High-Payload Buccal Delivery System of Amorphous Curcumin–Chitosan Nanoparticle Complex in Hydroxypropyl Methylcellulose and Starch Films
Abstract
:1. Introduction
2. Results and Discussion
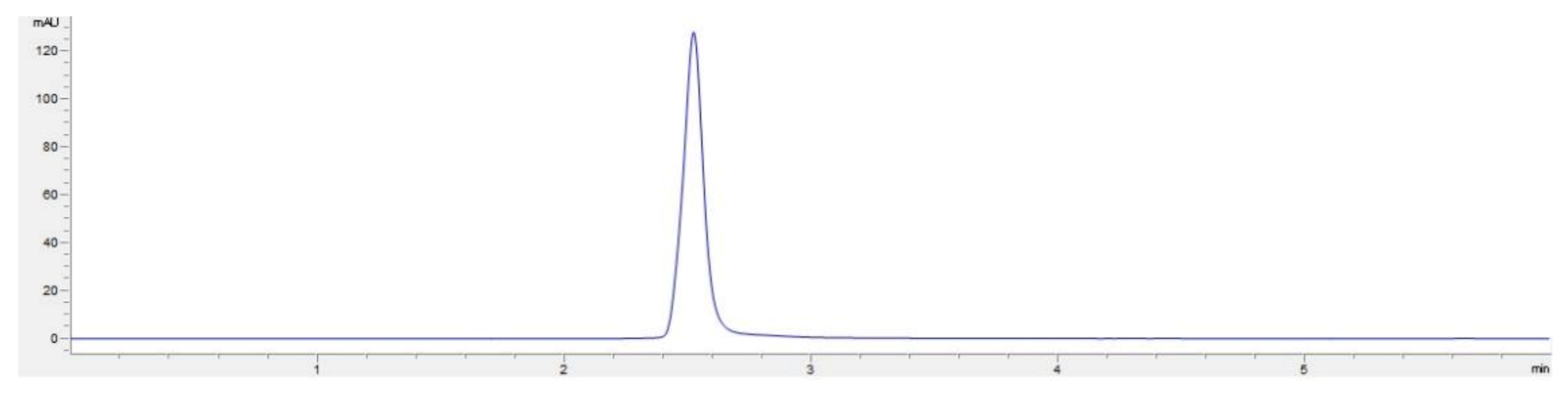
2.1. Physical Characteristics of Amorphous CUR–CHI Nanoplex
2.2. HPMC-Based Films
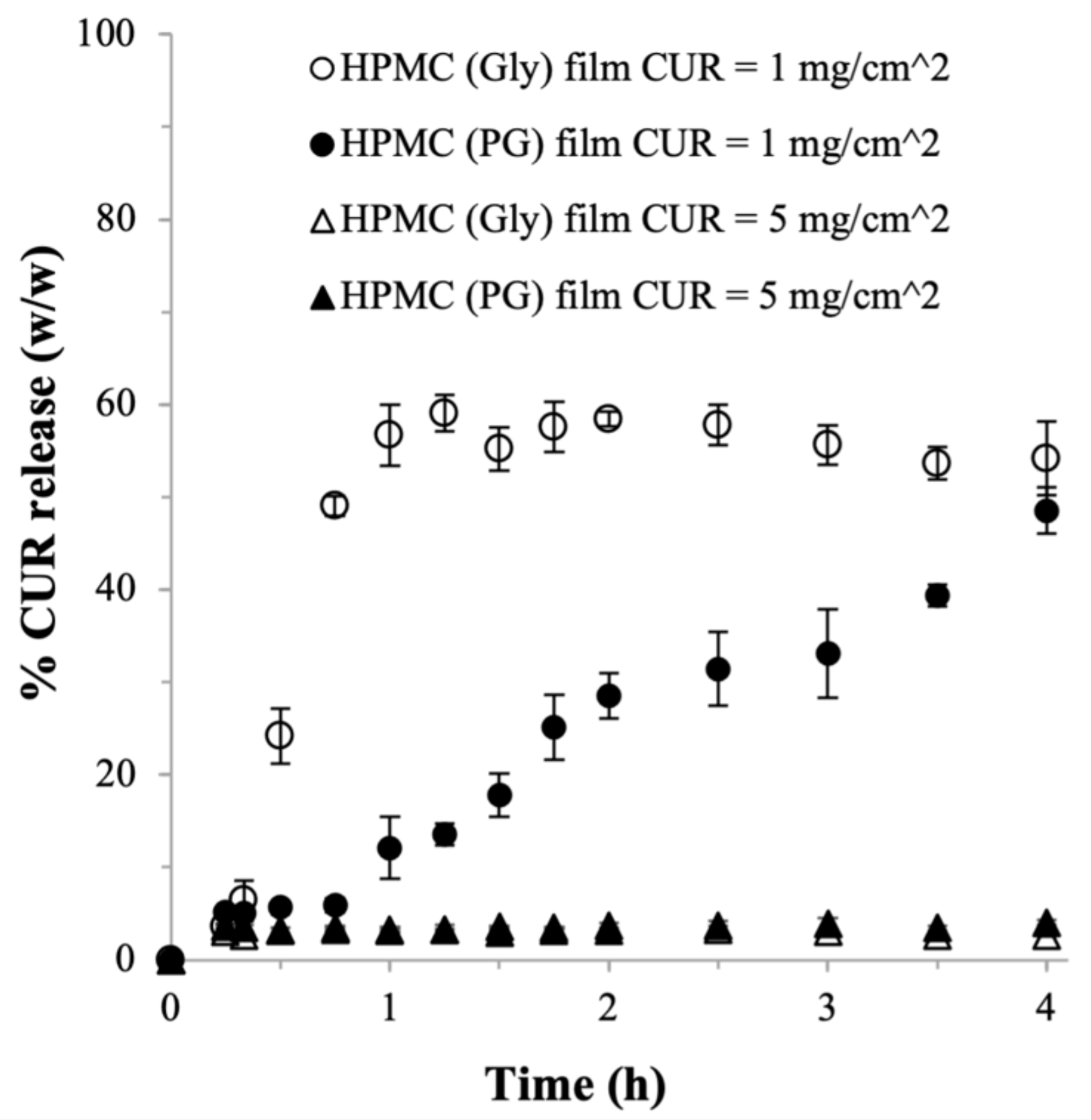
2.2.1. Effects of Plasticizer
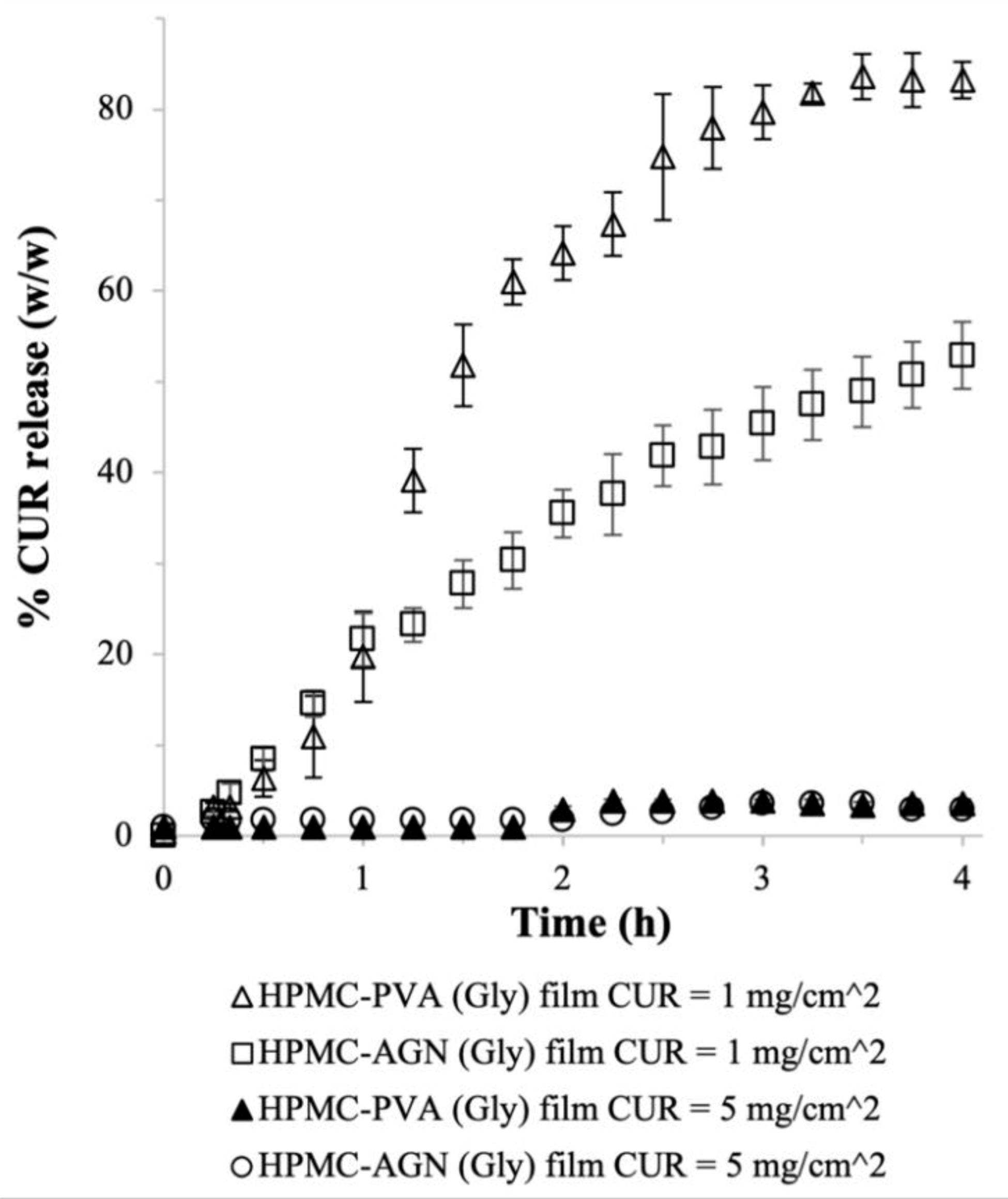
2.2.2. Effects of Adjuvants
2.3. Starch-Based Films
2.3.1. Physical Characteristics
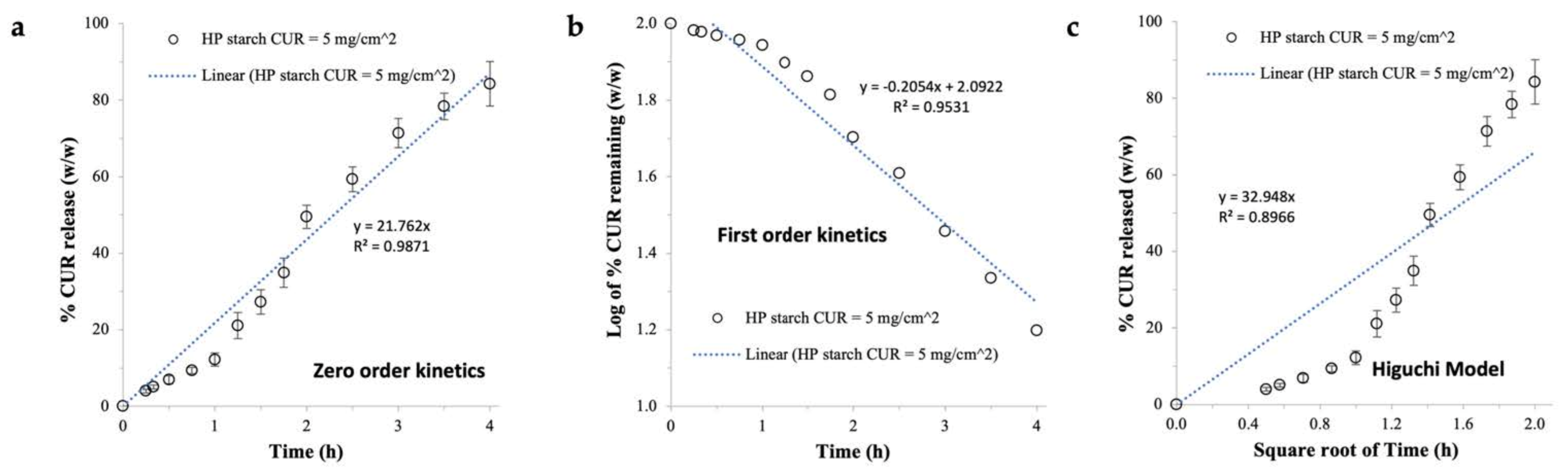
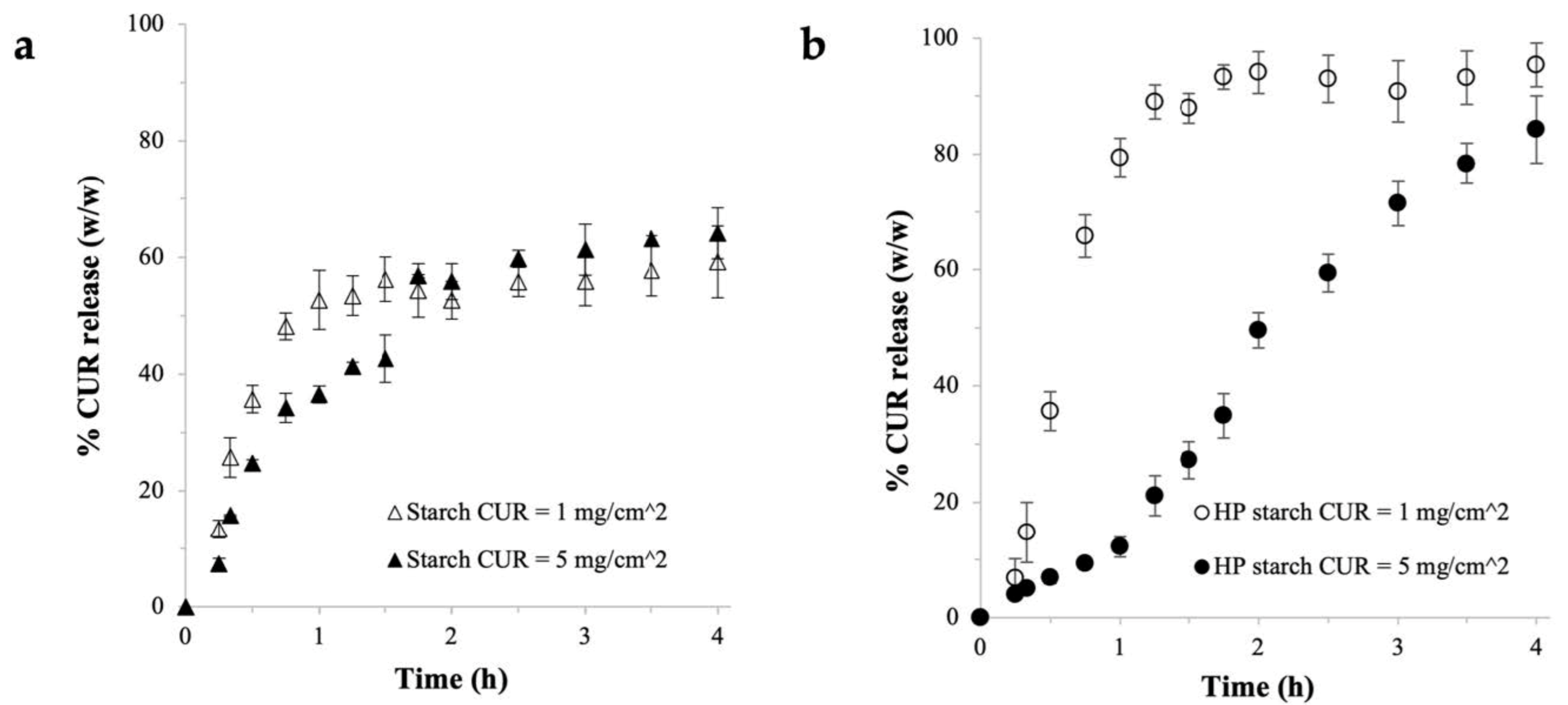
2.3.2. CUR Release Profile
2.4. Further Characterizations of HP Starch Films
2.4.1. FESEM and FTIR
2.4.2. CUR Payload Uniformity and Folding Endurance
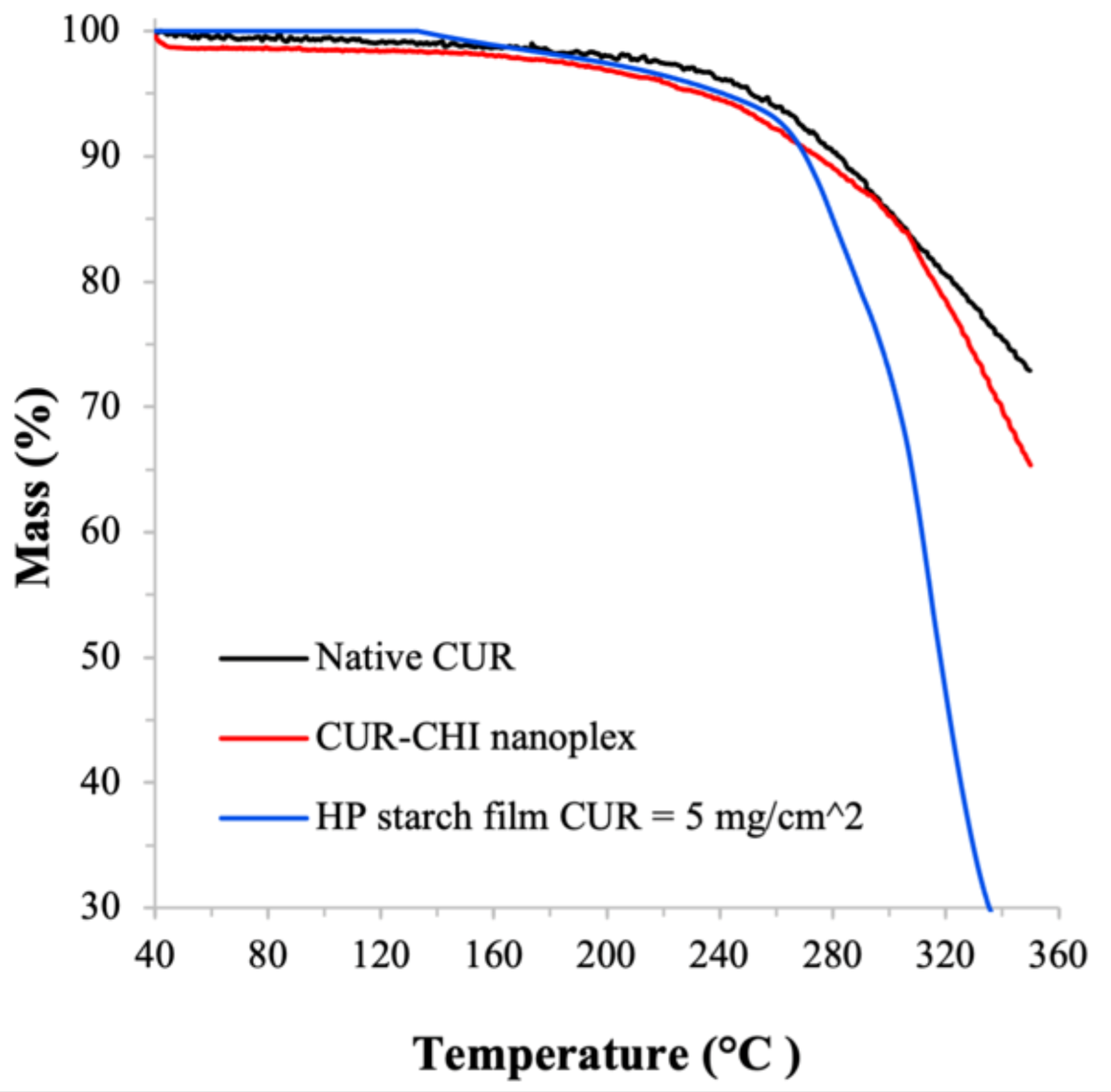
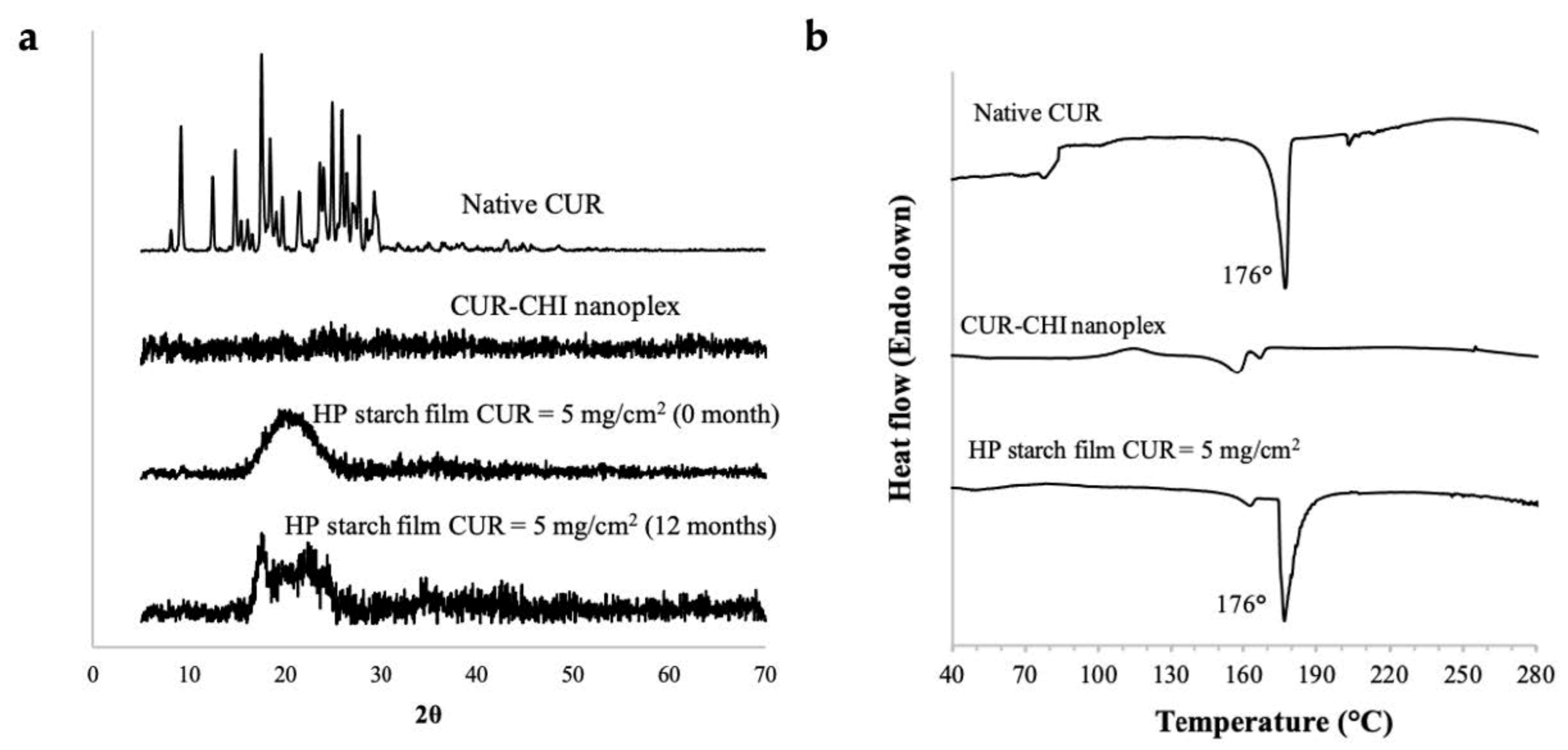
2.4.3. Amorphous form Stability and Thermal Stability
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation and Characterization of CUR–CHI Nanoplex
3.2.2. Preparation of CUR–CHI Nanoplex-Loaded Buccal Film
3.2.3. Experimental CUR Payload, CUR Payload Uniformity, CUR Entrapment Efficiency
3.2.4. Weight, Thickness, Folding Endurance of the Buccal Film
3.2.5. CUR Dissolution from Buccal Film and Film Disintegration
3.2.6. PXRD, DSC, and FTIR
3.2.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Acetic acid |
| AGN | Alginate |
| CSat | Thermodynamic saturation solubility |
| CHI | Chitosan |
| CUR | Curcumin |
| DLS | Dynamic light scattering |
| DSC | Differential scanning calorimetry |
| FESEM | Field emission scanning electron microscope |
| FTIR | Fourier transform infrared spectroscopy |
| Gly | Glycerol |
| HPMC | Hydroxypropyl methyl cellulose |
| HP | Hydroxypropyl |
| PG | Propylene glycol |
| PXRD | Powder X-ray diffraction |
| PVA | Polyvinyl alcohol |
| SSF | Simulated saliva fluid |
| TGA | Thermal gravimetry analysis |
| USP | United States Pharmacopeia |
Appendix A

References
- Hay, E.; Lucariello, A.; Contieri, M.; Esposito, T.; De Luca, A.; Guerra, G.; Perna, A. Therapeutic effects of turmeric in several diseases: An overview. Chem.-Biol. Interact. 2019, 310, 108729. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X.B. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033. [Google Scholar] [CrossRef] [Green Version]
- Barchitta, M.; Maugeri, A.; Favara, G.; San Lio, R.M.; Evola, G.; Agodi, A.; Basile, G. Nutrition and Wound Healing: An Overview Focusing on the Beneficial Effects of Curcumin. Int. J. Mol. Sci. 2019, 20, 1119. [Google Scholar] [CrossRef] [Green Version]
- Kunnumakkara, A.B.; Harsha, C.; Banik, K.; Vikkurthi, R.; Sailo, B.L.; Bordoloi, D.; Gupta, S.C.; Aggarwal, B.B. Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert Opin. Drug Metab. Toxicol. 2019, 15, 705–733. [Google Scholar] [CrossRef]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef]
- Bhutani, U.; Basu, T.; Majumdar, S. Oral Drug Delivery: Conventional to Long Acting New-Age Designs. Eur. J. Pharm. Biopharm. 2021, 162, 23–42. [Google Scholar] [CrossRef]
- Sharma, R.A.; Steward, W.P.; Gescher, A.J. Pharmacokinetics and pharmacodynamics of curcumin. Adv. Exp. Med. Biol. 2007, 595, 453–470. [Google Scholar] [PubMed] [Green Version]
- Iurciuc, C.-E.; Atanase, L.I.; Jérôme, C.; Sol, V.; Martin, P.; Popa, M.; Ochiuz, L. Polysaccharides-Based Complex Particles’ Protective Role on the Stability and Bioactivity of Immobilized Curcumin. Int. J. Mol. Sci. 2021, 22, 3075. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, K.; Buccarello, L.; Dragotto, J.; Mohammadi, A.; Corbo, M.; Feligioni, M. Obstacles against the Marketing of Curcumin as a Drug. Int. J. Mol. Sci. 2020, 21, 6619. [Google Scholar] [CrossRef] [PubMed]
- Arioglu-Tuncil, S.; Voelker, A.L.; Taylor, L.S.; Mauer, L.J. Amorphization of Thiamine Mononitrate: A Study of Crystallization Inhibition and Chemical Stability of Thiamine in Thiamine Mononitrate Amorphous Solid Dispersions. Int. J. Mol. Sci. 2020, 21, 9370. [Google Scholar] [CrossRef] [PubMed]
- Rho, K.; Hadinoto, K. Dry powder inhaler delivery of amorphous drug nanoparticles: Effects of the lactose carrier particle shape and size. Powder Technol. 2013, 233, 303–311. [Google Scholar]
- Cheow, W.S.; Hadinoto, K. Green Amorphous Nanoplex as a New Supersaturating Drug Delivery System. Langmuir 2012, 28, 6265–6275. [Google Scholar] [CrossRef]
- Wegiel, L.A.; Zhao, Y.; Mauer, L.J.; Edgar, K.J.; Taylor, L.S. Curcumin amorphous solid dispersions: The influence of intra and intermolecular bonding on physical stability. Pharm. Dev. Technol. 2014, 19, 976–986. [Google Scholar] [CrossRef]
- Sunagawa, Y.; Miyazaki, Y.; Funamoto, M.; Shimizu, K.; Shimizu, S.; Nurmila, S.; Katanasaka, Y.; Ito, M.; Ogawa, T.; Ozawa-Umeta, H.; et al. A novel amorphous preparation improved curcumin bioavailability in healthy volunteers: A single-dose, double-blind, two-way crossover study. J. Funct. Foods 2021, 81, 104443. [Google Scholar] [CrossRef]
- Sekitoh, T.; Okamoto, T.; Fujioka, A.; Tramis, O.; Takeda, K.; Matsuura, T.; Imanaka, H.; Ishida, N.; Imamura, K. Sole-amorphous-sugar-based solid dispersion of curcumin and the influence of formulation composition and heat treatment on the dissolution of curcumin. Dry. Technol. 2020, 1–10. [Google Scholar] [CrossRef]
- Skieneh, J.M.; Sathisaran, I.; Dalvi, S.V.; Rohani, S. Co-Amorphous Form of Curcumin–Folic Acid Dihydrate with Increased Dissolution Rate. Cryst. Growth Des. 2017, 17, 6273–6280. [Google Scholar] [CrossRef]
- Suresh, K.; Mannava, M.K.C.; Nangia, A. A novel curcumin–artemisinin coamorphous solid: Physical properties and pharmacokinetic profile. RSC Adv. 2014, 4, 58357–58361. [Google Scholar] [CrossRef]
- Bhagwat, A.; Pathan, I.B.; Chishti, N.A.H. Design and optimization of pellets formulation containing curcumin ascorbic acid co-amorphous mixture for ulcerative colitis management. Part. Sci. Technol. 2020, 1–9. [Google Scholar] [CrossRef]
- Lim, L.M.; Tran, T.T.; Long Wong, J.J.; Wang, D.; Cheow, W.S.; Hadinoto, K. Amorphous ternary nanoparticle complex of curcumin-chitosan-hypromellose exhibiting built-in solubility enhancement and physical stability of curcumin. Colloids Surf. B Biointerfaces 2018, 167, 483–491. [Google Scholar] [CrossRef]
- Araki, K.; Yoshizumi, M.; Kimura, S.; Tanaka, A.; Inoue, D.; Furubayashi, T.; Sakane, T.; Enomura, M. Application of a Microreactor to Pharmaceutical Manufacturing: Preparation of Amorphous Curcumin Nanoparticles and Controlling the Crystallinity of Curcumin Nanoparticles by Ultrasonic Treatment. AAPS PharmSciTech 2019, 21, 17. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Ciftci, O.N. A novel and green nanoparticle formation approach to forming low-crystallinity curcumin nanoparticles to improve curcumin’s bioaccessibility. Sci. Rep. 2019, 9, 19112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheow, W.S.; Kiew, T.Y.; Hadinoto, K. Amorphous nanodrugs prepared by complexation with polysaccharides: Carrageenan versus dextran sulfate. Carbohydr. Polym. 2015, 117, 549–558. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Lee, S.E.; Tran, T.T.; Bui, C.B.; Nguyen, T.H.; Vu, N.B.; Tran, T.T.; Nguyen, T.H.; Nguyen, T.T.; Hadinoto, K. A simple strategy to enhance the in vivo wound-healing activity of curcumin in the form of self-assembled nanoparticle complex of curcumin and oligochitosan. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Vu, N.B.; Nguyen, T.H.; Le, H.S.; Le, H.T.; Tran, T.T.; Le, X.C.; Le, V.T.; Nguyen, T.T.; Bui, C.B.; et al. In vivo comparison of wound healing and scar treatment effect between curcumin-oligochitosan nanoparticle complex and oligochitosan-coated curcumin-loaded-liposome. J. Microencapsul. 2019, 36, 156–168. [Google Scholar] [CrossRef]
- Adiwidjaja, J.; McLachlan, A.J.; Boddy, A.V. Curcumin as a clinically-promising anti-cancer agent: Pharmacokinetics and drug interactions. Expert Opin. Drug Metab. Toxicol. 2017, 13, 953–972. [Google Scholar] [CrossRef] [PubMed]
- Sintov, A.C. Transdermal delivery of curcumin via microemulsion. Int. J. Pharm. 2015, 481, 97–103. [Google Scholar] [CrossRef]
- Sun, Y.; Du, L.; Liu, Y.; Li, X.; Li, M.; Jin, Y.; Qian, X. Transdermal delivery of the in situ hydrogels of curcumin and its inclusion complexes of hydroxypropyl-β-cyclodextrin for melanoma treatment. Int. J. Pharm. 2014, 469, 31–39. [Google Scholar] [CrossRef]
- Eckert, R.W.; Wiemann, S.; Keck, C.M. Improved Dermal and Transdermal Delivery of Curcumin with SmartFilms and Nanocrystals. Molecules 2021, 26, 1633. [Google Scholar] [CrossRef]
- Mazzarino, L.; Borsali, R.; Lemos-Senna, E. Mucoadhesive films containing chitosan-coated nanoparticles: A new strategy for buccal curcumin release. J. Pharm. Sci. 2014, 103, 3764–3771. [Google Scholar] [CrossRef]
- Hazzah, H.A.; Farid, R.M.; Nasra, M.M.; El-Massik, M.A.; Abdallah, O.Y. Lyophilized sponges loaded with curcumin solid lipid nanoparticles for buccal delivery: Development and characterization. Int. J. Pharm. 2015, 492, 248–257. [Google Scholar] [CrossRef]
- Gowthamarajan, K.; Jawahar, N.; Wake, P.; Jain, K.; Sood, S. Development of buccal tablets for curcumin using Anacardium occidentale gum. Carbohydr. Polym. 2012, 88, 1177–1183. [Google Scholar] [CrossRef]
- Gilhotra, R.M.; Ikram, M.; Srivastava, S.; Gilhotra, N. A clinical perspective on mucoadhesive buccal drug delivery systems. J. Biomed Res. 2014, 28, 81–97. [Google Scholar]
- Forouzanfar, F.; Forouzanfar, A.; Sathyapalan, T.; Orafai, H.M.; Sahebkar, A. Curcumin for the Management of Periodontal Diseases: A Review. Curr. Pharm. Des. 2020, 26, 4277–4284. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Madhavi, D.; Kagan, D. Bioavailability of a Sustained Release Formulation of Curcumin. Integr. Med. 2014, 13, 24–30. [Google Scholar]
- Krull, S.M.; Susarla, R.; Afolabi, A.; Li, M.; Ying, Y.; Iqbal, Z.; Bilgili, E.; Davé, R.N. Polymer strip films as a robust, surfactant-free platform for delivery of BCS Class II drug nanoparticles. Int. J. Pharm. 2015, 489, 45–57. [Google Scholar] [CrossRef]
- Sneha, R.; Vedha Hari, B.N.; Ramya Devi, D. Design of antiretroviral drug-polymeric nanoparticles laden buccal films for chronic HIV therapy in paediatrics. Colloid Interface Sci. Commun. 2018, 27, 49–59. [Google Scholar] [CrossRef]
- Dos Santos, T.C.; Rescignano, N.; Boff, L.; Reginatto, F.H.; Simões, C.M.O.; de Campos, A.M.; Mijangos, C.U. Manufacture and characterization of chitosan/PLGA nanoparticles nanocomposite buccal films. Carbohydr. Polym. 2017, 173, 638–644. [Google Scholar] [CrossRef]
- Rana, P.; Murthy, R.S.R. Formulation and evaluation of mucoadhesive buccal films impregnated with carvedilol nanosuspension: A potential approach for delivery of drugs having high first-pass metabolism. Drug Deliv. 2013, 20, 224–235. [Google Scholar] [CrossRef] [Green Version]
- Chonkar, A.D.; Rao, J.V.; Managuli, R.S.; Mutalik, S.; Dengale, S.; Jain, P.; Udupa, N. Development of fast dissolving oral films containing lercanidipine HCl nanoparticles in semicrystalline polymeric matrix for enhanced dissolution and ex vivo permeation. Eur. J. Pharm. Biopharm. 2016, 103, 179–191. [Google Scholar] [CrossRef]
- Morales, J.O.; Brayden, D.J. Buccal delivery of small molecules and biologics: Of mucoadhesive polymers, films, and nanoparticles. Curr. Opin. Pharm. 2017, 36, 22–28. [Google Scholar] [CrossRef]
- Kraisit, P.; Limmatvapirat, S.; Nunthanid, J.; Sriamornsak, P.; Luangtana-Anan, M. Preparation and Characterization of Hydroxypropyl Methylcellulose/Polycarbophil Mucoadhesive Blend Films Using a Mixture Design Approach. Chem. Pharm. Bull. 2017, 65, 284–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peh, K.K.; Wong, C.F. Polymeric films as vehicle for buccal delivery: Swelling, mechanical, and bioadhesive properties. J. Pharm. Pharm. Sci. 1999, 2, 53–61. [Google Scholar] [PubMed]
- Chan, S.Y.; Goh, C.F.; Lau, J.Y.; Tiew, Y.C.; Balakrishnan, T. Rice starch thin films as a potential buccal delivery system: Effect of plasticiser and drug loading on drug release profile. Int. J. Pharm. 2019, 562, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Soe, M.T.; Pongjanyakul, T.; Limpongsa, E.; Jaipakdee, N. Modified glutinous rice starch-chitosan composite films for buccal delivery of hydrophilic drug. Carbohydr. Polym. 2020, 245, 116556. [Google Scholar] [CrossRef]
- Cheow, W.S.; Kiew, T.Y.; Hadinoto, K. Combining inkjet printing and amorphous nanonization to prepare personalized dosage forms of poorly-soluble drugs. Eur. J. Pharm. Biopharm. 2015, 96, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S. Handbook of Pharmaceutical Manufacturing Formulations, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Fan, N.; He, Z.; Ma, P.; Wang, X.; Li, C.; Sun, J.; Sun, Y.; Li, J. Impact of HPMC on inhibiting crystallization and improving permeability of curcumin amorphous solid dispersions. Carbohydr. Polym. 2018, 181, 543–550. [Google Scholar] [CrossRef]
- Diaz, D.A.; Colgan, S.T.; Langer, C.S.; Bandi, N.T.; Likar, M.D.; Van Alstine, L. Dissolution Similarity Requirements: How Similar or Dissimilar Are the Global Regulatory Expectations? AAPS J. 2016, 18, 15–22. [Google Scholar] [CrossRef]
- Pamlényi, K.; Kristó, K.; Jójárt-Laczkovich, O.; Regdon, G., Jr. Formulation and Optimization of Sodium Alginate Polymer Film as a Buccal Mucoadhesive Drug Delivery System Containing Cetirizine Dihydrochloride. Pharmaceutics 2021, 13, 619. [Google Scholar] [CrossRef]
- Okeke, O.C.; Boateng, J.S. Composite HPMC and sodium alginate based buccal formulations for nicotine replacement therapy. Int. J. Biol. Macromol. 2016, 91, 31–44. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, L.; Ren, M.-H.; BeMiller, J.N. Developments in Hydroxypropylation of Starch: A Review. Starch-Stärke 2019, 71, 1800167. [Google Scholar] [CrossRef] [Green Version]
- Warren, F.J.; Gidley, M.J.; Flanagan, B.M. Infrared spectroscopy as a tool to characterise starch ordered structure—A joint FTIR-ATR, NMR, XRD and DSC study. Carbohydr. Polym. 2016, 139, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- USP-34. <905> “Uniformity of Dosage Unit”. In United States Pharmacopeia (USP 34-NF29); The United States Pharmacopeial Convention: Rockville, MD, USA, 2011. [Google Scholar]


| Type of Film | Theoretical CUR Payload (mg/cm2) | % CUR Entrapment (w/w) | CUR Payload (mg/cm2) | Weight (mg/cm2) | Thickness (µm) |
|---|---|---|---|---|---|
| HPMC (Gly) | 1 | 81 ± 3 | 0.8 ± 0.03 | 13 ± 1 | 110 ± 3 |
| HPMC (Gly) | 5 | 82 ± 5 | 4 ± 0.3 | 30 ± 1 | 231 ± 7 |
| HPMC (PG) | 1 | 83 ± 3 | 0.8 ± 0.03 | 17 ± 2 | 138 ± 6 |
| HPMC (PG) | 5 | 79 ± 8 | 4 ± 0.4 | 31 ± 1 | 254 ± 5 |
| Type of Film | Theoretical CUR Payload (mg/cm2) | % CUR Entrapment (w/w) | CUR Payload (mg/cm2) | Weight (mg/cm2) | Thickness (µm) |
|---|---|---|---|---|---|
| HPMC–PVA (Gly) | 1 | 59 ± 1 | 0.6 ± 0.01 | 16 ± 1 | 130 ± 5 |
| HPMC–PVA (Gly) | 5 | 57 ± 9 | 3 ± 0.4 | 24 ± 1 | 194 ± 5 |
| HPMC–AGN (Gly) | 1 | 58 ± 1 | 0.6 ± 0.01 | 20 ± 2 | 159 ± 15 |
| HPMC–AGN (Gly) | 5 | 63 ± 2 | 3 ± 0.1 | 27 ± 3 | 230 ± 5 |
| Type of Film | Theoretical CUR Payload (mg/cm2) | % CUR Entrapment (w/w) | CUR Payload (mg/cm2) | Weight (mg/cm2) | Thickness (µm) |
|---|---|---|---|---|---|
| Starch | 1 | 74 ± 5 | 0.7 ± 0.05 | 65 ± 2 | 404 ± 7 |
| Starch | 5 | 79 ± 5 | 4 ± 0.2 | 65 ± 4 | 453 ± 23 |
| HP starch | 1 | 74 ± 3 | 0.7 ± 0.03 | 44 ± 1 | 307 ± 5 |
| HP starch | 5 | 77 ± 6 | 4 ± 0.3 | 51 ± 2 | 352 ± 10 |
| CUR Payload (mg/cm2) | AV for CUR Payload (%) | Folding Endurance |
|---|---|---|
| 1 | 13.0 | 2.5 0.07 |
| 5 | 15.2 | 2.7 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, L.M.; Hadinoto, K. High-Payload Buccal Delivery System of Amorphous Curcumin–Chitosan Nanoparticle Complex in Hydroxypropyl Methylcellulose and Starch Films. Int. J. Mol. Sci. 2021, 22, 9399. https://doi.org/10.3390/ijms22179399
Lim LM, Hadinoto K. High-Payload Buccal Delivery System of Amorphous Curcumin–Chitosan Nanoparticle Complex in Hydroxypropyl Methylcellulose and Starch Films. International Journal of Molecular Sciences. 2021; 22(17):9399. https://doi.org/10.3390/ijms22179399
Chicago/Turabian StyleLim, Li Ming, and Kunn Hadinoto. 2021. "High-Payload Buccal Delivery System of Amorphous Curcumin–Chitosan Nanoparticle Complex in Hydroxypropyl Methylcellulose and Starch Films" International Journal of Molecular Sciences 22, no. 17: 9399. https://doi.org/10.3390/ijms22179399






