; Majumdar, A.P.N. A novel mechanism of lncRNA and miRNA interaction: CCAT2 regulates miR-145 expression by suppressing its maturation process in colon cancer cells. Mol. Cancer 2017, 16, 155. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, T.; Matsuyama, T.; Toiyama, Y.; Takahashi, N.; Ishikawa, T.; Uetake, H.; Yamada, Y.; Kusunoki, M.; Calin, G.; Goel, A. CCAT1 and CCAT2 long noncoding RNAs, located within the 8q.24.21 “gene desert”, serve as important prognostic biomarkers in colorectal cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1882–1888. [Google Scholar] [CrossRef]
- Siddique, H.; Al-Ghafari, A.; Choudhry, H.; AlTurki, S.; Alshaibi, H.; Al Doghaither, H.; Alsufiani, H. Long Noncoding RNAs as Prognostic Markers for Colorectal Cancer in Saudi Patients. Genet. Test Mol. Biomark. 2019, 23, 509–514. [Google Scholar] [CrossRef]
- Gharib, E.; Nazemalhosseini-Mojarad, E.; Baghdar, K.; Nayeri, Z.; Sadeghi, H.; Rezasoltani, S.; Jamshidi-Fard, A.; Larki, P.; Sadeghi, A.; Hashemi, M.; et al. Identification of a stool long non-coding RNAs panel as a potential biomarker for early detection of colorectal cancer. J. Clin. Lab. Anal. 2021, 35, e23601. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Sun, D.; Guo, H.; Wu, Z.; Chen, J. LncRNA CCAT2 promotes proliferation and suppresses apoptosis of colorectal cancer cells. J. BUON Off. J. Balk Union Oncol. 2020, 25, 1840–1846. [Google Scholar]
- Thean, L.F.; Blöcker, C.; Li, H.H.; Lo, M.; Wong, M.; Tang, C.L.; Tan, E.K.W.; Rozen, S.G.; Cheah, P.Y. Enhancer-derived long non-coding RNAs CCAT1 and CCAT2 at rs6983267 has limited predictability for early stage colorectal carcinoma metastasis. Sci. Rep. 2021, 11, 404. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7801656/ (accessed on 24 May 2021). [CrossRef]
- Zhang, X.; Xu, Y.; He, C.; Guo, X.; Zhang, J.; He, C.; Zhang, L.; Kong, M.; Chen, B.; Zhu, C. Elevated expression of CCAT2 is associated with poor prognosis in esophageal squamous cell carcinoma. J. Surg. Oncol. 2015, 111, 834–839. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, M.; Xu, Y.; Li, M.; Dong, G.; Mao, Q.; Yin, R.; Xu, L. Long noncoding RNA CCAT2 correlates with smoking in esophageal squamous cell carcinoma. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 5523–5528. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X. Long non-coding RNA colon cancer-associated transcript 2 may promote esophageal cancer growth and metastasis by regulating the Wnt signaling pathway. Oncol. Lett. 2019, 18, 1745–1754. Available online: http://www.spandidos-publications.com/10.3892/ol.2019.10488 (accessed on 27 October 2019). [CrossRef]
- Ma, Y.; Hu, X.; Shang, C.; Zhong, M.; Guo, Y. Silencing of long non-coding RNA CCAT2 depressed malignancy of oral squamous cell carcinoma via Wnt/beta-catenin pathway. Tumour Biol. J. Int. Soc. Oncodevel. Biol. Med. 2017, 39, 1010428317717670. [Google Scholar]
- Wang, M.; Wang, L.; He, X.; Zhang, J.; Zhu, Z.; Zhang, M.; Li, X. lncRNA CCAT2 promotes radiotherapy resistance for human esophageal carcinoma cells via the miR-145/p70S6K1 and p53 pathway. Int. J. Oncol. 2020, 56, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Fan, Y.; Liu, Y.; Shen, B.; Lu, H.; Ma, H. Long Non-Coding RNA CCAT2 Promotes the Development of Esophageal Squamous Cell Carcinoma by Inhibiting miR-200b to Upregulate the IGF2BP2/TK1 Axis. Front. Oncol. 2021, 11, 680642. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Hua, L.; Yao, K.-H.; Chen, J.-T.; Zhang, J.-J.; Hu, J.-H. Long non-coding RNA CCAT2 is up-regulated in gastric cancer and associated with poor prognosis. Int. J. Clin. Exp. Pathol. 2015, 8, 779–785. [Google Scholar]
- Wang, Y.-J.; Liu, J.-Z.; Lv, P.; Dang, Y.; Gao, J.-Y.; Wang, Y. Long non-coding RNA CCAT2 promotes gastric cancer proliferation and invasion by regulating the E-cadherin and LATS2. Am. J. Cancer Res. 2016, 6, 2651–2660. [Google Scholar] [PubMed]
- Lin, S.; Wang, H.; Yang, W.; Wang, A.; Geng, C. Silencing of Long Non-Coding RNA Colon Cancer-Associated Transcript 2 Inhibits the Growth and Metastasis of Gastric Cancer Through Blocking mTOR Signaling. OncoTargets Ther. 2020, 13, 337–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Shi, H.; Xi, H.; Wu, X.; Cui, J.; Gao, Y.; Liang, W.; Hu, C.; Liu, Y.; Li, J. Genome-Wide lncRNA Microarray Profiling Identifies Novel Circulating lncRNAs for Detection of Gastric Cancer. Theranostics 2017, 7, 213–227. [Google Scholar] [CrossRef]
- Zhou, N.; Si, Z.; Li, T.; Chen, G.; Zhang, Z.; Qi, H. Long non-coding RNA CCAT2 functions as an oncogene in hepatocellular carcinoma, regulating cellular proliferation, migration and apoptosis. Oncol. Lett. 2016, 12, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Bai, G.; Li, Y.; Feng, Y.; Wang, L. A positive feedback loop of long noncoding RNA CCAT2 and FOXM1 promotes hepatocellular carcinoma growth. Am. J. Cancer Res. 2017, 7, 1423–1434. [Google Scholar]
- Xu, Y.; Wang, B.; Zhang, F.; Wang, A.; Du, X.; Hu, P.; Zhu, Y.; Fang, Z. Long non-coding RNA CCAT2 is associated with poor prognosis in hepatocellular carcinoma and promotes tumor metastasis by regulating Snail2-mediated epithelial-mesenchymal transition. OncoTargets Ther. 2017, 10, 1191–1198. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, D.; Li, Y.; Yan, S.; Dang, H.; Yue, H.; Ling, J.; Chen, F.; Zhao, Y.; Gou, L.; et al. Long noncoding RNA CCAT2 promotes hepatocellular carcinoma proliferation and metastasis through up-regulation of NDRG1. Exp. Cell Res. 2019, 379, 19–29. [Google Scholar] [CrossRef]
- Shi, J.; Guo, C.; Ma, J. CCAT2 enhances autophagy-related invasion and metastasis via regulating miR-4496 and ELAVL1 in hepatocellular carcinoma. J. Cell Mol. Med. 2021, 25, 8985–8996. [Google Scholar] [CrossRef]
- Cai, Y.; Li, X.; Shen, P.; Zhang, D. CCAT2 is an oncogenic long non-coding RNA in pancreatic ductal adenocarcinoma. Biol. Res. 2018, 51, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, H.-L.; Hu, G.-W.; Zhang, B.; Kuang, W.; Chen, Y.; Wu, L.; Xu, G.-H. Glioma cells enhance angiogenesis and inhibit endothelial cell apoptosis through the release of exosomes that contain long non-coding RNA CCAT2. Oncol. Rep. 2017, 38, 785–798. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Hu, G.; Yang, Q.; Zhang, P.; Kuang, W.; Zhu, X.; Wu, L. Knockdown of long non-coding RNA CCAT2 suppressed proliferation and migration of glioma cells. Oncotarget 2016, 7, 81806–81814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, J.; Du, T.; Song, Y.; Gao, Y.; Li, F.; Wu, R.; Chen, Y.; Li, W.; Zhou, H.; Yang, Y.; et al. Knockdown of Long Noncoding RNA CCAT2 Inhibits Cellular Proliferation, Invasion, and Epithelial-Mesenchymal Transition in Glioma Cells. Oncol. Res. 2017, 25, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Filippella, M.; Galland, F.; Kujas, M.; Young, J.; Faggiano, A.; Lombardi, G.; Colao, A.; Meduri, G.; Chanson, P. Pituitary tumour transforming gene (PTTG) expression correlates with the proliferative activity and recurrence status of pituitary adenomas: A clinical and immunohistochemical study. Clin. Endocrinol. 2006, 65, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, M.; Hou, Y.; Zhu, B. Expression of lncRNA CCAT2 in children with neuroblastoma and its effect on cancer cell growth. Mol. Cell Biochem. 2021, 476, 1871–1879. [Google Scholar] [CrossRef]
- Qiu, M.; Xu, Y.; Yang, X.; Wang, J.; Hu, J.; Xu, L.; Yin, R. CCAT2 is a lung adenocarcinoma-specific long non-coding RNA and promotes invasion of non-small cell lung cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014, 35, 5375–5380. [Google Scholar] [CrossRef]
- Chen, S.; Wu, H.; Lv, N.; Wang, H.; Wang, Y.; Tang, Q.; Shao, H.; Sun, C. LncRNA CCAT2 predicts poor prognosis and regulates growth and metastasis in small cell lung cancer. Biomed. Pharmacother. 2016, 82, 583–588. [Google Scholar] [CrossRef]
- Zhao, C.; Qiao, C.; Zong, L.; Chen, Y. Long non-coding RNA-CCAT2 promotes the occurrence of non-small cell lung cancer by regulating the Wnt/beta-catenin signaling pathway. Oncol. Lett. 2018, 16, 4600–4606. [Google Scholar]
- Esfandi, F.; Fallah, H.; Arsang-Jang, S.; Taheri, M.; Ghafouri-Fard, S. The Expression of CCAT2, UCA1, PANDA and GHET1 Long Non-coding RNAs in Lung Cancer. Rep. Biochem. Mol. Biol. 2019, 8, 36–41. [Google Scholar]
- Yu, W.-L.; Yao, J.-J.; Xie, Z.-Z.; Huang, Y.-J.; Xiao, S. LncRNA PRNCR1 rs1456315 and CCAT2 rs6983267 Polymorphisms on 8q24 Associated with Lung Cancer. Int. J. Gen. Med. 2021, 14, 255–266. [Google Scholar] [CrossRef]
- Ruan, R.; Zhao, X.-L. LncRNA CCAT2 enhances cell proliferation via GSK3beta/beta-catenin signaling pathway in human osteosarcoma. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 2978–2984. [Google Scholar]
- Yan, L.; Wu, X.; Yin, X.; Du, F.; Liu, Y.; Ding, X. LncRNA CCAT2 promoted osteosarcoma cell proliferation and invasion. J. Cell Mol. Med. 2018, 22, 2592–2599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, F.; Chen, C.; Fu, J.; Yu, L.; Geng, J. Inhibiting proliferation and metastasis of osteosarcoma cells by downregulation of long non-coding RNA colon cancer-associated transcript 2 targeting microRNA-143. Oncol. Lett. 2021, 21, 265. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Ye, X. Knockdown of long non-coding RNA CCAT2 suppresses the progression of thyroid cancer by inhibiting the Wnt/β-catenin pathway. Int. J. Mol. Med. 2020, 46, 2047–2056. [Google Scholar] [CrossRef]
- Fu, W.; Wang, X.-D.; Ye, J.-D.; Jin, J.; Chen, L.; Qi, Q.-Y. CCAT2 contributes to progression and treatment resistance of thyroid carcinoma. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 12224–12231. [Google Scholar]
- Xu, H.; Yin, Q.; Shen, X.; Ju, S. Long non-coding RNA CCAT2 as a potential serum biomarker for diagnosis and prognosis of multiple myeloma. Ann. Hematol. 2020, 99, 2159–2171. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-J.; Zhu, J.-F.; Zhang, F.; Zhang, P.-P.; Zhang, J.-J. Upregulation of lncRNA CCAT2 predicts poor prognosis in patients with acute myeloid leukemia and is correlated with leukemic cell proliferation. Int. J. Clin. Exp. Pathol. 2018, 11, 5658–5666. [Google Scholar]
- Redis, R.S.; Sieuwerts, A.M.; Look, M.P.; Tudoran, O.; Ivan, C.; Spizzo, R.; Zhang, X.; de Weerd, V.; Shimizu, M.; Ling, H. CCAT2, a novel long non-coding RNA in breast cancer: Expression study and clinical correlations. Oncotarget 2013, 4, 1748–1762. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; He, J.; Zhang, D. Suppression of long non-coding RNA CCAT2 improves tamoxifen-resistant breast cancer cells’ response to tamoxifen. Mol. Biol. 2016, 50, 821–827. [Google Scholar] [CrossRef]
- Sarrafzadeh, S.; Geranpayeh, L.; Tasharrofi, B.; Soudyab, M.; Nikpayam, E.; Iranpour, M.; Mirfakhraie, R.; Gharesouran, J.; Ghafouri-Fard, S.; Ghafouri-Fard, S. Expression Study and Clinical Correlations of MYC and CCAT2 in Breast Cancer Patients. Iran Biomed. J. 2017, 21, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Deng, X.; Zhao, Y.; Wu, X.; Song, G. Upregulation of CCAT2 promotes cell proliferation by repressing the P15 in breast cancer. Biomed. Pharmacother. 2017, 91, 1160–1166. [Google Scholar] [CrossRef]
- Wu, Z.-J.; Li, Y.; Wu, Y.-Z.; Wang, Y.; Nian, W.-Q.; Wang, L.-L.; Li, L.-C.; Luo, H.-L.; Wang, D.-L. Long non-coding RNA CCAT2 promotes the breast cancer growth and metastasis by regulating TGF-beta signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 706–714. [Google Scholar] [PubMed]
- Chen, X.; Liu, L.; Zhu, W. Up-regulation of long non-coding RNA CCAT2 correlates with tumor metastasis and poor prognosis in cervical squamous cell cancer patients. Int. J. Clin. Exp. Pathol. 2015, 8, 13261–13266. [Google Scholar] [PubMed]
- Wu, L.; Jin, L.; Zhang, W.; Zhang, L. Roles of Long Non-Coding RNA CCAT2 in Cervical Cancer Cell Growth and Apoptosis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016, 22, 875–879. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.-J.; Wang, D.; Zhao, M.; Sun, X.-J.; Li, Y.; Lin, H.; Che, Y.-Q.; Huang, C. Serum lncRNAs (CCAT2, LINC01133, LINC00511) with Squamous Cell Carcinoma Antigen Panel as Novel Non-Invasive Biomarkers for Detection of Cervical Squamous Carcinoma. Cancer Manag. Res. 2020, 12, 9495–9502. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Cai, H.; Jiang, H.; Li, W.; Shi, Y. Long coding RNA CCAT2 enhances the proliferation and epithelial-mesenchymal transition of cervical carcinoma cells via the microRNA-493-5p/CREB1 axis. Bioengineered 2021, 12, 6264–6274. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Qing, C.; Huang, Z.; Zhu, Y. The long non-coding RNA CCAT2 is up-regulated in ovarian cancer and associated with poor prognosis. Diagn. Pathol. 2016, 11, 49. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhou, S.; Guo, B. Vitamin D Suppresses Ovarian Cancer Growth and Invasion by Targeting Long Non-Coding RNA CCAT2. Int. J. Mol. Sci. 2020, 21, 2334. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Zhao, S.; He, X.; Zheng, Z.; Bai, W.; Duan, Y.; Che, Y.-Q.; Huang, C. The up-regulation of long non-coding RNA CCAT2 indicates a poor prognosis for prostate cancer and promotes metastasis by affecting epithelial-mesenchymal transition. Biochem. Biophys. Res. Commun. 2016, 480, 508–514. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, C.; Liu, Y.; Chen, M.; Zhou, Q.; Chen, Z.; He, A.; Zhao, G.; Guo, Y.; Wu, H. shRNA targeting long non-coding RNA CCAT2 controlled by tetracycline-inducible system inhibits progression of bladder cancer cells. Oncotarget 2016, 7, 28989–28997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Li, Y.; Wang, F.; Wang, X.; Cheng, B.; Ye, F.; Xie, X.; Zhou, C.; Lu, W. Suppressed miR-424 expression via upregulation of target gene Chk1 contributes to the progression of cervical cancer. Oncogene 2013, 32, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, F.; Jiang, Z.; Guan, H.; Jia, Q. CREB1 Suppresses Transcription of microRNA-186 to Promote Growth, Invasion and Epithelial-Mesenchymal Transition of Gastric Cancer Cells Through the KRT8/HIF-1α Axis. Cancer Manag. Res. 2020, 12, 9097–9111. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Li, J.; Meng, S.; Liang, Z.; Xu, X.; Zhu, Y.; Li, S.; Wu, J.; Xu, M.; et al. c-Met, CREB1 and EGFR are involved in miR-493-5p inhibition of EMT via AKT/GSK-3β/Snail signaling in prostate cancer. Oncotarget 2017, 8, 82303–82313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janikova, M.; Zizkova, V.; Skarda, J.; Kharaishvili, G.; Radova, L.; Kolar, Z. Prognostic significance of miR-23b in combination with P-gp, MRP and LRP/MVP expression in non-small cell lung cancer. Neoplasma 2016, 63, 576–587. [Google Scholar] [CrossRef]
- Wei, L.-X.; Zhou, R.-S.; Xu, H.-F.; Wang, J.-Y.; Yuan, M.-H. High expression of FOXC1 is associated with poor clinical outcome in non-small cell lung cancer patients. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2013, 34, 941–946. [Google Scholar] [CrossRef]
- Chand, V.; Pandey, A.; Kopanja, D.; Guzman, G.; Raychaudhuri, P. Opposing Roles of the Fork-head box genes FoxM1 and FoxA2 in Liver Cancer. Mol. Cancer Res. 2019, 1, 383604. [Google Scholar]
- Xu, X.; Chen, W.; Miao, R.; Zhou, Y.; Wang, Z.; Zhang, L.; Wan, Y.; Dong, Y.; Qu, K.; Liu, C. miR-34a induces cellular senescence via modulation of telomerase activity in human hepatocellular carcinoma by targeting FoxM1/c-Myc pathway. Oncotarget 2015, 6, 3988–4004. [Google Scholar] [CrossRef] [Green Version]
- Abdelmohsen, K.; Gorospe, M. Posttranscriptional regulation of cancer traits by HuR. Wiley Interdiscip Rev. RNA 2010, 1, 214–229. [Google Scholar] [CrossRef]
- Takagi, T.; Iio, A.; Nakagawa, Y.; Naoe, T.; Tanigawa, N.; Akao, Y. Decreased expression of microRNA-143 and -145 in human gastric cancers. Oncology 2009, 77, 12–21. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, J. Prognostic role of microRNA-145 in prostate cancer: A systems review and meta-analysis. Prostate Int. 2015, 3, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Sachdeva, M.; Zhu, S.; Wu, F.; Wu, H.; Walia, V.; Kumar, S.; Elble, R.; Watabe, K.; Mo, Y.-Y. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc. Natl. Acad. Sci. USA 2009, 106, 3207–3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Guo, H.; Zhang, H.; Wang, H.; Qian, G.; Fan, X.; Hoffman, A.R.; Hu, J.-F.; Ge, S. Putative tumor suppressor miR-145 inhibits colon cancer cell growth by targeting oncogene Friend leukemia virus integration 1 gene. Cancer 2011, 117, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Nangia-Makker, P.; Farhana, L.; GRajendra, S.; Levi, E.; Majumdar, A.P.N. miR-21 and miR-145 cooperation in regulation of colon cancer stem cells. Mol. Cancer 2015, 14, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciot, R. MDM2 Amplified Sarcomas: A Literature Review. Diagnostics 2021, 11, 496. [Google Scholar] [CrossRef]
- Meng, X.; Franklin, D.A.; Dong, J.; Zhang, Y. MDM2-p53 pathway in hepatocellular carcinoma. Cancer Res. 2014, 74, 7161–7167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Sun, Q.; Zhang, Z.; Ge, S.; Han, Z.-G.; Chen, W.-T. Loss of microRNA-143/145 disturbs cellular growth and apoptosis of human epithelial cancers by impairing the MDM2-p53 feedback loop. Oncogene 2013, 32, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-F.; Alshareef, A.; Wu, C.; Jiao, J.-W.; Sorensen, P.H.; Lai, R.; Xu, L.-Y.; Li, E.-M. miR-200b induces cell cycle arrest and represses cell growth in esophageal squamous cell carcinoma. Carcinogenesis 2016, 37, 858–869. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-F.; Zhang, K.; Liao, L.-D.; Li, L.-Y.; Du, Z.-P.; Wu, B.-L.; Wu, J.-Y.; Xu, X.-E.; Zeng, F.-M.; Chen, B.; et al. miR-200b suppresses invasiveness and modulates the cytoskeletal and adhesive machinery in esophageal squamous cell carcinoma cells via targeting Kindlin-2. Carcinogenesis 2014, 35, 292–301. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Hu, P.-S.; Zuo, Z.; Lin, J.-F.; Li, X.; Wu, Q.-N.; Chen, Z.-H.; Zeng, Z.-L.; Wang, F.; Zheng, J.; et al. METTL3 facilitates tumor progression via an m6A-IGF2BP2-dependent mechanism in colorectal carcinoma. Mol. Cancer 2019, 18, 112. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dai, G.; Yu, L.; Hu, Q.; Chen, J.; Guo, W. miR-143-3p inhibits the proliferation, migration and invasion in osteosarcoma by targeting FOSL2. Sci. Rep. 2018, 8, 606. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Guan, S.; Hou, Y.; Qin, Y.; Zeng, H.; Yang, L.; Qiao, Y.; Liu, S.; Li, Q.; Jin, T.; et al. FOSL2 promotes VEGF-independent angiogenesis by transcriptionnally activating Wnt5a in breast cancer-associated fibroblasts. Theranostics 2021, 11, 4975–4991. [Google Scholar] [CrossRef]
- Yin, J.; Hu, W.; Fu, W.; Dai, L.; Jiang, Z.; Zhong, S.; Deng, B.; Zhao, J. HGF/MET Regulated Epithelial-Mesenchymal Transitions And Metastasis By FOSL2 In Non-Small Cell Lung Cancer. OncoTargets Ther. 2019, 12, 9227–9237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, W.-J.; Peng, J.-B.; Yin, J.-Y.; Li, X.-P.; Zheng, W.; Xiao, L.; Tan, L.-M.; Xiao, D.; Chen, Y.-X.; Li, X.; et al. Association between well-characterized lung cancer lncRNA polymorphisms and platinum-based chemotherapy toxicity in Chinese patients with lung cancer. Acta Pharmacol. Sin. 2017, 38, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wei, Y.; Wang, L.; Debeb, B.G.; Yuan, Y.; Zhang, J.; Yuan, J.; Wang, M.; Chen, D.; Sun, Y.; et al. ATM-mediated stabilization of ZEB1 promotes DNA damage response and radioresistance through CHK1. Nat. Cell Biol. 2014, 16, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.-J.; Yin, J.-Y.; Li, X.-P.; Fang, C.; Xiao, D.; Zhang, W.; Zhou, H.-H.; Li, X.; Liu, Z.-Q. Association of well-characterized lung cancer lncRNA polymorphisms with lung cancer susceptibility and platinum-based chemotherapy response. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 8349–8358. [Google Scholar] [CrossRef] [PubMed]
- David, L.; Fernandez-Vidal, A.; Bertoli, S.; Grgurevic, S.; Lepage, B.; Deshaies, D.; Prade, N.; Cartel, M.; Larrue, C.; Sarry, J.-E.; et al. CHK1 as a therapeutic target to bypass chemoresistance in AML. Sci. Signal 2016, 9, ra90. [Google Scholar] [CrossRef]
- Ohba, S.; Yamashiro, K.; Hirose, Y. Inhibition of DNA Repair in Combination with Temozolomide or Dianhydrogalactiol Overcomes Temozolomide-Resistant Glioma Cells. Cancers 2021, 13, 2570. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Yang, Y.; An, W.; Xu, J.; Zhang, G.; Jie, J.; Zhang, Q. The long noncoding RNA-ROR promotes the resistance of radiotherapy for human colorectal cancer cells by targeting the p53/miR-145 pathway. J. Gastroenterol. Hepatol. 2017, 32, 837–845. [Google Scholar] [CrossRef]
- Liu, L.-Z.; Zhou, X.-D.; Qian, G.; Shi, X.; Fang, J.; Jiang, B.-H. AKT1 amplification regulates cisplatin resistance in human lung cancer cells through the mammalian target of rapamycin/p70S6K1 pathway. Cancer Res. 2007, 67, 6325–6332. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Liu, L.-Z.; Qian, X.; Chen, Q.; Jiang, Y.; Li, D.; Lai, L.; Jiang, B.-H. MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res. 2012, 40, 761–774. [Google Scholar] [CrossRef]
- Fu, D.; Zhang, Y.; Cui, H. Long noncoding RNA CCAT2 is activated by E2F1 and exerts oncogenic properties by interacting with PTTG1 in pituitary adenomas. Am. J. Cancer Res. 2018, 8, 245–255. [Google Scholar]
- Zang, Y.; Li, J.; Wan, B.; Tai, Y.; Liu, H.; Li, Q.; Ji, Y. Long non-coding RNA CCAT2 drives the growth of laryngeal squamous cell carcinoma via regulating YAP activity. Hum. Cell 2021, 34, 1878–1887. [Google Scholar] [CrossRef]
- Cheng, J.; Xie, H.-Y.; Xu, X.; Wu, J.; Wei, X.; Su, R.; Zhang, W.; Lv, Z.; Zheng, S.; Zhou, L. NDRG1 as a biomarker for metastasis, recurrence and of poor prognosis in hepatocellular carcinoma. Cancer Lett. 2011, 310, 35–45. [Google Scholar] [CrossRef]
- Yu, Z.-Y.; Wang, Z.; Lee, K.-Y.; Yuan, P.; Ding, J. Effect of silencing colon cancer-associated transcript 2 on the proliferation, apoptosis and autophagy of gastric cancer BGC-823 cells. Oncol. Lett. 2018, 15, 3127–3132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, H.; Arao, T.; Togashi, Y.; Kato, H.; Fujita, Y.; De Velasco, M.A.; Kimura, H.; Matsumoto, K.; Tanaka, K.; Okamoto, I.; et al. The OCT4 pseudogene POU5F1B is amplified and promotes an aggressive phenotype in gastric cancer. Oncogene 2015, 34, 199–208. [Google Scholar] [CrossRef]
- Tian, G.-W.; Li, N.; Xin, Y. Prognostic and clinicopathological significance of CCAT2 in Chinese patients with various tumors. Int. J. Biol. Markers 2017, 32, e344–e351. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Z.; Xu, H.; He, A.; Liu, Y.; Huang, W. Long noncoding RNA CCAT2 as a novel biomaker of metastasis and prognosis in human cancer: A meta-analysis. Oncotarget 2017, 8, 75664–75674. [Google Scholar] [CrossRef]
- Fan, Y.-H.; Fang, H.; Ji, C.-X.; Xie, H.; Xiao, B.; Zhu, X.-G. Long noncoding RNA CCAT2 can predict metastasis and poor prognosis: A meta-analysis. Clin. Chim. Acta 2017, 466, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Shaker, O.G.; Senousy, M.A.; Elbaz, E.M. Association of rs6983267 at 8q24, HULC rs7763881 polymorphisms and serum lncRNAs CCAT2 and HULC with colorectal cancer in Egyptian patients. Sci. Rep. 2017, 7, 16246. [Google Scholar] [CrossRef] [PubMed]
- Fosselteder, J.; Calin, G.A.; Pichler, M. Long non-coding RNA CCAT2 as a therapeutic target in colorectal cancer. Expert Opin. Ther. Targets 2018, 22, 973–976. [Google Scholar] [CrossRef]
| Cancer Type | Study Samples | CCAT2 Expression | Regulatory Targets | Biological Effect | Ref. |
|---|---|---|---|---|---|
| Colorectal cancer | Tissue: 191 CRC and ANTT Cell lines: COLO320D, MHCT116, RKO, HEK293 | ↑ | ↑Wnt/β-catenin ↑MYC, ↑TCF7L2 ↑ MS status | Invasion, Distant Metastasis | [16] |
| Cell lines: HCT116, KM12C, KM12SM, COLO320, DLD-1, HT29 In vivo model: CCAT2 transgenic mice, WT mice | ↑ | ↑BOP1 ↑AURK B | Chromosomal instability, Chemoresistance to 5 fluorouracil and oxaliplatin | [32] | |
| Tissue: 218 CRC and ANTT | ↑ | Differentiation grade TNM stage, Lymph nodes metastasis, Distant metastasis, Vascular invasion, Poor prognosis | [47] | ||
| Tissue: 149 CRC and ANTT | ↑ | Distant metastasis | [48] | ||
| Cell lines: HCT-116, HT-29 | ↑ | ↓ pre-miR-145, ↑ miR-21 | Proliferation, Invasion | [49] | |
| Tissue: 280 CRC and ANTT | ↑ | ↑MS status ↑ MYC | Poor prognosis, Lymph nodes metastasis, TNM stage | [50] | |
| Blood: 63 CRC and 40 Controls | N/A | - | - | [51] | |
| Tissue: 60 CRC, 30 Colon polyps, and 60 non-cancers. | ↑ along with CCAT1, CCAT2, H19, HOTAIR, HULC, MALAT1, PCAT1, MEG3, PTENP1, and TUSC7 | Part of a stool lncRNA panel for CRC detection | [52] | ||
| Tissue: 80 CRC and ANTT Cell lines: FHC, HT29, Lovo, HCT-116. | ↑ | - | Cellular growth, Proliferation, Antiapoptotic | [53] | |
| Tissue: 150 CRC and ANTT | ↑ | ↑MYC | Metastasis | [54] | |
| Esophageal cancer | Tissue: 229 ESCC and ANTT | ↑ | - | Poor prognosis, Lymph node metastasis, TNM stage | [55] |
| Tissue: 57 ESCC and ANTT Cell lines: TE13, KYSE410, ECA109, TE1/N: HEEC | ↑TE1, TE13, KYSE410 ↓ ECA109 | - | CCAT2 expression correlated with smoking status | [56] | |
| Cell lines: Eca-109, EC9706, KYSE150, TE-1/N: HEEC | ↑ | ↑BCL-2, ↓BAX, ↓CYCLIN D1, ↑Wnt pathway | Proliferation, Migration, Invasion | [57] | |
| Tissue: 62 OSCC and ANTT Cell lines: Tca8113, Cal27/hNOK | ↑ | ↑CCND1, ↑MYC, ↑Wnt/β-catenin | Poor prognosis, Invasion proliferation, T stage differentiation | [58] | |
| Tissue: 60 ESCC and 21 esophageal mucosa Cell lines: HEEC, TE-1, TE-3, ECA109, KYSE410, KYSE520 | ↑ | ↓miR-145, ↑p70S6K1, ↓p53 pathway | Radiotherapy resistance, cellular proliferation. | [59] | |
| Tissue: 33 ESCC and ANTT Cell lines: KYSE-410, KYSE-150, TE10, TE11, TE13/HET-1A | ↑ | β -catenin/ WISP1 signaling pathway | Cell proliferation, Invasion, Poor prognosis. | [26] | |
| Tissue: 93 ESCC and ANTT Cell lines: Eca109, TE-1, EC-1, ESC410/HET-1A | ↑ | ↓miR-200b ↑IGF2BP2/TK1 Axis | Migration, Invasion, Tumorigenesis | [60] | |
| Gastric cancer | Tissue: 85 GC and ANTT | ↑ | - | Lymph node metastasis Distant metastasis, Poor prognosis | [61] |
| Tissue: 108 GC and ANTT Cell lines: Tu: SGC7901, MKN45, BGC-823, MKN-28/N: GES-1 | ↑ | ↑ZEB2, ↑VIM, ↑CHD1, ↑CHD2 ↑EZH2, ↑3K27me3 ↑LSD1, ↑LATS | Poor prognosis Proliferation Migration, Invasion, EMT | [62] | |
| Tissue: 60 GC and ANTT Cell lines: GES-1, RGM-1, SGC-7901, SNU-1, HGC-27 | ↑ | ↑mTOR signaling | Proliferation, Metastasis | [63] | |
| Serum: 167 GC and 110 controls | ↑ | - | Tumor stage, Invasion, Lymph node metastasis | [64] | |
| Hepatocellular carcinoma | Tissue: 50 HCC and ANTT Cell lines: Tu: HepG2, HEP3B, HCCLM3, HuH7/L02 | ↑ | - | Proliferation, Migration Antiapoptotic | [65] |
| Tissue: 60 HCC and ANTT Cell lines: SMMC-7721, PLC/PRF/5, Huh7, SK-Hep-1, Hep3B | ↑ | ↑FOXM1 ↓ miR-34a | Poor prognosis, Proliferation, Tumor growth, Antiapoptotic | [66] | |
| Tissue: 96 HCC and ANTT Cell lines: HepG2, SMMC772, MHCC97H /MIHA | ↑ | ↑CDH1, ↑CDH2, ↑VIM, ↑SNAI2 | Poor prognosis, TNM stage, Vascular invasion, Alcoholism history, Migration, Invasion, EMT | [67] | |
| Cell lines: SMMC7721, SK-hep1, HepG2, Huh7/L02 | ↑ | ↑NDRG1 promoter | Proliferation, Migration, Invasion | [68] | |
| Cell lines: Hep3B, HepG2, and THLE-3, MHCC97H | ↑ | ↓miR-145 ↑MDM2 | Proliferation, Metastasis | [45] | |
| Tissue: 61 HCC and ANTT Cell lines: HepG2 HCCLM3 | ↑ | ↓miR- 4496 ↑ELAVL1 | Advanced stage, Venous invasion, Migration, Invasion | [69] | |
| Pancreatic cancer | Tissue: 80 PDAC and ANTT Cell lines: PANC-1, SW1990, PC-3/HPDE6-C7 | ↑ | ↑KRAS, ↑MEK/ERK | Poor prognosis, Proliferation, Invasion Tumor growth | [70] |
| Glioma | Cell lines: A172, U87-MG, U251, T98G/HUVECs | ↑VEGF, ↑TGFβ, ↑FGF | Angiogenesis, Migration Proliferation | [71] | |
| Tissue:134 Glioma and ANTT Cell lines: U87-MG, U251 | ↑ | ↑Wnt/β-catenin | TNM stage, Proliferation Cell cycle, Migration Tumor growth | [72] | |
| Tissue: 134 Glial tumors and ANTT Cell lines: U87, U251, A172, SHG44/Normal human astrocyte cell line | ↑ | ↑CDH1, ↑CDH2, ↑VIM, ↑TWIST, ↑ SNAI1 | Poor prognosis, Tumor grade, Tumor size, Proliferation, Migration, Invasion, Apoptosis, EMT | [73] | |
| Tissue: 74 PA and ANTT Cell line: HP75 | ↑ | ↑E2F1 ↑PTTG1 | Poor prognosis, Proliferation, Antiapoptotic, Cell cycle, Migration, Invasion | [74] | |
| Tissue: 138 Gliomas and ANTT Cell lines: U251, U87, A172, SHG44. | ↑ | ↓ miR-424 ↑CHK1 | Proliferation, Invasion, Migration via miR-424 sponging and CHK1 regulation | [44] | |
| Cell lines: A172, U251 | ↑ | ↓ miR-424 ↑ VEGFA | Proliferation, Migration, Angiogenesis | [43] | |
| Neuroblastoma | Tissue: 96 Neuroblastomas and ANTT Cell lines: SH-SY5Y, SK-N-SH/HUVEC | ↑ | ↓P53 ↑BCL-2 | Antiapoptotic, Cell growth, Poor prognosis | [75] |
| Lung cancer | Tissue: 57 NSCLC and ANTT cell lines: A549, NCI-H1975, NCI-H358, NCI-H1650, NCI-H1299, SK-MES-1, Pc-9/HBE | ↑ ↑ H1975, Pc9, NCI-H358 ↓NCI-H1299 NCI-H1650, A549 SK-MES-1 | Proliferation, Invasion | [76] | |
| Tissue: 112 SCLC and ANTT Cell lines: DMS-53, H446/16 HBE | ↑ | Poor progression, Clinical stage, Tumor size, Distant metastasis Proliferation, Invasion | [77] | ||
| Tissue: 36 NSCLC and ANTT Cell lines: NCI-H1975 | ↑ | ↑Wnt/β-catenin | Tumor size, Lymph node metastasis | [78] | |
| Cell lines:A549, SPC-A- 1, H1395, H441, H1975/BEAS-2B | ↑ | ↑FOXC1 ↓ miR-23b-5p | Proliferation, Migration | [42] | |
| Tissue: 32 NSCLC and ANTT | N/A | - | - | [79] | |
| Serum: 438 LC and 438 controls | ↑ | - | - | [80] | |
| Osteosarcoma | Tissue: 50 OS and ANTT Cell lines: SAOS-2, MG63, U2-OS/Normal osteoblast cell line | ↑ | ↑GSK3β/β-catenin | Tumor size, Poor prognosis, Proliferation | [81] |
| Tissue: 40 OS/ANTT Cell lines: SOSP-9607, MG-63, U2OS, SAOS-2/hFOB | ↑ | ↑LATS2, ↑MYC ↑CDH1, ↑CHD2, ↑SNAI1 | Poor prognosis, Proliferation. EMT | [82] | |
| Cell lines: SOSP-9607, MG-63, U2OS, SAOS-2 /hFOB | ↑ | ↓miR-143, ↑FOSL2 | Proliferation, Metastasis | [83] | |
| Thyroid cancer | Tissues: 30 pairs TC and ANTT (papillary, follicular, and anaplastic) Cell lines: TPC- 1, TH83, IHH4, FTC- 133, FTC- 238/Nthy-ori3-1 | ↑ | ↑Wnt/β-catenin | Proliferation, Migration, Invasion, Apoptosis | [84] |
| Tissue: 60 anaplastic and papillary TC and ANTT Cell lines: TC cell lines | ↑ | - | Doxorubicin and cisplatin resistance, Increased tumor size, Poor prognosis, | [85] | |
| Multiple myeloma | Serum: 106 MM and 106 matched normal controls | ↑ | - | ISS stages, Renal dysfunction, Serum creatinine | [86] |
| Acute myeloid leukemia | Bone marrow samples: 46 patients and 46 healthy volunteers Cell lines: KG-1 | ↑ | Cell cycle arrest in S phase | Cellular proliferation, Poor prognosis | [87] |
| Breast cancer | Tissue: 997 BC and ANTT and 56 BC and ANTT Cell lines: MDA-MB-231, MDA-MB-436 | ↑ | - | Poor prognosis, Therapeutic response | [88] |
| Tissue: 67 BC and ANTT Cell lines: MDA-MB-231, MCF-7/Hs578Bst | ↑ | ↑Wnt/β-catenin ↑CCND1 ↑MYC | Poor prognosis, Proliferation, Invasion, Tumorigenesis | [30] | |
| Cell lines: MCF-7, T47 D tamoxifen resistant/MCF-7, T47D–tamoxifen responsive | ↑ tamoxifen-resistant cell lines | Suppressing CCAT2 expression improves sensitivity to tamoxifen in resistant cells | [89] | ||
| Tissue: 48 BC and ANTT | ↑ | - | Lymph node metastasis | [90] | |
| Tissue: 67 BC and ANTT Cell lines: MDA-MB-231, MCF-7/MCF10A | ↑ | ↑P15 ↑EZH2 | Poor prognosis, Proliferation, Invasion, Cell cycle, Tumor growth | [91] | |
| Tissue: 60 BC and ANTT Cell lines: LCC9, MDA-MB-231 MCF-7/HCC1937 | ↑ | ↑TGF-β, ↑Smad2, ↑α-SMA | Lymph node metastasis Proliferation, Invasion, Migration, Apoptosis, Cell cycle | [92] | |
| Endometrial cancer | Tissue: 30 EC and ANTT Cell lines: HEC-1-A and RL95-2 | ↑ | ↓miR-216b ↑PI3K/AKT | Proliferation, Migration, Invasion, Apoptosis | [41] |
| Cervical cancer | Tissue: 123 SCCC and ANTT | ↑ | - | FIGO stage, Lymph node metastasis, Cervical invasion, Poor prognosis | [93] |
| Cell lines: CaSki, HeLa, SiHa | ↑ | - | Proliferation, Apoptosis | [94] | |
| Serum: 115 SCCC, 79 CIN, and 110 healthy controls | ↑ | CCAT2, LINC01133, LINC00511 upregulated in serum of SCCC and CIN patients. | [95] | ||
| Tissue: 30 SCCC and ANTT Cell lines: GH329, CaSki, HeLa, SiHa, C4-1. Xenografts: 2 groups pSilencer, pSilencer/sh-CCAT2 | ↑ | ↓miR-493-5p ↑CREB1 | EMT, Proliferation | [96] | |
| Ovarian Cancer | Tissue: 31 EOC and ANTT Cell lines: SKOV3, MC685, A2780, HO8910/IOSE 386 | ↑ | ↓miR-424 | Proliferation, Apoptosis | [39] |
| Cell lines: SKOV3, A2780, HO8910/HOSE, HUM-CELL-0088 | ↑ | ↑CDH1, ↑CHD2, ↑SNAI1, ↑SNAI2, ↑TWIST1, ↑Wnt/β-catenin | Migration, Invasion, EMT | [28] | |
| Tissue: 109 EOC and ANTT Cell lines: SKOV3, IGROV1, A2780, OVCAR3/HOSE 6.3 | ↑ | - | FIGO stage, Tumor grade, Distant metastasis, Poor prognosis, Proliferation Migration, Invasion | [97] | |
| Cell lines: SKOV3 and A2780 | ↑ | ↑TCF7L2, ↑MYC | Vitamin D suppresses CCAT2 expression | [98] | |
| Prostate cancer | Tissue: 96 PC and ANTT Cell lines: DU-145, 22RV1/WPMY-1 | ↑ | - | Poor prognosis, Proliferation, Migration Invasion, EMT | [99] |
| Renal cell cancer | Tissue: 61ccRCC and ANTT Cell lines: 786-O, AHCN ccRCC/HK-2 | ↑ | ↑Wnt/β-catenin | Poor prognosis, Proliferation, Migration, Apoptosis, Invasion | [29] |
| Bladder cancer | Tissue: 48 BC and ANTT Cell lines: SV-HUC-1/T24, 5637 | ↑ | - | Tumor grade, TNM stage, Proliferation, Migration, Apoptosis | [100] |
| Cancer Type | Role of CCAT2 in Therapeutic Resistance | Ref. |
|---|---|---|
| Thyroid cancer | Upregulation is associated with chemoresistance to doxorubicin and cisplatin. | [85] |
| Colorectal cancer | Upregulation is associated with chromosomal instability and chemotherapy resistance to 5-fluorouracil and oxaliplatin. | [32] |
| Lung cancer | Presence of the rs6983267 SNP was associated with reduced hematological toxicity to platinum-based chemotherapy and platinum-based chemotherapy response. | [123,125] |
| Breast cancer | Upregulation enhances tamoxifen resistance in breast cancer cell lines. | [89] |
| Glioblastoma | Upregulation in glioblastoma cell lines increases resistance to teniposide, temozolomide, vincristine, and cisplatin. | [44] |
| Esophageal squamous cell carcinoma | Upregulation promotes radiotherapy resistance in ESCC cell lines by inhibiting miR-145, the expression level of P70 ribosomal protein S6 kinase 1, p53, and p21. | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirlog, R.; Drula, R.; Nutu, A.; Calin, G.A.; Berindan-Neagoe, I. The Roles of the Colon Cancer Associated Transcript 2 (CCAT2) Long Non-Coding RNA in Cancer: A Comprehensive Characterization of the Tumorigenic and Molecular Functions. Int. J. Mol. Sci. 2021, 22, 12491. https://doi.org/10.3390/ijms222212491
Pirlog R, Drula R, Nutu A, Calin GA, Berindan-Neagoe I. The Roles of the Colon Cancer Associated Transcript 2 (CCAT2) Long Non-Coding RNA in Cancer: A Comprehensive Characterization of the Tumorigenic and Molecular Functions. International Journal of Molecular Sciences. 2021; 22(22):12491. https://doi.org/10.3390/ijms222212491
Chicago/Turabian StylePirlog, Radu, Rares Drula, Andreea Nutu, George Adrian Calin, and Ioana Berindan-Neagoe. 2021. "The Roles of the Colon Cancer Associated Transcript 2 (CCAT2) Long Non-Coding RNA in Cancer: A Comprehensive Characterization of the Tumorigenic and Molecular Functions" International Journal of Molecular Sciences 22, no. 22: 12491. https://doi.org/10.3390/ijms222212491








