Design and Engineering of “Green” Nanoemulsions for Enhanced Topical Delivery of Bakuchiol Achieved in a Sustainable Manner: A Novel Eco-Friendly Approach to Bioretinol
Abstract
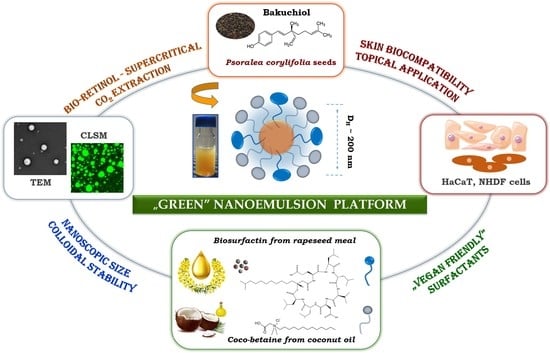
:1. Introduction
2. Results and Discussion
2.1. Supercritical Fluid Extraction—Obtaining the Purest Extracts
2.2. Physicochemical Characteristics and Optimization Studies
2.3. Evaluation of Skin Biological Responses: In Vitro, Ex Vivo, and In Vivo
3. Materials and Methods
3.1. Chemicals
3.2. Surfactin Synthesis
3.2.1. Synthetic Protocol
3.2.2. Analytical Evaluation
3.3. Supercritical CO2 Extraction Procedure (Bakuchiol-Rich Extract Fabrication)
3.3.1. SC-CO2 Protocol
3.3.2. Analytical Evaluation
3.4. Ternary Phase Diagrams and Nanoemulsion Preparation
3.5. Centrifugation Test and Heating and Cooling Cycles
3.6. Nanoemulsion Characterization Methods
3.6.1. Dynamic Light Scattering (DLS) and Electrophoretic Light Scattering (ELS)
3.6.2. Backscattering (BS)
3.6.3. Transmission Electron Microscopy (TEM)
3.6.4. Confocal Laser Scanning Microscopy (CLSM)
3.7. In Vitro, Ex Vivo, and In Vivo Topical Experiments
3.7.1. Cell Cultures
3.7.2. Colorimetric Cell Viability Assay
3.7.3. Ex Vivo Diffusion Test on Franz Cells
3.7.4. Ex Vivo Penetration Studies by Confocal Laser Scanning Microscopy (CLSM)
3.7.5. In Vivo Skin Contact Study
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Pucek, A.; Tokarek, B.; Waglewska, E.; Bazylińska, U. Recent advances in the structural design of photosensitive agent formulations using “soft” colloidal nanocarriers. Pharmaceutics 2020, 12, 587. [Google Scholar] [CrossRef] [PubMed]
- Naseema, A.; Kovooru, L.; Behera, A.K.; Kumar, K.P.P.; Srivastava, P. A critical review of synthesis procedures, applications and future potential of nanoemulsion. Adv. Colloid Interface Sci. 2021, 287, 102318. [Google Scholar]
- Gupta, A.; Burak Eral, H.; Alan Hatton, T.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswal, T.; Kumar, S.; Jena, B.; Pradhan, D. Sustainable biomaterials and their applications: A short review. Mater. Today Proc. 2020, 30, 274–282. [Google Scholar] [CrossRef]
- Bhadani, A.; Kafle, A.; Ogura, T.; Akamatsu, M.; Sakai, K.; Sakai, H.; Abe, M. Current perspective of sustainable surfactants based on renewable building blocks. Curr. Opin. Colloid Interface Sci. 2020, 45, 124–135. [Google Scholar] [CrossRef]
- Banat, I.M.; Carbou, Q.; Saucedo-Castaneda, G.; Cazares-Marinero, J.J. The green generation of speciality chemicals and potential production using solid-state fermentation (SSF) technology. Biores. Technol. 2021, 320, 124222. [Google Scholar] [CrossRef]
- Clendennen, S.K.; Boaz, N.W. Biobased Surfactants, 2nd ed.; Hayes, D.G., Solaiman, D.K.Y., Ashby, R.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Hunter, J.E.; Fowler, J.F. Safety to human skin of cocamidopropyl betaine: A mild surfactant for personal-care products. J. Surfactants Deterg. 1998, 1, 235–239. [Google Scholar] [CrossRef]
- Jahan, R.; Bodratti, A.M.; Tsianou, M.; Alexandridis, P. Biosurfactants, natural alternatives to synthetic surfactants: Physicochemical properties and applications. Adv. Colloid Interface Sci. 2020, 275, 102061. [Google Scholar] [CrossRef]
- Shaligram, N.S.; Singhal, R.S. Surfactin—A review on biosynthesis, fermentation, purification and applications. Food Technol. Biotechnol. 2010, 48, 119–134. [Google Scholar]
- Jajor, P.; Piłakowska-Pietras, D.; Krasowska, A.; Łukaszewicz, M. Surfactin analogues produced by Bacillus subtilis strains grown on rapeseed cake. J. Mol. Struct. 2016, 1126, 141–146. [Google Scholar] [CrossRef]
- Lewińska, A. Optimizing the process design of oil-in-water nanoemulsion for delivering poorly soluble cannabidiol oil. Processes 2021, 9, 1180. [Google Scholar] [CrossRef]
- Bazylinska, U.; Saczko, J. Nanoemulsion-templated polylelectrolyte multifunctional nanocapsules for DNA entrapment and bioimaging. Colloids Surf. B Biointerfaces 2016, 137, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Kim, Y.J.; Kim, B.Y.; Jeong, S.J. Bakuchiol suppresses inflammatory responses via the downregulation of the p38 MAPK/ERK signaling pathway. Int. J. Mol. Sci. 2019, 20, 3574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, R.K. Bakuchiol. In Cosmeceuticals and Active Cosmetics, 3rd ed.; Sivamani, R., Jagdeo, J., Elsner, P., Maibach, H., Eds.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Khuranna, D.; Sharma, S.; Mir, S.R.; Aqil, M.; Ahmad, A.; Rehman, M.U.; Ahmad, P.; Alwahibi, M.S.; Elshikh, M.S.; Mujeeb, M. Extraction, quantification, and cytokine inhibitory response of bakuchiol in Psoralea coryfolia Linn. Separations 2020, 7, 48. [Google Scholar] [CrossRef]
- Uikey, S.K.; Yadav, A.S.; Sharma, A.K.; Rai, A.K.; Raghuwanshi, D.K.; Badkhane, Y. The botany, chemistry, pharmacological and therapeutic application of Psoralea corylifolia L.: A review. Int. J. Phytomed. 2010, 2, 100–107. [Google Scholar] [CrossRef] [Green Version]
- Marcus, Y. Some advances in supercritical fluid extraction for fuels, biomaterials and purification. Processes 2019, 7, 156. [Google Scholar] [CrossRef] [Green Version]
- Manohar, B.; Sankar, K.U. Prediction of solubility of Psoralea corylifolia L. seed extract in supercritical carbon dioxide by equation of state models. Theor. Found. Chem. Eng. 2011, 45, 409. [Google Scholar] [CrossRef]
- Bubalo, M.C.; Vidovic, S.; Redovnikovic, I.R.; Jokic, S. Green solvents for green technologies. J. Chem. Technol. Biotechnol. 2015, 90, 1631. [Google Scholar] [CrossRef]
- Cheikh, A.; Tabka, H.; Tlili, Y.; Santulli, A.; Bouzouaya, N.; Bouhaouala-Zahar, B.; Benkhalifa, R. Xenopus oocyte’s conductance for bioactive compounds screening and characterization. Int. J. Mol. Sci. 2019, 20, 2083. [Google Scholar] [CrossRef] [Green Version]
- Bhawna, C.; Dhingra, A.K.; Dhar, K.L. Antimicrobial activity of Psoralea corylifolia Linn. (Baguchi) seeds extracts by organic solvents and supercritical fluids. Int. J. Pharm Clinic. Res. 2013, 5, 13–16. [Google Scholar]
- Wang, X.; Wang, Y.; Yuan, J.; Sun, Q.; Liu, J.; Zheng, C. An effcient new method for extraction, separation and purification of psoralen and isopsoralen from Fructus Psoraleae by supercritical fluid extraction and high-speed counter-current chromatography. J. Chromatogr. A 2004, 1055, 135–140. [Google Scholar] [CrossRef]
- Marzorati, S.; Schievano, A.; Ida, A.; Verotta, L. Carotenoids, chlorophylls and phycocyanin from Spirulina: Supercritical CO2 and water extraction methods for added value products cascade. Green Chem. 2020, 22, 187–196. [Google Scholar] [CrossRef]
- Lewińska, A.; Jaromin, A.; Jezierska, J. Role of architecture of N-oxide surfactants in the design of nanoemulsions for Candida skin infection. Colloids Surf. B Biointerfaces 2020, 187, 110639. [Google Scholar] [CrossRef] [PubMed]
- Raju, N.S.; Krishnaswami, V.; Vijayaraghavalu, S.; Kandasamy, R. Nanocosmetics Fundamentals, Application, Toxicology, Micro and Nano Technologies; Nanda, A., Nanda, S., Nguyen, T.A., Rajendran, S., Slimani, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; Available online: https://www.sciencedirect.com/science/book/9780128222867 (accessed on 31 December 2020).
- Bazylińska, U. Rationally designed double emulsion process for co-encapsulation of hybrid cargo in stealth nanocarriers. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 476–482. [Google Scholar] [CrossRef]
- National Toxicology Program Technical Report Series Ser 2012: Photocarcinogenesis Study of Retinoic Acid and Retinyl Palmitate [CAS Nos. 302-79-4 (All-Trans-Retinoic Acid) and 79-81-2 (All-Trans-Retinyl Palmitate)] in SKH-1 Mice (Simulated Solar Light and Topical Application Study); National Toxicology Program: 2012. Available online: https://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr568_508 (accessed on 16 September 2021).
- Garcia-Bilbao, A.; Gómez-Fernández, P.; Larush, L.; Soroka, Y.; Suarez-Merino, B.; Frušić-Zlotkin, M.; Magdassi, S.; Goñi-de-Cerio, F. Preparation, characterization, and biological evaluation of retinyl palmitate and Dead Sea water loaded nanoemulsions toward topical treatment of skin diseases. J. Bioact. Compat. Polym. 2020, 35, 24–38. [Google Scholar] [CrossRef]
- Kim, T.I.; Kim, T.G.; Lim, D.H.; Kim, S.B.; Park, S.M.; Hur, T.Y.; Ki, K.S.; Kwon, E.G.; Vijayakumar, M.; Kim, Y.J. Preparation of nanoemulsions of Vitamin A and C by microfluidization: Efficacy on the expression pattern of milk-specific proteins in MAC-T cells. Molecules 2019, 24, 2566. [Google Scholar] [CrossRef] [Green Version]
- Ohno, O.; Watabe, T.; Nakamura, K.; Kawagoshi, M.; Uotsu, N.; Chiba, T.; Yamada, M.; Yamaguchi, K.; Yamada, K.; Miyamoto, K.; et al. Inhibitory effects of bakuchiol, bavachin, and isobavachalcone isolated from piper longum on melanin production in b16 mouse melanoma cells. Biosci. Biotechnol. Biochem. 2010, 74, 1504–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Trotta, F.; Rao, R. Encapsulation of babchi oil in cyclodextrin-based nanosponges: Physicochemical characterization, photodegradation, and in vitro cytotoxicity studies. Pharmaceutics 2018, 10, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diridollou, S.; Vabre, V.; Berson, M.; Vaillant, L.; Black, D.; Lagarde, J.M.; Grégoire, J.M.; Gall, Y.; Patat, F. Skin ageing: Changes of physical properties of human skin in vivo. Int. J. Cosm. Sci. 2001, 23, 353–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewińska, A.; Kulbacka, J.; Domżał-Kędzia, M.; Witwicki, M. Antiradical properties of n-oxide surfactants-two in one. Int. J. Mol. Sci. 2021, 22, 8040. [Google Scholar] [CrossRef]







| Sample Number/ Seed Weight (g) | P (bar) | Static/Dynamic Interval (min/min) | Extraction Time (min) | Extract (g) | Yield (%) | Bakuchiol (%) | Psoralen (%) | Iso-Psoralen (%) | Sum of the Other Components (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1/80.003 | 280 ± 5 | 10/50 | 250 | 4.731 | 5.91 | 79.68 | 1.23 | 3.14 | 15.95 |
| 2/80.003 | 280 ± 5 | 15/15 | 420 | 5.035 | 6.29 | 80.96 | 1.52 | 4.15 | 13.37 |
| 3/80.005 | 280 ± 5 | 10/20 | 330 | 6.862 | 8.58 | 80.25 | 1.23 | 3.15 | 15.37 |
| 4/80.002 | 250 ± 5 | 10/20 | 420 | 5.257 | 6.57 | 81.42 | 1.65 | 4.27 | 12.66 |
| System | Nanoemulsion Composition/% | DH d/nm | PdI e | ζ f/mV | ||
|---|---|---|---|---|---|---|
| S a | O b | W c | ||||
| 1 | 10 | 5 | 85 | g | - | - |
| 2 | 10 | 2 | 88 | - | - | - |
| 3 | 5 | 2 | 93 | 243 ± 6 | 0.221 ± 0.02 | −66 ± 3 |
| 4 | 5 | 1 | 94 | 221 ± 4 | 0.182 ± 0.01 | −73 ± 5 |
| 5 | 3 | 1 | 96 | 200 ± 3 | 0.276 ± 0.02 | −70 ± 5 |
| Probant Age | Active Ingredient | Wrinkles (mm) | Discolorations (%) | Vessels (mm2) | |||
|---|---|---|---|---|---|---|---|
| 0 | 28th Day | 0 | 28th Day | 0 | 28th Day | ||
| 30+ | R | 0.085 ± 0.018 | 0.082 ± 0.02 | 10.87 ± 1.44 | 10.48 ± 1.36 | 2.61 ± 1.36 | 2.58 ± 1.19 |
| B | 0.086 ± 0.013 | 0.082 ± 0.017 | 10.97 ± 1.65 | 10.28 ± 1.79 | 2.04 ± 1.18 | 1.90 ± 1.35 | |
| 40+ | R | 0.100 ± 0.020 | 0.070 ± 0.040 | 2.87 ± 1.24 | 2.05 ± 1,63 | 11.51 ± 1.92 | 10.95 ± 1.31 |
| B | 0.090 ± 0.010 | 0.090 ± 0.020 | 2.87 ± 1.24 | 2.05 ± 1.63 | 10.60 ± 1,21 | 10.85 ± 1.20 | |
| 50+ | R | 0.099 ± 0.010 | 0.079 ± 0.035 | 12.23 ± 1.76 | 12.03 ± 1.57 | 2.82 ± 0.77 | 2.52 ± 1.35 |
| B | 0.098 ± 0.013 | 0.092 ± 0.007 | 12.34 ± 1.9 | 10.75 ± 1.71 | 3.68 ± 1.39 | 3.44 ± 0.75 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewińska, A.; Domżał-Kędzia, M.; Maciejczyk, E.; Łukaszewicz, M.; Bazylińska, U. Design and Engineering of “Green” Nanoemulsions for Enhanced Topical Delivery of Bakuchiol Achieved in a Sustainable Manner: A Novel Eco-Friendly Approach to Bioretinol. Int. J. Mol. Sci. 2021, 22, 10091. https://doi.org/10.3390/ijms221810091
Lewińska A, Domżał-Kędzia M, Maciejczyk E, Łukaszewicz M, Bazylińska U. Design and Engineering of “Green” Nanoemulsions for Enhanced Topical Delivery of Bakuchiol Achieved in a Sustainable Manner: A Novel Eco-Friendly Approach to Bioretinol. International Journal of Molecular Sciences. 2021; 22(18):10091. https://doi.org/10.3390/ijms221810091
Chicago/Turabian StyleLewińska, Agnieszka, Marta Domżał-Kędzia, Ewa Maciejczyk, Marcin Łukaszewicz, and Urszula Bazylińska. 2021. "Design and Engineering of “Green” Nanoemulsions for Enhanced Topical Delivery of Bakuchiol Achieved in a Sustainable Manner: A Novel Eco-Friendly Approach to Bioretinol" International Journal of Molecular Sciences 22, no. 18: 10091. https://doi.org/10.3390/ijms221810091